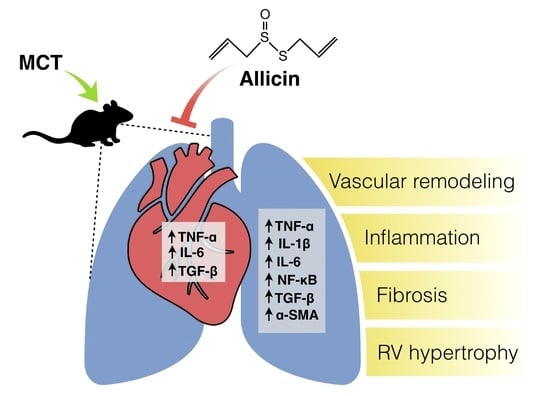
Anti-Inflammatory Effect of Allicin Associated with Fibrosis in Pulmonary Arterial Hypertension
Abstract
:1. Introduction
2. Results
2.1. Effect of Allicin on Right Ventricle (RV) Hypertrophy and Lung Morphology (Pulmonary Arteries Wall)
2.2. Anti-Inflammatory Effect of Allicin
2.3. Antifibrotic Effects of Allicin
2.4. Effect of Allicin on Expression Levels of miR-21-5p and Signaling Pathway Bmpr2/Smad5
2.5. Effects of Allicin on Inflammation and Fibrosis in the RVs of MCT-Induced PAH Rats
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Experimental Model of MCT-Induced PAH
4.3. RV Hypertrophy
4.4. Histopathology
4.5. Analysis of Protein Expression
4.6. miRNAs and mRNA Determination by RT-qPCR
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Humbert, M.; Guignabert, C.; Bonnet, S.; Dorfmüller, P.; Klinger, J.R.; Nicolls, M.R.; Olschewski, A.J.; Pullamsetti, S.S.; Schermuly, R.T.; Stenmark, K.R.; et al. Pathology and pathobiology of pulmonary hypertension: State of the art and research perspectives. Eur. Respir. J. 2019, 53, 1801887. [Google Scholar] [CrossRef] [Green Version]
- Vonk Noordegraaf, A.; Westerhof, B.E.; Westerhof, N. The Relationship Between the Right Ventricle and its Load in Pulmonary Hypertension. J. Am. Coll. Cardiol. 2017, 69, 236–243. [Google Scholar] [CrossRef]
- Ahmed, L.A.; Obaid, A.A.Z.; Zaki, H.F.; Agha, A.M. Role of oxidative stress, inflammation, nitric oxide and transforming growth factor-beta in the protective effect of diosgenin in monocrotaline-induced pulmonary hypertension in rats. Eur. J. Pharmacol. 2014, 740, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Hiepen, C.; Jatzlau, J.; Hildebrandt, S.; Kampfrath, B.; Goktas, M.; Murgai, A.; Cuellar Camacho, J.L.; Haag, R.; Ruppert, C.; Sengle, G.; et al. BMPR2 acts as a gatekeeper to protect endothelial cells from increased TGFβ responses and altered cell mechanics. PLoS Biol. 2019, 17, e3000557. [Google Scholar] [CrossRef] [Green Version]
- Gali, N.; Hoeper, M.M.; Humbert, M.; Torbicki, A.; Vachiery, J.L.; Barbera, J.A.; Beghetti, M.; Corris, P.; Gaine, S.; Gibbs, J.S.; et al. Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur. Heart J. 2009, 30, 2493–2537. [Google Scholar] [CrossRef] [Green Version]
- Itoh, A.; Nishihira, J.; Makita, H.; Miyamoto, K.; Yamaguchi, E.; Nishimura, M. Effects of IL-1β, TNF-α and macrophage migration inhibitory factor on prostacyclin synthesis in rat pulmonary artery smooth muscle cells. Respirology 2003, 8, 467–472. [Google Scholar] [CrossRef]
- Sutendra, G.; Dromparis, P.; Bonnet, S.; Haromy, A.; McMurtry, M.S.; Bleackley, R.C.; Michelakis, E.D. Pyruvate dehydrogenase inhibition by the inflammatory cytokine TNFα contributes to the pathogenesis of pulmonary arterial hypertension. J. Mol. Med. 2011, 89, 771–783. [Google Scholar] [CrossRef]
