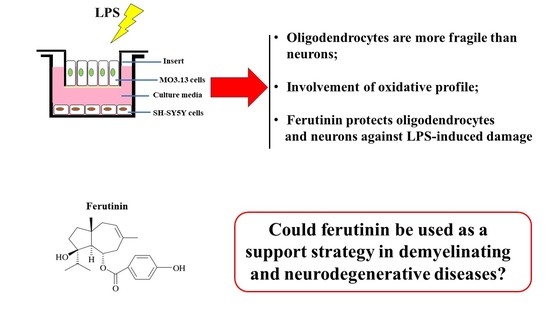
The Effect of Ferula communis Extract in Escherichia coli Lipopolysaccharide-Induced Neuroinflammation in Cultured Neurons and Oligodendrocytes
Abstract
:1. Introduction
2. Results
2.1. Both MO3.13 and SH-SY5Y Express Estrogen Receptor-β (ER-β)
2.2. 17-Beta Estradiol (17-β-E2) Protects Oligodendrocytes and Neurons from LPS-Induced Damage
2.3. Effects Induced by Treatment with FER
2.4. Evaluation of Cell Death through an Annexin V-PI Assay
2.5. Oxidative Profile in SH-SY5Y and MO3.13 Cells
2.6. Determination of Antioxidative Status in SH-SY5Y and MO3.13 Cells
3. Discussion and Future Perspectives
- (a)
- In this experimental system, neurons and oligodendrocytes were subjected to the accumulation of LPS-induced ROS and MDA, and FER was able to protect these cell lines from both;
- (b)
- The enzymatic activity of CAT, SOD, and GSH-Px was reduced after only 6 h of treatment with LPS, and the antioxidative status of the oligodendrocytes appeared to be particularly damaged. Presumably, therefore, neurons are not supported by oligodendrocytes in the management of antioxidative status. In any case, FER-LPS co-treatment was shown to support neurons and oligodendrocytes by protecting them from oxidative insult.
- Co-cultured Oligodendrocytes and neurons were a good experimental model to investigate because of the cross talk that physiologically exists between these cell lines. In particular, oligodendrocytes were found to be more vulnerable than neurons; oligodendrocytes were able to support neurons metabolically but were fragile from the perspective of antioxidative status;
- Pretreatment with FER was highly protective against the damage caused by LPS in both cell lines. These results highlight the protective action of estrogen, since FER exerts estrogenic actions, and indicate the use of FER as a potential therapeutic strategy to reduce the damage caused to neurons and oligodendrocytes in inflammatory neurodegenerative diseases. Thus, for the first time, we can include the use of sesquiterpene FER in the scenario of neurodegeneration. Finally, in this direction, FER could be an excellent candidate to replace the neuro-protective action of estrogen in menopause;
- Under inflammatory conditions, the antioxidative profiles of oligodendrocytes are greatly compromised. This information could, therefore, be of prime importance in the treatment of demyelinating diseases. Indeed, the protective role of natural compounds in neuroinflammatory diseases is becoming an important topic in scientific research [65,66,67].
4. Materials and Methods
4.1. LPS and Ferutinin
4.2. Cell Cultures
4.3. Cell Viability
4.4. Cell Lysis and Immunoblot Analysis
4.5. Intracellular ROS Detection
4.6. Malondialdehyde Assay
4.7. Annexin V Staining
4.8. Total RNA Extraction and RT-PCR
4.9. Superoxide Dismutase (SOD), Glutathione Peroxidase (GSH-Px), and Catalase (CAT) Activities
4.10. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Roleira, F.M.; Tavares-da-Silva, E.J.; Varela, C.L.; Costa, S.C.; Silva, T.; Garrido, J.; Borges, F. Plant derived and dietary phenolic antioxidants: Anticancer properties. Food Chem. 2015, 183, 235–258. [Google Scholar] [CrossRef]
- Azab, A.; Nassar, A.; Azab, A.N. Anti-Inflammatory activity of natural products. Molecules 2016, 21, 1321. [Google Scholar] [CrossRef]
- Tetali, S.D. Terpenes and isoprenoids: A wealth of compounds for global use. Planta 2019, 249, 1–8. [Google Scholar] [CrossRef]
- Pichersky, E.; Raguso, R.A. Why do plants produce so many terpenoid compounds? New Phytol. 2018, 220, 692–702. [Google Scholar] [CrossRef] [PubMed]
