Bacterial Cellulose Membrane Containing Epilobium angustifolium L. Extract as a Promising Material for the Topical Delivery of Antioxidants to the Skin
Abstract
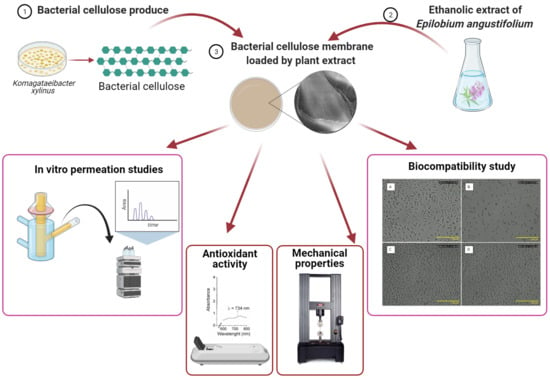
:1. Introduction
2. Results
2.1. Chemical Composition and Antioxidant Activity of the FEEs
2.2. The TG, DTG, FTIR SEM, and Mechanical Properties of BC and BC-FEEs
2.3. Antioxidant Properties of Phenolic Acids Contain in BC and BC-FEEs
2.4. Biocompatibility Study
2.5. In Vitro Penetration Studies
3. Discussion
4. Materials and Methods
- Atest—cells with medium containing the extracts,
- Ablank—medium with the respective extract (without cells),
- Acontrol—cells with a free medium.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Raiszadeh-Jahromi, Y.; Rezazadeh-Bari, M.; Almasi, H.; Amiri, S. Optimization of Bacterial Cellulose Production by Komagataeibacter Xylinus PTCC 1734 in a Low-Cost Medium Using Optimal Combined Design. J. Food Sci. Technol. 2020, 57, 2524–2533. [Google Scholar] [CrossRef] [PubMed]
- Hur, D.H.; Rhee, H.-S.; Lee, J.H.; Shim, W.Y.; Kim, T.Y.; Lee, S.Y.; Park, J.H.; Jeong, K.J. Enhanced Production of Cellulose in Komagataeibacter Xylinus by Preventing Insertion of IS Element into Cellulose Synthesis Gene. Biochem. Eng. J. 2020, 156, 107527. [Google Scholar] [CrossRef]
- Almeida, I.F.; Pereira, T.; Silva, N.H.C.S.; Gomes, F.P.; Silvestre, A.J.D.; Freire, C.S.R.; Sousa Lobo, J.M.; Costa, P.C. Bacterial Cellulose Membranes as Drug Delivery Systems: An In Vivo Skin Compatibility Study. Eur. J. Pharm. Biopharm. 2014, 86, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Rajwade, J.M.; Paknikar, K.M.; Kumbhar, J.V. Applications of Bacterial Cellulose and Its Composites in Biomedicine. Appl. Microbiol. Biotechnol. 2015, 99, 2491–2511. [Google Scholar] [CrossRef] [PubMed]
- Ullah, H.; Santos, H.A.; Khan, T. Applications of Bacterial Cellulose in Food, Cosmetics and Drug Delivery. Cellulose 2016, 23, 2291–2314. [Google Scholar] [CrossRef]
- Oprea, M.; Voicu, S.I. Recent Advances in Composites Based on Cellulose Derivatives for Biomedical Applications. Carbohydr. Polym. 2020, 247, 116683. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Song, J.E.; Kim, H.R. Comparative Study on the Physical Entrapment of Soy and Mushroom Proteins on the Durability of Bacterial Cellulose Bio-Leather. Cellulose 2021, 28, 3183–3200. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, X.; Jiang, Q.; Yu, D.; Xu, Y.; Wang, B.; Xia, W. Development and Properties of Bacterial Cellulose, Curcumin, and Chitosan Composite Biodegradable Films for Active Packaging Materials. Carbohydr. Polym. 2021, 260, 117778. [Google Scholar] [CrossRef]
- Swingler, S.; Gupta, A.; Gibson, H.; Kowalczuk, M.; Heaselgrave, W.; Radecka, I. Recent Advances and Applications of Bacterial Cellulose in Biomedicine. Polymers 2021, 13, 412. [Google Scholar] [CrossRef]
- Ahmed, J.; Gultekinoglu, M.; Edirisinghe, M. Bacterial Cellulose Micro-Nano Fibres for Wound Healing Applications. Biotechnol. Adv. 2020, 41, 107549. [Google Scholar] [CrossRef]
- Islam, S.U.; Ul-Islam, M.; Ahsan, H.; Ahmed, M.B.; Shehzad, A.; Fatima, A.; Sonn, J.K.; Lee, Y.S. Potential Applications of Bacterial Cellulose and Its Composites for Cancer Treatment. Int. J. Biol. Macromol. 2021, 168, 301–309. [Google Scholar] [CrossRef]