- Marsh, L.M.; Jandl, K.; Grünig, G.; Foris, V.; Bashir, M.; Ghanim, B.; Klepetko, W.; Olschewski, H.; Olschewski, A.; Kwapiszewska, G. The inflammatory cell landscape in the lungs of patients with idiopathic pulmonary arterial hypertension. Eur. Respir. J. 2018, 51, 1701214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Wang, Y.; Li, Y.; Qian, Z.; Zhu, L.; Yang, D. Osthole attenuates pulmonary arterial hypertension in monocrotaline-treated rats. Mol. Med. Rep. 2017, 16, 2823–2829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Q.; Zuo, X.R.; Wang, Y.Y.; Xie, W.P.; Wang, H.; Zhang, M. Monocrotaline-induced pulmonary arterial hypertension is attenuated by TNF-α antagonists via the suppression of TNF-α expression and NF-κB pathway in rats. Vascul. Pharmacol. 2013, 58, 71–77. [Google Scholar] [CrossRef]
- Nasim, M.T.; Ogo, T.; Chowdhury, H.M.; Zhao, L.; Chen, C.N.; Rhodes, C.; Trembath, R.C. BMPR-II deficiency elicits pro-proliferative and anti-apoptotic responses through the activation of TGFβ-TAK1-MAPK pathways in PAH. Hum. Mol. Genet. 2012, 21, 2548–2558. [Google Scholar] [CrossRef] [Green Version]
- Pardali, E.; Sanchez-Duffhues, G.; Gomez-Puerto, M.C.; Ten Dijke, P. TGF-β-induced endothelial-mesenchymal transition in fibrotic diseases. Int. J. Mol. Sci. 2017, 18, 2157. [Google Scholar] [CrossRef] [Green Version]
- Upton, P.D.; Morrell, N.W. The transforming growth factor-β-bone morphogenetic protein type signalling pathway in pulmonary vascular homeostasis and disease. Exp. Physiol. 2013, 98, 1262–1266. [Google Scholar] [CrossRef] [Green Version]
- Dewachter, L.; Dewachter, C. Inflammation in right ventricular failure: Does it matter? Front. Physiol. 2018, 9, 1056. [Google Scholar] [CrossRef] [PubMed]
- Xiang, L.; Li, Y.; Deng, X.; Kosanovic, D.; Schermuly, R.T.; Li, X. Natural plant products in treatment of pulmonary arterial hypertension. Pulm. Circ. 2018, 8, 2045894018784033. [Google Scholar] [CrossRef] [Green Version]
- Jasemi, S.V.; Khazaei, H.; Aneva, I.Y.; Farzaei, M.H.; Echeverría, J. Medicinal Plants and Phytochemicals for the Treatment of Pulmonary Hypertension. Front. Pharmacol. 2020, 11, 145. [Google Scholar] [CrossRef]
- Rybak, M.E.; Calvey, E.M.; Harnly, J.M. Quantitative Determination of Allicin in Garlic: Supercritical Fluid Extraction and Standard Addition of Alliin. J. Agric. Food Chem. 2004, 52, 682–687. [Google Scholar] [CrossRef] [PubMed]
- Park, B.M.; Chun, H.; Chae, S.W.; Kim, S.H. Fermented garlic extract ameliorates monocrotaline-induced pulmonary hypertension in rats. J. Funct. Foods 2017, 30, 247–253. [Google Scholar] [CrossRef]
- Suleria, H.A.R.; Butt, M.S.; Khalid, N.; Sultan, S.; Raza, A.; Aleem, M.; Abbas, M. Garlic (Allium sativum): Diet based therapy of 21st century-a review. Asian Pacific J. Trop. Dis. 2015, 5, 271–278. [Google Scholar] [CrossRef]
- Elkayam, A.; Peleg, E.; Grossman, E.; Shabtay, Z.; Sharabi, Y. Effects of allicin on cardiovascular risk factors in spontaneously hypertensive rats. Isr. Med. Assoc. J. 2013, 15, 170–173. [Google Scholar]
- Chan, J.Y.Y.; Yuen, A.C.Y.; Chan, R.Y.K.; Chan, S.W. A review of the cardiovascular benefits and antioxidant properties of allicin. Phyther. Res. 2013, 27, 637–646. [Google Scholar] [CrossRef]