- Ponce-Rodríguez, H.D.; Herráez-Hernández, R.; Verdú-Andrés, J.; Campíns-Falcó, P. Quantitative Analysis of Terpenic Compounds in microsamples of resins by capillary liquid chromatography. Molecules 2019, 24, 4068. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González-Burgos, E.; Gómez-Serranillos, M.P. Terpene compounds in nature: A review of their potential antioxidant activity. Curr. Med. Chem. 2012, 19, 5319–5341. [Google Scholar] [CrossRef]
- Chen, J.; Jiang, Q.D.; Chai, Y.P.; Zhang, H.; Peng, P.; Yang, X.X. Natural terpenes as penetration enhancers for transdermal drug delivery. Molecules 2016, 21, 1709. [Google Scholar] [CrossRef] [Green Version]
- Yuyama, K.T.; Fortkamp, D.; Abraham, W.R. Eremophilane-type sesquiterpenes from fungi and their medicinal potential. Biol. Chem. 2017, 399, 13–28. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Chen, M.; Lang, Y.; Wang, X.; Zhuang, P. Sesquiterpenes from the fruits of Illicium Simonsii maxim. Nat. Prod. Res. 2020, 34, 903–908. [Google Scholar] [CrossRef] [PubMed]
- Chadwick, M.; Trewin, H.; Gawthrop, F.; Wagstaff, C. Sesquiterpenoids lactones: Benefits to plants and people. Int. J. Mol. Sci. 2013, 14, 12780–12805. [Google Scholar] [CrossRef] [Green Version]
- Jeena, K.; Liju, V.B.; Kuttan, R. A preliminary 13-week oral toxicity study of ginger oil in male and female Wistar rats. Int. J. Toxicol. 2011, 30, 662–670. [Google Scholar] [CrossRef] [PubMed]
- Ghareeb, D.A.; ElAhwany, A.M.D.; El-mallawany, S.M.; Saif, A.A. In vitro screening for anti-acetylcholiesterase, anti-oxidant, anti-glucosidase, anti-inflammatory and anti-bacterial effect of three traditional medicinal plants. Biotechnol. Biotechnol. Equip. 2014, 28, 1155–1164. [Google Scholar] [CrossRef] [Green Version]
- Ishnava, J.B.; Chauhan, M.B. Anticariogenic and phytochemical evaluation of Eucalyptus globules Labill. Saudi J. Biol. Sci. 2013, 20, 69–74. [Google Scholar] [CrossRef] [Green Version]
- Li-Weber, M.; Giaisi, M.; Treibe, M.K.; Krammer, P.H. The anti-inflammatory sesquiterpene lactone parthenolide suppresses IL-4 gene expression in peripheral blood T. Eur. J. Immunol. 2002, 32, 3587–3597. [Google Scholar] [CrossRef]
- Abramov, A.Y.; Zamaraeva, M.V.; Hagelgans, A.I.; Azimov, R.R.; Krasilnikov, O.V. Influence of plant terpenoids on the permeability of mitochondria and lipid bilayers. Biochim. Biophys. Acta 2001, 1512, 98–110. [Google Scholar] [CrossRef] [Green Version]
- Berridge, M.J.; Bootman, M.D.; Roderick, H.L. Calcium signaling: Dynamics, homeostasis and remodelling. Nat. Rev. Mol. Cell. Biol. 2003, 4, 517–529. [Google Scholar] [CrossRef] [Green Version]
- Sonigra, P.; Meena, M. Metabolic profile, bioactivities, and variations in the chemical constituents of essential oils of the Ferula genus (Apiaceae). Front. Pharmacol. 2021, 11, 608649. [Google Scholar] [CrossRef]
- Zofia, N.Ł.; Martyna, Z.D.; Aleksandra, Z.; Tomasz, B. Comparison of the antiaging and protective properties of plants from the apiaceae family. Oxid. Med. Cell. Longev. 2020, 2020, 5307614. [Google Scholar] [CrossRef] [PubMed]
- Akaberi, M.; Iranshahy, M.; Iranshahi, M. Review of the traditional uses, phytochemistry, pharmacology and toxicology of giant fennel (Ferula communis L. subsp. communis). Iran J. Basic Med. Sci 2015, 18, 1050–1062. [Google Scholar]