- Nicolai, M.; Mota, J.; Fernandes, A.S.; Pereira, F.; Pereira, P.; Reis, P.C.; Robles Velasco, M.V.; Baby, A.R.; Rosado, C.; Rijo, P. Assessment of the Potential Skin Application of Plectranthus Ecklonii Benth. Pharmaceuticals 2020, 13, 120. [Google Scholar] [CrossRef]
- De Fernandes, I.A.A.; Maciel, G.M.; Oliveira, A.L.M.S.; Miorim, A.J.F.; Fontana, J.D.; Ribeiro, V.R.; Haminiuk, C.W.I. Hybrid Bacterial Cellulose-Collagen Membranes Production in Culture Media Enriched with Antioxidant Compounds from Plant Extracts. Polym. Eng. Sci. 2020, 60, 2814–2826. [Google Scholar] [CrossRef]
- Fatima, A.; Yasir, S.; Khan Mohd, S.; Manan, S.; Ullah, M.W.; Ul-Islam, M. Plant Extract-Loaded Bacterial Cellulose Composite Membrane for Potential Biomedical Applications. J. Bioresour. Bioprod. 2021, 6, 26–32. [Google Scholar] [CrossRef]
- Karl, B.; Alkhatib, Y.; Beekmann, U.; Bellmann, T.; Blume, G.; Steiniger, F.; Thamm, J.; Werz, O.; Kralisch, D.; Fischer, D. Development and Characterization of Bacterial Nanocellulose Loaded with Boswellia Serrata Extract Containing Nanoemulsions as Natural Dressing for Skin Diseases. Int. J. Pharm. 2020, 587, 119635. [Google Scholar] [CrossRef]
- Indrianingsih, A.W.; Rosyida, V.T.; Apriyana, W.; Hayati, S.N.; Darsih, C.; Nisa, K.; Ratih, D. Antioxidant and Antibacterial Properties of Bacterial Cellulose—Indonesian Plant Extract Composites for Mask Sheet. J. Appl. Pharm. Sci. 2020, 37–42. [Google Scholar] [CrossRef]
- Moradian, S.; Almasi, H.; Moini, S. Development of Bacterial Cellulose-Based Active Membranes Containing Herbal Extracts for Shelf Life Extension of Button Mushrooms (Agaricus bisporus). Food Process. Preserv. 2017, 42, e13537. [Google Scholar] [CrossRef]
- Sukhtezari, S.; Almasi, H.; Pirsa, S.; Zandi, M.; Pirouzifard, M. Development of Bacterial Cellulose Based Slow-Release Active Films by Incorporation of Scrophularia striata Boiss. Extract. Carbohydr. Polym. 2017, 156, 340–350. [Google Scholar] [CrossRef]
- Pourali, P.; Yahyaei, B. The Healing Property of a Bioactive Wound Dressing Prepared by the Combination of Bacterial Cellulose (BC) and Zingiber Officinale Root Aqueous Extract in Rats. 3 Biotech 2019, 9, 59. [Google Scholar] [CrossRef]
- Asanarong, O.; Minh Quan, V.; Boonrungsiman, S.; Sukyai, P. Bioactive Wound Dressing Using Bacterial Cellulose Loaded with Papain Composite: Morphology, Loading/Release and Antibacterial Properties. Eur. Polym. J. 2021, 143, 110224. [Google Scholar] [CrossRef]
- Silva, N.H.C.S.; Drumond, I.; Almeida, I.F.; Costa, P.; Rosado, C.F.; Neto, C.P.; Freire, C.S.R.; Silvestre, A.J.D. Topical Caffeine Delivery Using Biocellulose Membranes: A Potential Innovative System for Cellulite Treatment. Cellulose 2014, 21, 665–674. [Google Scholar] [CrossRef] [Green Version]
- Trovatti, E.; Freire, C.S.R.; Pinto, P.C.; Almeida, I.F.; Costa, P.; Silvestre, A.J.D.; Neto, C.P.; Rosado, C. Bacterial Cellulose Membranes Applied in Topical and Transdermal Delivery of Lidocaine Hydrochloride and Ibuprofen: In Vitro Diffusion Studies. Int. J. Pharm. 2012, 435, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Silva, N.H.C.S.; Mota, J.P.; Santos de Almeida, T.; Carvalho, J.P.F.; Silvestre, A.J.D.; Vilela, C.; Rosado, C.; Freire, C.S.R. Topical Drug Delivery Systems Based on Bacterial Nanocellulose: Accelerated Stability Testing. Int. J. Mol. Sci. 2020, 21, 1262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luan, J.; Wu, J.; Zheng, Y.; Song, W.; Wang, G.; Guo, J.; Ding, X. Impregnation of Silver Sulfadiazine into Bacterial Cellulose for Antimicrobial and Biocompatible Wound Dressing. Biomed. Mater. 2012, 7, 065006. [Google Scholar] [CrossRef]