- Lang, A.; Lahav, M.; Sakhnini, E.; Barshack, I.; Fidder, H.H.; Avidan, B.; Bardan, E.; Hershkoviz, R.; Bar-Meir, S.; Chowers, Y. Allicin inhibits spontaneous and TNF-α induced secretion of proinflammatory cytokines and chemokines from intestinal epithelial cells. Clin. Nutr. 2004, 23, 1199–1208. [Google Scholar] [CrossRef]
- Daley, E.; Emson, C.; Guignabert, C.; De Waal Malefyt, R.; Louten, J.; Kurup, V.P.; Hogaboam, C.; Taraseviciene-Stewart, L.; Voelkel, N.F.; Rabinovitch, M.; et al. Pulmonary arterial remodeling induced by a Th2 immune response. J. Exp. Med. 2008, 205, 361–372. [Google Scholar] [CrossRef]
- Sánchez-Gloria, J.L.; Osorio-Alonso, H.; Arellano-Buendía, A.S.; Carbó, R.; Hernández-Díazcouder, A.; Guzmán-Martín, C.A.; Rubio-Gayosso, I.; Sánchez-Muñoz, F. Nutraceuticals in the treatment of pulmonary arterial hypertension. Int. J. Mol. Sci. 2020, 21, 4827. [Google Scholar] [CrossRef] [PubMed]
- Itoh, T.; Nagaya, N.; Ishibashi-Ueda, H.; Kyotani, S.; Oya, H.; Sakamaki, F.; Kimura, H.; Nakanishi, N. Increased plasma monocyte chemoattractant protein-1 level in idiopathic pulmonary arterial hypertension. Respirology 2006, 11, 158–163. [Google Scholar] [CrossRef]
- Soon, E.; Holmes, A.M.; Treacy, C.M.; Doughty, N.J.; Southgate, L.; MacHado, R.D.; Trembath, R.C.; Jennings, S.; Barker, L.; Nicklin, P.; et al. Elevated levels of inflammatory cytokines predict survival in idiopathic and familial pulmonary arterial hypertension. Circulation 2010, 122, 920–927. [Google Scholar] [CrossRef] [Green Version]
- Fujita, M.; Shannon, J.M.; Irvin, C.G.; Fagan, K.A.; Cool, C.; Augustin, A.; Mason, R.J. Overexpression of tumor necrosis factor-α produces an increase in lung volumes and pulmonary hypertension. Am. J. Physiol. Lung Cell. Mol. Physiol. 2001, 280, L39–L49. [Google Scholar] [CrossRef]
- Li, X.; Wang, H.; Yang, C.; Zhang, X.; Han, D.; Wang, H. Fluoxetine inhibited extracellular matrix of pulmonary artery and inflammation of lungs in monocrotaline-treated rats. Acta Pharmacol. Sin. 2011, 32, 217–222. [Google Scholar] [CrossRef] [Green Version]
- Chen, D.; Zhou, D.; Qian, J.; Chen, F.; Guan, L.; Dong, L.; Ge, J. Atorvastatin prevents dehydromonocrotaline-induced pulmonary hypertension in beagles. Exp. Lung Res. 2012, 38, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Luan, Y.; Zhang, X.; Kong, F.; Cheng, G.H.; Qi, T.G.; Zhang, Z.H. Mesenchymal stem cell prevention of vascular remodeling in high flow-induced pulmonary hypertension through a paracrine mechanism. Int. Immunopharmacol. 2012, 14, 432–437. [Google Scholar] [CrossRef]
- Savale, L.; Tu, L.; Rideau, D.; Izziki, M.; Maitre, B.; Adnot, S.; Eddahibi, S. Impact of interleukin-6 on hypoxia-induced pulmonary hypertension and lung inflammation in mice. Respir. Res. 2009, 10, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Steiner, M.K.; Syrkina, O.L.; Kolliputi, N.; Mark, E.J.; Hales, C.A.; Waxman, A.B. Interleukin-6 overexpression induces pulmonary hypertension. Circ. Res. 2009, 104, 236–244. [Google Scholar] [CrossRef]