- Zhou, Y.; Xin, F.; Zhang, G.; Qu, H.; Yang, D.; Han, X. Recent advances on bioactive constituents in Ferula. Drug Dev. Res. 2017, 78, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Appendino, G.; Maxia, L.; Bascope, M.; Houghton, P.J.; Gonzalo, S.D.; Munoz, E.; Sterner, O. A meroterpenoid NF-κB inhibitor and drimane sesquiterpenoids from asafetida. J. Nat. Prod. 2006, 69, 1101–1104. [Google Scholar] [CrossRef] [PubMed]
- Li, G.Z.; Wang, J.C.; Li, X.J.; Cao, L.; Lv, N.; Si, J.Y. An unusual sesquiterpene coumarin from the seeds of Ferula sinkiangensis. J. Asian Nat. Prod. Res. 2016, 18, 891–896. [Google Scholar] [CrossRef]
- Iranshahy, M.; Iranshahi, M. Traditional uses, Phytochem. and pharmacology of asafoetida (Ferula assafoetida oleo-gumresin)—A review. J. Ethnopharmacol. 2011, 134, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Mansour, Z.; Insaf, F.; Aymen, J.; Joseph, C.; Jalloul, B.; Hichem, B.J. Chemical composition and in vitro evaluation of antimicrobial, antioxidant and antigerminative properties of the seed oil from the tunisian endemic Ferula tunetana POMEl ex BATT. Chem. Biodivers. 2017, 14, e1600116. [Google Scholar]
- Xin, Y.C.; Li, N.; Zhou, D.; Chen, G.; Jiao, K.; Wang, W.L.; Si, Y.Y.; Hou, Y. Sesquiterpene coumarins from Ferula sinkiangensis act as neuroinflammation inhibitors. Planta Med. 2017, 83, 135–142. [Google Scholar]
- Iranshahi, M.; Rezaee, R.; Najaf Najafi, M.; Haghbin, A.; Kasaian, J. Cytotoxic activity of the genus Ferula (Apiaceae) and its bioactive constituents. Avicenna J. Phytomed. 2018, 8, 296–312. [Google Scholar]
- Mirakabad, H.Z.; Farsi, M.; Shafaroudi, S.M.; Bagheri, A.; Iranshahi, M.; Moshtaghi, N. Comparison The effect of ferutinin and 17β-estradiol on Bone mineralization of developing Zebrafish (Danio rerio) larvae. Int. J. Mol. Sci. 2019, 20, 1507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Macrì, R.; Musolino, V.; Gliozzi, M.; Carresi, C.; Maiuolo, J.; Nucera, S.; Scicchitano, M.; Bosco, F.; Scarano, F.; Ruga, S.; et al. Ferula L. Plant Extracts and Dose-Dependent Activity of Natural Sesquiterpene Ferutinin: From Antioxidant Potential to Cytotoxic effects. Molecules 2020, 25, 5768. [Google Scholar] [CrossRef]
- Maiuolo, J.; Macrì, R.; Bava, I.; Gliozzi, M.; Musolino, V.; Nucera, S.; Carresi, C.; Sicchitano, M.; Bosco, F.; Scarano, F.; et al. Myelin disturbances produced by sub-toxic concentration of heavy metals: The role of oligodendrocyte dysfunction. Int. J. Mol. Sci. 2019, 20, 4554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, P.N.; Shah, R.Y.; Ferguson, J.F.; Reilly, M.P. Human experimental endotoxemia in modeling the pathophysiology, genomics, and therapeutics of innate immunity in complex cardiometabolic diseases. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 525–534. [Google Scholar] [CrossRef] [Green Version]
- Chae, B.S. Pretreatment of low-dose and super-low-dose LPS on the production of in vitro LPS-induced inflammatory mediators. Toxicol. Res. 2018, 34, 65–73. [Google Scholar] [CrossRef] [Green Version]
- Park, J.; Min, J.S.; Kim, B.; Chae, U.B.; Yun, J.W.; Choi, M.S.; Kong, I.-K.; Chang, K.-T.; Lee, D.-S. Mitochondrial ROS govern the LPS-induced pro-inflammatory response in microglia cells by regulating MAPK and NF-kB pathways. Neurosci. Lett. 2015, 584, 191–196. [Google Scholar] [CrossRef]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative stress: A Key modulator in neurodegenerative diseases. Molecules 2019, 24, 1583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Islam, M.T. Oxidative stress and mitochondrial dysfunction linked neurodegenerative disorders. Neurol. Res. 2017, 39, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Mosselman, S.; Polman, J.; Dijkema, R. ER-β: Identification and characterisation of a novel human estrogen receptor. FEBS Lett. 1996, 392, 49–53. [Google Scholar] [CrossRef] [Green Version]