- Pavaloiu, R.-D.; Stoica, A.; Stroescu, M.; Dobre, T. Controlled Release of Amoxicillin from Bacterial Cellulose Membranes. Open Chem. 2014, 12, 962–967. [Google Scholar] [CrossRef]
- Taokaew, S.; Nunkaew, N.; Siripong, P.; Phisalaphong, M. Characteristics and Anticancer Properties of Bacterial Cellulose Films Containing Ethanolic Extract of Mangosteen Peel. J. Biomater. Sci. Polym. Ed. 2014, 25, 907–922. [Google Scholar] [CrossRef]
- Kalle, R.; Belichenko, O.; Kuznetsova, N.; Kolosova, V.; Prakofjewa, J.; Stryamets, N.; Mattalia, G.; Šarka, P.; Simanova, A.; Prūse, B.; et al. Gaining Momentum: Popularization of Epilobium angustifolium as Food and Recreational Tea on the Eastern Edge of Europe. Appetite 2020, 150, 104638. [Google Scholar] [CrossRef]
- Shi, H.; Sun, S.; Liu, X.; Fan, J.; Wang, J.; Zhao, K.; Wang, W. Allelopathic Potential and Mechanism of Rosebay Willowherb [Chamaenerion angustifolium (L.) Scop.] Demonstrated on Model Plant Lettuce. Phyton 2021, 90, 159–170. [Google Scholar] [CrossRef]
- Güven, S.; Makbul, S.; Mertayak, F.; Coşkunçelebi, K. Anatomical Properties of Epilobium and Chamaenerion from a Taxonomical Perspective in Turkey. Protoplasma 2021. [Google Scholar] [CrossRef]
- Ferrante, C.; Chiavaroli, A.; Angelini, P.; Venanzoni, R.; Flores, G.A.; Brunetti, L.; Petrucci, M.; Politi, M.; Menghini, L.; Leone, S.; et al. Phenolic Content and Antimicrobial and Anti-Inflammatory Effects of Solidago virga-aurea, Phyllanthus niruri, Epilobium angustifolium, Peumus boldus, and Ononis spinosa Extracts. Antibiotics 2020, 9, 783. [Google Scholar] [CrossRef]
- Zagórska-Dziok, M.; Ziemlewska, A.; Bujak, T.; Nizioł-Łukaszewska, Z.; Hordyjewicz-Baran, Z. Cosmetic and Dermatological Properties of Selected Ayurvedic Plant Extracts. Molecules 2021, 26, 614. [Google Scholar] [CrossRef]
- Battinelli, L.; Tita, B.; Evandri, M.G.; Mazzanti, G. Antimicrobial Activity of Epilobium Spp. Extracts. Il Farmaco 2001, 56, 345–348. [Google Scholar] [CrossRef]
- Lasinskas, M.; Jariene, E.; Vaitkeviciene, N.; Hallmann, E.; Najman, K. Effect of Different Durations of Solid-Phase Fermentation for Fireweed (Chamerion angustifolium (L.) Holub) Leaves on the Content of Polyphenols and Antioxidant Activity In Vitro. Molecules 2020, 25, 1011. [Google Scholar] [CrossRef] [Green Version]
- Nowak, A.; Klimowicz, A.; Duchnik, W.; Kucharski, Ł.; Florkowska, K.; Muzykiewicz, A.; Wira, D.; Zielonkabrzezicka, J.; Siedłowska, A.; Nadarzewska, K. Application of Green-Extraction Technique to Evaluate of Antioxidative Capacity of Wild Population of Fireweed (Epilobium angustifolium). Herba Pol. 2019, 65, 18–30. [Google Scholar] [CrossRef]
- Karakaya, S.; Süntar, I.; Yakinci, O.F.; Sytar, O.; Ceribasi, S.; Dursunoglu, B.; Ozbek, H.; Guvenalp, Z. In Vivo Bioactivity Assessment on Epilobium Species: A Particular Focus on Epilobium angustifolium and Its Components on Enzymes Connected with the Healing Process. J. Ethnopharmacol. 2020, 262, 113207. [Google Scholar] [CrossRef]
- Adamska-Szewczyk, A.; Zgórka, G. Plant Polyphenols in Cosmetics—A Review. Eur. J. Med. Technol. 2019, 3, 1–10. [Google Scholar]
- Dacrema, M.; Sommella, E.; Santarcangelo, C.; Bruno, B.; Marano, M.G.; Insolia, V.; Saviano, A.; Campiglia, P.; Stornaiuolo, M.; Daglia, M. Metabolic Profiling, In Vitro Bioaccessibility and In Vivo Bioavailability of a Commercial Bioactive Epilobium angustifolium L. Extract. Biomed. Pharmacother. 2020, 131, 110670. [Google Scholar] [CrossRef]