- Gomez-Arroyo, J.G.; Farkas, L.; Alhussaini, A.A.; Farkas, D.; Kraskauskas, D.; Voelkel, N.F.; Bogaard, H.J. The monocrotaline model of pulmonary hypertension in perspective. Am. J. Physiol. Lung Cell. Mol. Physiol. 2012, 302, 363–369. [Google Scholar] [CrossRef]
- Nogueira-Ferreira, R.; Vitorino, R.; Ferreira, R.; Henriques-Coelho, T. Exploring the monocrotaline animal model for the study of pulmonary arterial hypertension: A network approach. Pulm. Pharmacol. Ther. 2015, 35, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Sztuka, K.; Jasińska-Stroschein, M. Animal models of pulmonary arterial hypertension: A systematic review and meta-analysis of data from 6126 animals. Pharmacol. Res. 2017, 125, 201–214. [Google Scholar] [CrossRef]
- Xiao, R.; Su, Y.; Feng, T.; Sun, M.; Liu, B.; Zhang, J.; Lu, Y.; Li, J.; Wang, T.; Zhu, L.; et al. Monocrotaline induces endothelial injury and pulmonary hypertension by targeting the extracellular calcium-sensing receptor. J. Am. Heart Assoc. 2017, 6, e004865. [Google Scholar] [CrossRef] [Green Version]
- Haase, H.; Hieke, N.; Plum, L.M.; Gruhlke, M.C.H.; Slusarenko, A.J.; Rink, L. Impact of allicin on macrophage activity. Food Chem. 2012, 134, 141–148. [Google Scholar] [CrossRef]
- Chen, W.; Qi, J.; Feng, F.; Wang, M.D.; Bao, G.; Wang, T.; Xiang, M.; Xie, W.F. Neuroprotective effect of allicin against traumatic brain injury via Akt/endothelial nitric oxide synthase pathway-mediated anti-inflammatory and anti-oxidative activities. Neurochem. Int. 2014, 68, 28–37. [Google Scholar] [CrossRef]
- Qian, Y.Q.; Feng, Z.H.; Li, X.B.; Hu, Z.C.; Xuan, J.W.; Wang, X.Y.; Xu, H.C.; Chen, J.X. Downregulating PI3K/Akt/NF-κB signaling with allicin for ameliorating the progression of osteoarthritis: In vitro and vivo studies. Food Funct. 2018, 9, 4865–4875. [Google Scholar] [CrossRef]
- Rabinovitch, M.; Guignabert, C.; Humbert, M.; Nicolls, M.R. Inflammation and immunity in the pathogenesis of pulmonary arterial hypertension. Circ. Res. 2014, 115, 165–175. [Google Scholar] [CrossRef]
- Hassoun, P.M.; Mouthon, L.; Barberà, J.A.; Eddahibi, S.; Flores, S.C.; Grimminger, F.; Jones, P.L.; Maitland, M.L.; Michelakis, E.D.; Morrell, N.W.; et al. Inflammation, Growth Factors, and Pulmonary Vascular Remodeling. J. Am. Coll. Cardiol. 2009, 54, S10–S19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, C.; Luo, Y.; Li, S.; Huang, B.; Xu, S.; Li, L. Characteristics of inflammation process in monocrotaline-induced pulmonary arterial hypertension in rats. Biomed. Pharmacother. 2021, 133, 111081. [Google Scholar] [CrossRef]
- Yin, J.; You, S.; Liu, H.; Chen, L.; Zhang, C.; Hu, H.; Xue, M.; Cheng, W.; Wang, Y.; Li, X.; et al. Role of P2X7R in the development and progression of pulmonary hypertension. Respir. Res. 2017, 18, 127. [Google Scholar] [CrossRef] [PubMed]
- Varshney, R.; Ali, Q.; Wu, C.; Sun, Z. Monocrotaline-induced pulmonary hypertension involves downregulation of antiaging protein klotho and eNOS activity. Hypertension 2016, 68, 1255–1263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zawia, A.; Arnold, N.D.; West, L.; Pickworth, J.A.; Turton, H.; Iremonger, J.; Braithwaite, A.T.; Cañedo, J.; Johnston, S.A.; Thompson, A.A.R.; et al. Altered Macrophage Polarization Induces Experimental Pulmonary Hypertension and Is Observed in Patients with Pulmonary Arterial Hypertension. Arterioscler. Thromb. Vasc. Biol. 2020, 41, 430–445. [Google Scholar] [CrossRef]