- Speirs, V.; Malone, C.; Walton, D.S.; Kerin, M.J.; Atkin, S.L. Increased expression of estrogen receptor b mRNA in tamoxifen-resistant breast cancer patients. Cancer Res. 1999, 59, 5421–5424. [Google Scholar]
- Ding, X.; Gao, T.; Gao, P.; Meng, Y.; Zheng, Y.; Dong, L.; Luo, P.; Zhang, G.; Shi, X.; Rong, W. Activation of the G protein-coupled estrogen receptor elicits store calcium release and phosphorylation of the Mu-opioid receptors in the human neuroblastoma SH-SY5Y Cells. Front. Neurosci. 2019, 13, 1351. [Google Scholar] [CrossRef] [PubMed]
- Marraudino, M.; Carrillo, B.; Bonaldo, B.; Llorente, R.; Campioli, E.; Garate, I.; Pinos, H.; Garcia-Segura, L.M.; Collado, P.; Grassi, D. G protein-coupled estrogen receptor immunoreactivity in the rat hypothalamus is widely distributed in neurons, astrocytes and oligodendrocytes, fluctuates during the estrous cycle and is sexually dimorphic. Neuroendocrinology 2021, 111, 660–677. [Google Scholar] [CrossRef] [PubMed]
- Lei, J.; Wei, Y.; Song, P.; Li, Y.; Zhang, T.; Feng, Q.; Xu, G. Cordycepin inhibits LPS-induced acute lung injury by inhibiting inflammation and oxidative stress. Eur. J. Pharmacol. 2018, 818, 110–114. [Google Scholar] [CrossRef]
- Del Prete, C.; Stout, T.; Montagnaro, S.; Pagnini, U.; Uccello, M.; Florio, P.; Ciani, F.; Tafuri, S.; Palumbo, V.; Pasolini, M.P.; et al. Combined addition of superoxide dismutase, catalase and glutathione peroxidase improves quality of cooled stored stallion semen. Anim. Reprod. Sci. 2019, 210, 106195. [Google Scholar] [CrossRef]
- Stephenson, J.; Nutma, E.; van der Valk, P.; Amor, S. Inflammation in CNS neurodegenerative diseases. Immunology 2018, 154, 204–219. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; He, D.; Bai, Y. Microglia-mediated inflammation and neurodegenerative disease. Mol. Neurobiol. 2016, 53, 6709–6715. [Google Scholar] [CrossRef]
- Amor, S.; Peferoen, L.A.; Vogel, D.Y.; van der Valk, P.; Baker, D.; van Noort, J.M. Inflammation in neurodegenerative diseases—An update. Immunology 2014, 142, 151–166. [Google Scholar] [CrossRef]
- Magrone, T.; Marzulli, G.; Jirillo, E. Immunopathogenesis of neurodegenerative diseases: Current therapeutic models of neuroprotection with special reference to natural products. Curr. Pharm. Des. 2012, 18, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Oppedisano, F.; Maiuolo, J.; Gliozzi, M.; Musolino, V.; Carresi, C.; Nucera, S.; Scicchitano, M.; Scarano, F.; Bosco, F.; Macri, R.; et al. The potential for natural antioxidant supplementation in the early stages of neurodegenerative disorders. Int. J. Mol. Sci. 2020, 21, 2618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Höftberger, R.; Guo, Y.; Flanagan, E.P.; Lopez-Chiriboga, A.S.; Endmayr, V.; Hochmeister, S.; Joldic, D.; Pittock, S.J.; Tilemma, J.M.; Gorman, M.; et al. The pathology of central nervous system inflammatory demyelinating disease accompanying myelin oligodendrocyte glycoprotein autoantibody. Acta Neuropathol. 2020, 139, 875–892. [Google Scholar] [CrossRef] [Green Version]
- Maiuolo, J.; Gliozzi, M.; Musolino, V.; Carresi, C.; Nucera, S.; Scicchitano, M.; Scarano, F.; Bosco, F.; Oppedisano, F.; Macri, R.; et al. Environmental and nutritional “stressors” and oligodendrocyte dysfunction: Role of mitochondrial and endoplasmatic reticulum impairment. Biomedicines 2020, 8, 553. [Google Scholar] [CrossRef] [PubMed]