- Liu, J.; Du, C.; Beaman, H.T.; Monroe, M.B.B. Characterization of Phenolic Acid Antimicrobial and Antioxidant Structure-Property Relationships. Pharmaceutics 2020, 12, 419. [Google Scholar] [CrossRef]
- Barbi, S.; Taurino, C.; La China, S.; Anguluri, K.; Gullo, M.; Montorsi, M. Mechanical and Structural Properties of Environmental Green Composites Based on Functionalized Bacterial Cellulose. Cellulose 2021, 28, 1431–1442. [Google Scholar] [CrossRef]
- Pang, M.; Huang, Y.; Meng, F.; Zhuang, Y.; Liu, H.; Du, M.; Ma, Q.; Wang, Q.; Chen, Z.; Chen, L.; et al. Application of Bacterial Cellulose in Skin and Bone Tissue Engineering. Eur. Polym. J. 2020, 122, 109365. [Google Scholar] [CrossRef]
- Dreger, M.; Adamczak, A.; Seidler-Łożykowska, K.; Wielgus, K. Pharmacological Properties of Fireweed (Epilobium angustifolium L.) and Bioavailability of Ellagitannins. A Review. Herba Pol. 2020, 66, 52–64. [Google Scholar] [CrossRef]
- Nowak, A.; Cybulska, K.; Makuch, E.; Kucharski, Ł.; Różewicka-Czabańska, M.; Prowans, P.; Czapla, N.; Bargiel, P.; Petriczko, J.; Klimowicz, A. In Vitro Human Skin Penetration, Antioxidant and Antimicrobial Activity of Ethanol-Water Extract of Fireweed (Epilobium angustifolium L.). Molecules 2021, 26, 329. [Google Scholar] [CrossRef]
- Kaškonienė, V.; Stankevičius, M.; Drevinskas, T.; Akuneca, I.; Kaškonas, P.; Bimbiraitė-Survilienė, K.; Maruška, A.; Ragažinskienė, O.; Kornyšova, O.; Briedis, V.; et al. Evaluation of Phytochemical Composition of Fresh and Dried Raw Material of Introduced Chamerion angustifolium L. Using Chromatographic, Spectrophotometric and Chemometric Techniques. Phytochemistry 2015, 115, 184–193. [Google Scholar] [CrossRef]
- Zeng, Q.Y.; Wu, J.; Lin, P.C. Chemical Composition and Antimicrobial Activity of the Essential Oil from Epilobium angustifolium. Chem. Nat. Compd. 2016, 52, 1113–1115. [Google Scholar] [CrossRef]
- Bajer, T.; Šilha, D.; Ventura, K.; Bajerová, P. Composition and Antimicrobial Activity of the Essential Oil, Distilled Aromatic Water and Herbal Infusion from Epilobium Parviflorum Schreb. Ind. Crop. Prod. 2017, 100, 95–105. [Google Scholar] [CrossRef]
- Canli, K.; Yetgin, A.; Akata, I.; Altuner, E.M. Antimicrobial Activity and Chemical Composition Screening of Epilobium Montanum Root. Indian J. Pharm. Educ. Res. 2017, 51, s239–s243. [Google Scholar] [CrossRef]
- Jariene, E.; Lasinskas, M.; Danilcenko, H.; Vaitkeviciene, N.; Slepetiene, A.; Najman, K.; Hallmann, E. Polyphenols, Antioxidant Activity and Volatile Compounds in Fermented Leaves of Medicinal Plant Rosebay Willowherb (Chamerion angustifolium (L.) Holub). Plants 2020, 9, 1683. [Google Scholar] [CrossRef] [PubMed]
- Agatonovic-Kustrin, S.; Chan, C.K.Y.; Gegechkori, V.; Morton, D.W. Models for Skin and Brain Penetration of Major Components from Essential Oils Used in Aromatherapy for Dementia Patients. J. Biomol. Struct. Dyn. 2020, 38, 2402–2411. [Google Scholar] [CrossRef]
- Ruszová, E.; Cheel, J.; Pávek, S.; Moravcová, M.; Hermannová, M.; Matějková, I.; Spilková, J.; Velebný, V.; Kubala, L. Epilobium angustifolium Extract Demonstrates Multiple Effects on Dermal FIbroblasts In Vitro and Skin Photo-Protection In Vivo. Gen. Physiol. Biophys. 2013, 32, 347–359. [Google Scholar] [CrossRef] [Green Version]