- Sen, R.; Baltimore, D. Inducibility of κ immunoglobulin enhancer-binding protein NF-κB by a posttranslational mechanism. Cell 1986, 47, 921–928. [Google Scholar] [CrossRef]
- Kimura, H.; Okada, O.; Tanabe, N.; Tanaka, Y.; Terai, M.; Takiguchi, Y.; Masuda, M.; Nakajima, N.; Hiroshima, K.; Inadera, H.; et al. Plasma monocyte chemoattractant protein-1 and pulmonary vascular resistance in chronic thromboembolic pulmonary hypertension. Am. J. Respir. Crit. Care Med. 2001, 164, 319–324. [Google Scholar] [CrossRef]
- Shinde, A.V.; Humeres, C.; Frangogiannis, N.G. The role of α-smooth muscle actin in fibroblast-mediated matrix contraction and remodeling. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 298–309. [Google Scholar] [CrossRef]
- Guignabert, C.; Humbert, M. Targeting transforming growth factor-β receptors in pulmonary hypertension. Eur. Respir. J. 2021, 57, 2002341. [Google Scholar] [CrossRef]
- Zaiman, A.L.; Podowski, M.; Medicherla, S.; Gordy, K.; Xu, F.; Zhen, L.; Shimoda, L.A.; Neptune, E.; Higgins, L.; Murphy, A.; et al. Role of the TGF-β/Alk5 signaling pathway in monocrotaline-induced pulmonary hypertension. Am. J. Respir. Crit. Care Med. 2008, 177, 896–905. [Google Scholar] [CrossRef]
- Buendía, A.S.A.; González, M.T.; Reyes, O.S.; Arroyo, F.E.G.; García, R.A.; Tapia, E.; Lozada, L.G.S.; Alonso, H.O. Immunomodulatory effects of the nutraceutical garlic derivative allicin in the progression of diabetic nephropathy. Int. J. Mol. Sci. 2018, 19, 3107. [Google Scholar] [CrossRef] [Green Version]
- Jones, R.; Jacobson, M.; Steudel, W. alpha-smooth-muscle actin and microvascular precursor smooth-muscle cells in pulmonary hypertension. Am. J. Respir. Cell Mol. Biol. 1999, 20, 582–594. [Google Scholar] [CrossRef]
- Boleto, G.; Guignabert, C.; Pezet, S.; Cauvet, A.; Sadoine, J.; Tu, L.; Nicco, C.; Gobeaux, C.; Batteux, F.; Allanore, Y.; et al. T-cell costimulation blockade is effective in experimental digestive and lung tissue fibrosis. Arthritis Res. Ther. 2018, 20, 1–12. [Google Scholar] [CrossRef]
- Hurst, L.A.; Dunmore, B.J.; Long, L.; Crosby, A.; Al-Lamki, R.; Deighton, J.; Southwood, M.; Yang, X.; Nikolic, M.Z.; Herrera, B.; et al. TNFα drives pulmonary arterial hypertension by suppressing the BMP type-II receptor and altering NOTCH signalling. Nat. Commun. 2017, 8, 14079. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, L.; Sun, C.; Kong, F.; Wang, J.; Xin, Q.; Jiang, W.; Li, K.; Chen, O.; Luan, Y. Baicalin attenuates monocrotaline-induced pulmonary hypertension through bone morphogenetic protein signaling pathway. Oncotarget 2017, 8, 63430–63441. [Google Scholar] [CrossRef] [Green Version]
- Atkinson, C.; Stewart, S.; Upton, P.D.; Machado, R.; Thomson, J.R.; Trembath, R.C.; Morrell, N.W. Primary Pulmonary Hypertension Is Associated With Reduced Pulmonary Vascular Expression of Type II Bone Morphogenetic Protein Receptor. Circulation 2002, 105, 1672–1678. [Google Scholar] [CrossRef] [Green Version]