- Catorce, M.N.; Gevorkian, G. LPS-induced Murine Neuroinflammation model: Main features and suitability for pre-clinical assessment of nutraceuticals. Curr. Neuropharmacol. 2016, 14, 155–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, F.; Zhang, J.G.; Yang, W.; Xu, P.; Xiao, Y.L.; Zhang, H.T. 6-Gingerol attenuates LPS-induced neuroinflammation and cognitive impairment partially via suppressing astrocyte overactivation. Biomed. Pharmacother. 2018, 107, 1523–1529. [Google Scholar] [CrossRef]
- Walter, S.; Doering, A.; Letiembre, M.; Liu, Y.; Hao, W.; Diem, R.; Bernreuther, C.; Glatzel, M.; Engelhardt, B.; Fassbender, K. The LPS receptor, CD14 in experimental autoimmune encephalomyelitis and multiple sclerosis. Cell. Physiol. Biochem. 2006, 17, 167–172. [Google Scholar] [CrossRef]
- Lamoke, F.; Mazzone, V.; Persichini, T.; Maraschi, A.; Harris, M.B.; Venema, R.C.; Colasanti, M.; Gliozzi, M.; Muscoli, C.; Bartoli, K.; et al. Amyloid β peptide-induced inhibition of endothelial nitric oxide production involves oxidative stress-mediated constitutive eNOS/HSP90 interaction and disruption of agonist-mediated Akt activation. J. Neuroinflamm. 2015, 12, 84. [Google Scholar] [CrossRef] [Green Version]
- Maiuolo, J.; Gliozzi, M.; Musolino, V.; Scicchitano, M.; Carresi, C.; Scarano, F.; Bosco, F.; Nucera, S.; Ruga, S.; Zito, M.C.; et al. The “frail” brain blood barrier in neurodegenerative diseases: Role of early disruption of endothelial cell-to-cell connections. Int. J. Mol. Sci. 2018, 19, 2693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Philips, T.; Rothstein, J.D. Oligodendroglia: Metabolic supporters of neurons. J. Clin. Investig. 2017, 127, 3271–3280. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Dallenga, T.; Winkler, A.; Roemer, S.; Maruschak, B.; Siebert, H.; Bruck, W.; Stadelmann, C. Relationship of acute axonal damage, Wallerian degeneration, and clinical disability in multiple sclerosis. J. Neuroinflamm. 2017, 14, 57. [Google Scholar] [CrossRef] [Green Version]
- Vegeto, E.; Belcredito, S.; Ghisletti, S.; Meda, C.; Etteri, S.; Maggi, A. The endogenous estrogen status regulates microglia reactivity in animal models of neuroinflammation. Endocrinology 2016, 147, 2263–2272. [Google Scholar] [CrossRef] [Green Version]
- Sárvári, M.; Hrabovszky, E.; Kalló, I.; Solymosi, N.; Likó, I.; Berchtold, N.; Cortman, C.; Liposits, Z. Menopause leads to elevated expression of macrophage-associated genes in the aging frontal cortex: Rat and human studies identify strikingly similar changes. J. Neuroinflamm. 2012, 9, 264. [Google Scholar] [CrossRef] [Green Version]
- Villa, A.; Vegeto, E.; Poletti, A.; Maggi, A. Estrogens, neuroinflammation and neurodegeneration. Endocr. Rev. 2016, 37, 372–402. [Google Scholar] [CrossRef] [Green Version]
- Smith, J.A.; Das, A.; Butler, J.; Ray, S.K.; Banik, N.L. Estrogen or estrogen receptor agonist inhibits lipopolysaccharide induced microglial activation and death. Neurochem. Res. 2011, 36, 1587–1593. [Google Scholar] [CrossRef]
- Safi, R.; Hamade, A.; Bteich, N.; El Saghir, J.; Assaf, M.D.; El-Sabban, M.; Najjar, F. A ferutinin analogue with enhanced potency and selectivity against ER-positive breast cancer cells in vitro. Biomed. Pharmacother. 2018, 105, 267–273. [Google Scholar] [CrossRef]
- Zanoli, P.; Zavatti, M.; Geminiani, E.; Corsi, L.; Baraldi, M. The phytoestrogen ferutinin affects female sexual behavior modulating ERalpha expression in the hypothalamus. Behav Brain Res. 2009, 199, 283–287. [Google Scholar] [CrossRef]
- Russo, R.; Navarra, M.; Maiuolo, J.; Rotiroti, D.; Bagetta, G.; Corasaniti, M.T. 17β-estradiol protects SH-SY5Y cells against HIV-1 gp120-induced cell death: Evidence for a role of estrogen receptors. Neurotoxicology 2005, 26, 905–913. [Google Scholar] [CrossRef]