- Shikov, A.N.; Poltanov, E.A.; Dorman, H.J.D.; Makarov, V.G.; Tikhonov, V.P.; Hiltunen, R. Chemical Composition and In Vitro Antioxidant Evaluation of Commercial Water-Soluble Willow Herb (Epilobium angustifolium L.) Extracts. J. Agric. Food Chem. 2006, 54, 3617–3624. [Google Scholar] [CrossRef]
- Bazargani, M.M.; Falahati-Anbaran, M.; Rohloff, J. Comparative Analyses of Phytochemical Variation Within and Between Congeneric Species of Willow Herb, Epilobium hirsutum and E. parviflorum: Contribution of Environmental Factors. Front. Plant Sci. 2021, 11, 595190. [Google Scholar] [CrossRef]
- Oney-Montalvo, J.E.; Avilés-Betanzos, K.A.; de Ramírez-Rivera, E.J.; Ramírez-Sucre, M.O.; Rodríguez-Buenfil, I.M. Polyphenols Content in Capsicum Chinense Fruits at Different Harvest Times and Their Correlation with the Antioxidant Activity. Plants 2020, 9, 1394. [Google Scholar] [CrossRef]
- Schepetkin, I.A.; Ramstead, A.G.; Kirpotina, L.N.; Voyich, J.M.; Jutila, M.A.; Quinn, M.T. Therapeutic Potential of Polyphenols from Epilobium angustifolium (Fireweed): Polyphenols from Fireweed. Phytother. Res. 2016, 30, 1287–1297. [Google Scholar] [CrossRef] [Green Version]
- Vitalone, A.; Allkanjari, O. Epilobium Spp: Pharmacology and Phytochemistry. Phytother. Res. 2018, 32, 1229–1240. [Google Scholar] [CrossRef]
- Efenberger-Szmechtyk, M.; Nowak, A.; Czyzowska, A. Plant Extracts Rich in Polyphenols: Antibacterial Agents and Natural Preservatives for Meat and Meat Products. Crit. Rev. Food Sci. Nutr. 2021, 61, 149–178. [Google Scholar] [CrossRef]
- Ikram, M.; Ali, N.; Jan, G.; Jan, F.G.; Romman, M.; Ishaq, M.; Islam, Y.; Khan, N. Antimicrobial and Antioxidant Activities of Methanolic Extract and Fractions of (Epilobium roseum) (Schreb.) against Bacterial Strains. Am. J. Plant Sci. 2021, 12, 275–284. [Google Scholar] [CrossRef]
- Ribeiro, A.; Estanqueiro, M.; Oliveira, M.; Sousa Lobo, J. Main Benefits and Applicability of Plant Extracts in Skin Care Products. Cosmetics 2015, 2, 48–65. [Google Scholar] [CrossRef] [Green Version]
- Działo, M.; Mierziak, J.; Korzun, U.; Preisner, M.; Szopa, J.; Kulma, A. The Potential of Plant Phenolics in Prevention and Therapy of Skin Disorders. Int. J. Mol. Sci. 2016, 17, 160. [Google Scholar] [CrossRef] [Green Version]
- Esposito, S.; De Simone, G.; Pan, A.; Brambilla, P.; Gattuso, G.; Mastroianni, C.; Kertusha, B.; Contini, C.; Massoli, L.; Francisci, D.; et al. Epidemiology and Microbiology of Skin and Soft Tissue Infections: Preliminary Results of a National Registry. J. Chemother. 2018, 31, 9–14. [Google Scholar] [CrossRef]
- Amparo, T.R.; Seibert, J.B.; de Vieira, P.M.A.; Teixeira, L.F.M.; dos Santos, O.D.H.; Souza, G.H.B. Herbal Medicines to the Treatment of Skin and Soft Tissue Infections: Advantages of the Multi-targets Action. Phytother. Res. 2019, 34, 94–103. [Google Scholar] [CrossRef]
- Onar, H.C.; Yusufoglu, A.; Turker, G.; Yanardag, R. Elastase, Tyrosinase and Lipoxygenase Inhibition and Antioxidant Activity of an Aqueous Extract from Epilobium angustifolium L. Leaves. J. Med. Plants Res. 2012, 6, 716–726. [Google Scholar] [CrossRef]
- Surma-Ślusarska, B.; Presler, S.; Danielewicz, D. Characteristics of Bacterial Cellulose Obtained from Acetobacter Xylinum Culture for Application in Papermaking. Fibres Text. East. Eur. 2008, 16, 108–111. [Google Scholar]
- Mohammadkazemi, F.; Azin, M.; Ashori, A. Production of Bacterial Cellulose Using Different Carbon Sources and Culture Media. Carbohydr. Polym. 2015, 117, 518–523. [Google Scholar] [CrossRef]