- Huertas, A.; Guignabert, C.; Barberà, J.A.; Bärtsch, P.; Bhattacharya, J.; Bhattacharya, S.; Bonsignore, M.R.; Dewachter, L.; Dinh-Xuan, A.T.; Dorfmüller, P.; et al. Pulmonary vascular endothelium: The orchestra conductor in respiratory diseases. Eur. Respir. J. 2018, 51, 1700745. [Google Scholar] [CrossRef]
- Happé, C.; Kurakula, K.; Sun, X.Q.; da Silva Goncalves Bos, D.; Rol, N.; Guignabert, C.; Tu, L.; Schalij, I.; Wiesmeijer, K.C.; Tura-Ceide, O.; et al. The BMP Receptor 2 in Pulmonary Arterial Hypertension: When and Where the Animal Model Matches the Patient. Cells 2020, 9, 1422. [Google Scholar] [CrossRef]
- Parikh, V.N.; Jin, R.C.; Rabello, S.; Gulbahce, N.; White, K.; Hale, A.; Cottrill, K.A.; Shaik, R.S.; Waxman, A.B.; Zhang, Y.-Y.; et al. MicroRNA-21 Integrates Pathogenic Signaling to Control Pulmonary Hypertension. Circulation 2012, 125, 1520–1532. [Google Scholar] [CrossRef]
- Trio, P.; You, S.; He, X.; He, J.; Sakao, K.; Hou, D. Chemopreventive functions and molecular mechanisms of garlic organosulfur compounds. Food Funct. 2014, 5, 833–844. [Google Scholar] [CrossRef]
- Liu, C.; Cao, F.; Tang, Q.-Z.; Yan, L.; Dong, Y.-G.; Zhu, L.-H.; Wang, L.; Bian, Z.-Y.; Li, H. Allicin protects against cardiac hypertrophy and fibrosis via attenuating reactive oxygen species-dependent signaling pathways. J. Nutr. Biochem. 2010, 21, 1238–1250. [Google Scholar] [CrossRef]
- Horev-Azaria, L.; Eliav, S.; Izigov, N.; Pri-Chen, S.; Mirelman, D.; Miron, T.; Rabinkov, A.; Wilchek, M.; Jacob-Hirsch, J.; Amariglio, N.; et al. Allicin up-regulates cellular glutathione level in vascular endothelial cells. Eur. J. Nutr. 2009, 48, 67–74. [Google Scholar] [CrossRef]
- Li, X.; Li, C.; Xiang, Z.; Hu, J.; Lu, J.; Tian, R.; Jia, W. Allicin ameliorates cardiac hypertrophy and fibrosis through enhancing of Nrf2 antioxidant signaling pathways. Cardiovasc. Drugs Ther. 2012, 26, 457–465. [Google Scholar] [CrossRef]
- García-Trejo, E.; Arellano-Buendía, A.; Argüello-García, R.; Loredo-Mendoza, M.; García-Arroyo, F.; Arellano-Mendoza, M.; Castillo-Hernández, M.; Guevara-Balcázar, G.; Tapia, E.; Sánchez-Lozada, L.; et al. Effects of Allicin on Hypertension and Cardiac Function in Chronic Kidney Disease. Oxid. Med. Cell. Longev. 2016, 2016, 3850402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arellano-Buendía, A.S.; Castañeda-Lara, L.G.; Loredo-Mendoza, M.L.; García-Arroyo, F.E.; Rojas-Morales, P.; Argüello-García, R.; Juárez-Rojas, J.G.; Tapia, E.; Pedraza-Chaverri, J.; Sánchez-Lozada, L.G.; et al. Effects of allicin on pathophysiological mechanisms during the progression of nephropathy associated to diabetes. Antioxidants 2020, 9, 1134. [Google Scholar] [CrossRef]
- Saradeth, T.; Seidl, S.; Resch, K.; Ernst, E. Does garlic alter the lipid pattern in normal volunteers? Phytomedicine 1994, 1, 183–185. [Google Scholar] [CrossRef]