- Cantarella, G.; Risuglia, N.; Lombardo, G.; Lempereur, L.; Nicoletti, F.; Memo, M.; Bernardini, R. Protective effects of estradiol on TRAIL-induced apoptosis in a human oligodendrocytic cell line: Evidence for multiple sites of interactions. Cell Death Differ. 2004, 11, 503–511. [Google Scholar] [CrossRef]
- Bhat, A.H.; Dar, K.B.; Anees, S.; Zargar, M.A.; Masood, A.; Sofi, M.A.; Ganie, S.A. Oxidative stress, mitochondrial dysfunction and neurodegenerative diseases; a mechanistic insight. Biomed. Pharmacother. 2015, 74, 101–110. [Google Scholar] [CrossRef]
- Lim, J.L.; van der Pol, S.M.; Baron, W.; McCord, J.M.; de Vries, H.E.; van Horssen, J. Protandim protects oligodendrocytes against an oxidative insult. Antioxidants 2016, 5, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiocchio, I.; Prata, C.; Mandrone, M.; Ricciardiello, F.; Marrazzo, P.; Tomasi, P.; Angeloni, C.; Fiorentini, D.; Malaguti, M.; Poli, F.; et al. Leaves and spiny burs of Castanea sativa from an experimental chestnut grove: Metabolomic analysis and anti-neuroinflammatory activity. Metabolites 2020, 10, 408. [Google Scholar] [CrossRef]
- Cerulli, A.; Napolitano, A.; Hošek, J.; Masullo, M.; Pizza, C.; Piacente, S. Antioxidant and In Vitro preliminary anti-inflammatory activity of Castanea sativa (Italian Cultivar “Marrone di Roccadaspide” PGI) burs, leaves and chestnuts extracts and their metabolite profiles by LC-ESI/LTQOrbitrap/MS/MS. Antioxidants 2021, 10, 278. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Chen, B.; Kang, X.; Zhang, R.; Guo, Y.; Zhao, J.; Yang, H. Neuroprotective effects of natural compounds on LPS-induced inflammatory responses in microglia. Am. J. Transl. Res. 2020, 12, 2353–2378. [Google Scholar]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maiuolo, J.; Bava, I.; Carresi, C.; Gliozzi, M.; Musolino, V.; Scicchitano, M.; Macri, R.; Oppedisano, F.; Scarano, F.; Caterina Zito, M.; et al. The Effect of Ferula communis Extract in Escherichia coli Lipopolysaccharide-Induced Neuroinflammation in Cultured Neurons and Oligodendrocytes. Int. J. Mol. Sci. 2021, 22, 7910. https://doi.org/10.3390/ijms22157910
Maiuolo J, Bava I, Carresi C, Gliozzi M, Musolino V, Scicchitano M, Macri R, Oppedisano F, Scarano F, Caterina Zito M, et al. The Effect of Ferula communis Extract in Escherichia coli Lipopolysaccharide-Induced Neuroinflammation in Cultured Neurons and Oligodendrocytes. International Journal of Molecular Sciences. 2021; 22(15):7910. https://doi.org/10.3390/ijms22157910
Chicago/Turabian StyleMaiuolo, Jessica, Irene Bava, Cristina Carresi, Micaela Gliozzi, Vincenzo Musolino, Miriam Scicchitano, Roberta Macri, Francesca Oppedisano, Federica Scarano, Maria Caterina Zito, and et al. 2021. "The Effect of Ferula communis Extract in Escherichia coli Lipopolysaccharide-Induced Neuroinflammation in Cultured Neurons and Oligodendrocytes" International Journal of Molecular Sciences 22, no. 15: 7910. https://doi.org/10.3390/ijms22157910
APA StyleMaiuolo, J., Bava, I., Carresi, C., Gliozzi, M., Musolino, V., Scicchitano, M., Macri, R., Oppedisano, F., Scarano, F., Caterina Zito, M., Bosco, F., Ruga, S., Nucera, S., Ilari, S., Palma, E., Muscoli, C., & Mollace, V. (2021). The Effect of Ferula communis Extract in Escherichia coli Lipopolysaccharide-Induced Neuroinflammation in Cultured Neurons and Oligodendrocytes. International Journal of Molecular Sciences, 22(15), 7910. https://doi.org/10.3390/ijms22157910