- Roman, M.; Winter, W.T. Effect of Sulfate Groups from Sulfuric Acid Hydrolysis on the Thermal Degradation Behavior of Bacterial Cellulose. Biomacromolecules 2004, 5, 1671–1677. [Google Scholar] [CrossRef]
- Kumbhar, J.V.; Rajwade, J.M.; Paknikar, K.M. Fruit Peels Support Higher Yield and Superior Quality Bacterial Cellulose Production. Appl. Microbiol. Biotechnol. 2015, 99, 6677–6691. [Google Scholar] [CrossRef]
- Piluzza, G.; Bullitta, S. Correlations between Phenolic Content and Antioxidant Properties in Twenty-Four Plant Species of Traditional Ethnoveterinary Use in the Mediterranean Area. Pharm. Biol. 2011, 49, 240–247. [Google Scholar] [CrossRef]
- Kalisz, S.; Oszmiański, J.; Kolniak-Ostek, J.; Grobelna, A.; Kieliszek, M.; Cendrowski, A. Effect of a Variety of Polyphenols Compounds and Antioxidant Properties of Rhubarb (Rheum rhabarbarum). LWT 2020, 118, 108775. [Google Scholar] [CrossRef]
- Wiegand, C.; Elsner, P.; Hipler, U.-C.; Klemm, D. Protease and ROS Activities Influenced by a Composite of Bacterial Cellulose and Collagen Type I In Vitro. Cellulose 2006, 13, 689–696. [Google Scholar] [CrossRef]
- Alonso, C.; Lucas, R.; Barba, C.; Marti, M.; Rubio, L.; Comelles, F.; Morales, J.C.; Coderch, L.; Parra, J.L. Skin Delivery of Antioxidant Surfactants Based on Gallic Acid and Hydroxytyrosol. J. Pharm. Pharmacol. 2015, 67, 900–908. [Google Scholar] [CrossRef]
- Fronza, M.; Heinzmann, B.; Hamburger, M.; Laufer, S.; Merfort, I. Determination of the Wound Healing Effect of Calendula Extracts Using the Scratch Assay with 3T3 Fibroblasts. J. Ethnopharmacol. 2009, 126, 463–467. [Google Scholar] [CrossRef]
- Da Pitz, H.S.; Pereira, A.; Blasius, M.B.; Voytena, A.P.L.; Affonso, R.C.L.; Fanan, S.; Trevisan, A.C.D.; Ribeiro-do-Valle, R.M.; Maraschin, M. In Vitro Evaluation of the Antioxidant Activity and Wound Healing Properties of Jaboticaba (Plinia peruviana) Fruit Peel Hydroalcoholic Extract. Oxid. Med. Cell. Longev. 2016, 2016, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacobi, U.; Kaiser, M.; Toll, R.; Mangelsdorf, S.; Audring, H.; Otberg, N.; Sterry, W.; Lademann, J. Porcine Ear Skin: An In Vitro Model for Human Skin. Skin Res. Technol. 2007, 13, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Janus, E.; Ossowicz, P.; Klebeko, J.; Nowak, A.; Duchnik, W.; Kucharski, Ł.; Klimowicz, A. Enhancement of Ibuprofen Solubility and Skin Permeation by Conjugation with L-Valine Alkyl Esters. RSC Adv. 2020, 10, 7570–7584. [Google Scholar] [CrossRef] [Green Version]
- Bertges, F.S.; da Penha Henriques do Amaral, M.; Rodarte, M.P.; Vieira Fonseca, M.J.; Sousa, O.V.; Pinto Vilela, F.M.; Alves, M.S. Assessment of Chemical Changes and Skin Penetration of Green Arabica Coffee Beans Biotransformed by Aspergillus oryzae. Biocatal. Agric. Biotechnol. 2020, 23, 101512. [Google Scholar] [CrossRef]
- Pirvu, L.; Nicorescu, I.; Hlevca, C.; Albu, B.; Nicorescu, V. Epilobi Hirsuti Herba Extracts Influence the In Vitro Activity of Common Antibiotics on Standard Bacteria. Open Chem. 2016, 14, 65–75. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Li, P.; Shangguan, L.; Ma, J.; Mao, K.; Zhang, Q.; Wang, Y.; Liu, Z.; Mao, K. A Novel Bacterial Cellulose Membrane Immobilized with Human Umbilical Cord Mesenchymal Stem Cells-Derived Exosome Prevents Epidural Fibrosis. Int. J. Nanomed. 2018, 13, 5257–5273. [Google Scholar] [CrossRef] [Green Version]