- Liu, D.-S.; Wang, S.-L.; Li, J.-M.; Liang, E.-S.; Yan, M.-Z.; Gao, W. Allicin improves carotid artery intima-media thickness in coronary artery disease patients with hyperhomocysteinemia. Exp. Ther. Med. 2017, 14, 1722–1726. [Google Scholar] [CrossRef] [Green Version]
- Ashraf, R.; Aamir, K.; Shaikh, A.R.; Ahmed, T. Effects of garlic on dyslipidemia in patients with type 2 diabetes mellitus. J. Ayub Med. Coll. Abbottabad 2005, 17, 60–64. [Google Scholar]
- Le Hiress, M.; Tu, L.; Ricard, N.; Phan, C.; Thuillet, R.; Fadel, E.; Dorfmuller, P.; Montani, D.; De Man, F.; Humbert, M.; et al. Proinflammatory signature of the dysfunctional endothelium in pulmonary hypertension role of the macrophage migration inhibitory factor/CD74 complex. Am. J. Respir. Crit. Care Med. 2015, 192, 983–997. [Google Scholar] [CrossRef]
- Ha, H.; Lee, H. Reactive oxygen species amplify glucose signalling in renal cells cultured under high glucose and in diabetic kidney. Nephrology 2005, 10, S7–S10. [Google Scholar] [CrossRef] [PubMed]









| Gene | GenBank ID | Direction | Primer (5′-3′) | UPL |
|---|---|---|---|---|
| Bmpr2 | NM_080407.1 | Forward | gagccctccctggacttg | 67 |
| Reverse | atatcgaccccgtccaatc | |||
| Smad5 | NM_021692.1 | Forward | gcctatggacacaagcaaca | 107 |
| Reverse | aggcaacaggctgaacatct | |||
| Cd68 | NM_001031638.1 | Forward | cgccagtgaccaatctctc | 34 |
| Reverse | gggtaacgcagaaggcaat | |||
| Rplp0 | NM_022402.2 | Forward | gatgcccagggaagacag | 85 |
| Reverse | gaagcattttgggtagtcatcc |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Gloria, J.L.; Martínez-Olivares, C.E.; Rojas-Morales, P.; Hernández-Pando, R.; Carbó, R.; Rubio-Gayosso, I.; Arellano-Buendía, A.S.; Rada, K.M.; Sánchez-Muñoz, F.; Osorio-Alonso, H. Anti-Inflammatory Effect of Allicin Associated with Fibrosis in Pulmonary Arterial Hypertension. Int. J. Mol. Sci. 2021, 22, 8600. https://doi.org/10.3390/ijms22168600
Sánchez-Gloria JL, Martínez-Olivares CE, Rojas-Morales P, Hernández-Pando R, Carbó R, Rubio-Gayosso I, Arellano-Buendía AS, Rada KM, Sánchez-Muñoz F, Osorio-Alonso H. Anti-Inflammatory Effect of Allicin Associated with Fibrosis in Pulmonary Arterial Hypertension. International Journal of Molecular Sciences. 2021; 22(16):8600. https://doi.org/10.3390/ijms22168600
Chicago/Turabian StyleSánchez-Gloria, José L., Constanza Estefanía Martínez-Olivares, Pedro Rojas-Morales, Rogelio Hernández-Pando, Roxana Carbó, Ivan Rubio-Gayosso, Abraham S. Arellano-Buendía, Karla M. Rada, Fausto Sánchez-Muñoz, and Horacio Osorio-Alonso. 2021. "Anti-Inflammatory Effect of Allicin Associated with Fibrosis in Pulmonary Arterial Hypertension" International Journal of Molecular Sciences 22, no. 16: 8600. https://doi.org/10.3390/ijms22168600
APA StyleSánchez-Gloria, J. L., Martínez-Olivares, C. E., Rojas-Morales, P., Hernández-Pando, R., Carbó, R., Rubio-Gayosso, I., Arellano-Buendía, A. S., Rada, K. M., Sánchez-Muñoz, F., & Osorio-Alonso, H. (2021). Anti-Inflammatory Effect of Allicin Associated with Fibrosis in Pulmonary Arterial Hypertension. International Journal of Molecular Sciences, 22(16), 8600. https://doi.org/10.3390/ijms22168600