- Subtaweesin, C.; Woraharn, W.; Taokaew, S.; Chiaoprakobkij, N.; Sereemaspun, A.; Phisalaphong, M. Characteristics of Curcumin-Loaded Bacterial Cellulose Films and Anticancer Properties against Malignant Melanoma Skin Cancer Cells. Appl. Sci. 2018, 16, 1188. [Google Scholar] [CrossRef] [Green Version]
- Čuříková, B.A.; Procházková, K.; Filková, B.; Diblíková, P.; Svoboda, J.; Kováčik, A.; Vávrová, K.; Zbytovská, J. Simplified Stratum Corneum Model Membranes for Studying the Effects of Permeation Enhancers. Int. J. Pharm. 2017, 534, 287–296. [Google Scholar] [CrossRef]
- Badran, M.M.; Kuntsche, J.; Fahr, A. Skin Penetration Enhancement by a Microneedle Device (Dermaroller®) In Vitro: Dependency on Needle Size and Applied Formulation. Eur. J. Pharm. Sci. 2009, 36, 511–523. [Google Scholar] [CrossRef]
- Simon, A.; Amaro, M.I.; Healy, A.M.; Cabral, L.M.; de Sousa, V.P. Comparative Evaluation of Rivastigmine Permeation from a Transdermal System in the Franz Cell Using Synthetic Membranes and Pig Ear Skin with In Vivo-In Vitro Correlation. Int. J. Pharm. 2016, 512, 234–241. [Google Scholar] [CrossRef]
- Haq, A.; Michniak-Kohn, B. Effects of Solvents and Penetration Enhancers on Transdermal Delivery of Thymoquinone: Permeability and Skin Deposition Study. Drug Deliv. 2018, 25, 1943–1949. [Google Scholar] [CrossRef] [Green Version]
- Kopečná, M.; Macháček, M.; Prchalová, E.; Štěpánek, P.; Drašar, P.; Kotora, M.; Vávrová, K. Galactosyl Pentadecene Reversibly Enhances Transdermal and Topical Drug Delivery. Pharm. Res. 2017, 34, 2097–2108. [Google Scholar] [CrossRef]
- Davies, D.J.; Ward, R.J.; Heylings, J.R. Multi-Species Assessment of Electrical Resistance as a Skin Integrity Marker for In Vitro Percutaneous Absorption Studies. Toxicol. In Vitro 2004, 18, 351–358. [Google Scholar] [CrossRef]








| Evaluated Compound/Parameter | (mg/100 mL) |
|---|---|
| Chlorogenic acid (ChA) | 26.78 ± 0.55 |
| Gallic acid (GA) | 78.02 ± 1.00 |
| 4- hydroxybenzoic acid (4-HA) | 34.97 ± 0.07 |
| 3- hydroxybenzoic acid (3-HB) | 12.64 ± 1.20 |
| 3,4-dihydroxybenzoic acid (3,4-DHA) | 15.55 ± 0.38 |
| Caffeic acid (CA) | 7.13 ± 0.33 |
| Total polyphenol content (mmol GA/l) | 41.04 ± 0.10 |
| DPPH (mmol Trolox/l) | 19.36 ± 0.24 |
| ABTS (mmol Trolox/l) | 21.51 ± 0.86 |
| Sample | Young Modulus [MPa] | Elongation at Break [%] | Tensile Strength [MPa] |
|---|---|---|---|
| BC | 13,807.88 ± 596.43 | 0.85 ± 0.34 | 115.53 ± 15.28 |
| BC-5%FEE | 20,974.64 ± 115.12 | 1.08 ± 0.16 | 137.38 ± 40.86 |
| BC-10%FEE | 11,327.83 ± 144.20 | 0.76 ± 0.14 | 76.48 ± 19.06 |
| BC-5%FEE | BC-10%FEE | BC (Control) | ||
|---|---|---|---|---|
| Phenolic acid (µg/g membrane) | ChA | 83.69 ± 2.57 | 140.52 ± 6.44 | nd |
| GA | 275.44 ± 56.44 | 453.66 ± 6.95 | nd | |
| 4-HB | 150. 31 ± 11.31 | 285.15 ± 21.28 | nd | |
| 3-HB | 43.74 ± 4.83 | 72.50 ± 6.48 | nd | |
| 3,4-DHA | 75.93 ± 1.13 | 116.17 ± 8.65 | nd | |
| CA | 31.83 ± 1.23 | 57.80 ± 4.12 | nd | |
| Total polyphenol content (mmol GA/l) | 0.45 ± 0.01 | 0.63 ± 0.02 | na | |
| DPPH (mmol Trolox/l) | 0.44 ± 0.05 | 0.55 ± 0.01 | na | |
| ABTS (mmol Trolox/l) | 1.59 ± 0.01 | 2.09 ± 0.01 | na | |
| BC + FEE5% | BC + FEE 10% | BC | |
|---|---|---|---|
| Total polyphenol content (mmol GA/dm3) | 0.29 ± 0.02 | 0.59 ± 0.09 | nd |
| Cell viability (% of the control medium) | 73.99 ± 7.14 | 14.16 ± 5.91 | 102.21 ± 3.73 |
| BC + FEE5% | BC + FEE 10% | BC (Control) | ||
|---|---|---|---|---|
| Phenolic acid (µg) | ChA | 1.28 ± 0.25 | 2.16 ± 0.430 | nd |
| GA | 9.22 ± 0.84 | 12.26 ± 1.96 | nd | |
| 4-HB | 5.07 ± 0.78 | 6.56 ± 0.09 | nd | |
| 3-HB | < 0.50 | < 0.50 | nd | |
| 3,4-DHA | 2.01 ± 0.23 | 3.56 ± 0.46 | nd | |
| CA | < 0.50 | 1.40 ± 0.31 | nd | |
| Total polyphenol content (mmol GA/l) | 0.016 ± 0.01 | 0.051 ± 0.01 | na | |
| DPPH (mmol Trolox/l) | na | na | na | |
| ABTS (mmol Trolox/l) | 0.084 ± 0.02 | 0.15 ± 0.03 | na | |
| Phenolic Acid | BC-5%FEE | BC-10%FEE | ||||
|---|---|---|---|---|---|---|
| JSS, μg cm−2 h−1 | KP 10−5, cm h−1 | LT, h | JSS, μg cm−2 h−1 | KP 10−5, cm h−1 | LT, h | |
| ChA | -iv | -iv | ~5 | 0.267 ± 0.021 | 379.315 ± 36.493 | 2.379 |
| GA | 0.389 ± 0.096 | 309.168 ± 76.150 | 2.009 | 0.549 ± 0.079 | 242.032 ± 34.83 | 1.479 |
| 4-HB | 0.389 ± 0.043 | 517.582 ± 57.202 | 2.444 | 0.345 ± 0.008 | 241.976 ± 5.516 | 1.359 |
| 3-HB | -iv | -iv | ~24 | -iv | -iv | ~5 |
| 3,4-DHB | 0.195 ± 0.031 | 512.116 ± 81.140 | 1.820 | 0.345 ± 0.019 | 593.944 ± 33.447 | 1.359 |
| CA | -iv | -iv | 2.204 | 0.138 ± 0.022 | 477.516 ± 77.129 | 1.377 |
| BC + FEE5% | BC + FEE 10% | BC (Control) | ||
|---|---|---|---|---|
| Phenolic acid (µg/g skin) | ChA | 30.77 ± 0.95 | 41.05 ± 1.99 | nd |
| GA | 151.34 ± 13.85 | 222.94 ± 16.60 | nd | |
| 4-HB | 45.36 ± 1.33 | 127.67 ± 1.59 | nd | |
| 3-HB | 17.93 ± 0.93 | 30.62 ± 4.06 | nd | |
| 3,4-DHA | 31.91 ± 0.62 | 52.03 ± 5.11 | nd | |
| CA | 21.58 ±1.42 | 41.18 ± 3.16 | nd | |
| Total polyphenol content (mmol GA/l) | 0.33 ± 0.01 | 0.44 ± 0.01 | na | |
| DPPH (mmol Trolox/l) | 0.27 ± 0.005 | 0.39 ± 0.01 | na | |
| ABTS (mmol Trolox/l) | 1.02 ± 0.01 | 1.52 ± 0.06 | na | |
| Sample | mg FEE/g Membrane * |
|---|---|
| BC | - |
| BC-5%FEE | 465.0 |
| BC-10%FEE | 857.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nowak, A.; Ossowicz-Rupniewska, P.; Rakoczy, R.; Konopacki, M.; Perużyńska, M.; Droździk, M.; Makuch, E.; Duchnik, W.; Kucharski, Ł.; Wenelska, K.; et al. Bacterial Cellulose Membrane Containing Epilobium angustifolium L. Extract as a Promising Material for the Topical Delivery of Antioxidants to the Skin. Int. J. Mol. Sci. 2021, 22, 6269. https://doi.org/10.3390/ijms22126269
Nowak A, Ossowicz-Rupniewska P, Rakoczy R, Konopacki M, Perużyńska M, Droździk M, Makuch E, Duchnik W, Kucharski Ł, Wenelska K, et al. Bacterial Cellulose Membrane Containing Epilobium angustifolium L. Extract as a Promising Material for the Topical Delivery of Antioxidants to the Skin. International Journal of Molecular Sciences. 2021; 22(12):6269. https://doi.org/10.3390/ijms22126269
Chicago/Turabian StyleNowak, Anna, Paula Ossowicz-Rupniewska, Rafał Rakoczy, Maciej Konopacki, Magdalena Perużyńska, Marek Droździk, Edyta Makuch, Wiktoria Duchnik, Łukasz Kucharski, Karolina Wenelska, and et al. 2021. "Bacterial Cellulose Membrane Containing Epilobium angustifolium L. Extract as a Promising Material for the Topical Delivery of Antioxidants to the Skin" International Journal of Molecular Sciences 22, no. 12: 6269. https://doi.org/10.3390/ijms22126269
APA StyleNowak, A., Ossowicz-Rupniewska, P., Rakoczy, R., Konopacki, M., Perużyńska, M., Droździk, M., Makuch, E., Duchnik, W., Kucharski, Ł., Wenelska, K., & Klimowicz, A. (2021). Bacterial Cellulose Membrane Containing Epilobium angustifolium L. Extract as a Promising Material for the Topical Delivery of Antioxidants to the Skin. International Journal of Molecular Sciences, 22(12), 6269. https://doi.org/10.3390/ijms22126269