Promising Nanoparticle-Based Heat Transfer Fluids—Environmental and Techno-Economic Analysis Compared to Conventional Fluids
Abstract
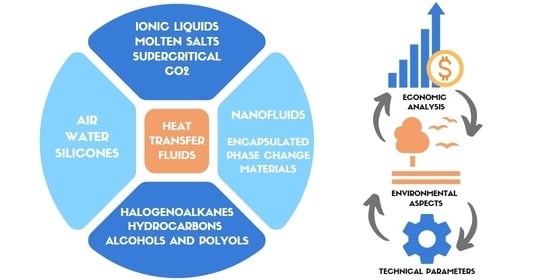
:1. Introduction
2. Conventional HTFs
2.1. Air
2.2. Halogenoalkanes
2.3. Water/Steam
2.4. Hydrocarbons
2.5. Silicones
2.6. Monohydroxyl Alcohols and Polyols
2.7. Summary of Environmental, Technical, and Economic Aspects of Traditional HTFs
3. Novel HTFs
3.1. Nanofluids
3.1.1. Metal Oxides
3.1.2. Metals
3.1.3. Layered Double Hydroxide (LDH)
3.1.4. Carbon Species
3.1.5. Hybrid Nanofluids
3.2. Supercritical CO2
3.3. Molten Salts/Molten Salts NFs
3.4. Ionic Liquids/Ionanofluids
3.5. Nano- and Micro-Encapsulated Phase Change Materials
3.6. Summary of Environmental, Technical, and Economic Aspects of Novel HTFs
4. Application, Advantages, and Limitations of Conventional and Novel HTFs
5. Conclusions and Future Recommendations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| [Bmim][NTf2] | 1-butyl-3-methylimidazolium-bis(trifluoromethylsulfonyl)imide |
| [bmim][Tf2N] | 1-butyl-3-methylimidazolium bis[(trifluoromethyl)sulfonyl]imide |
| [C2C1im][SCN] | 1-ethyl-3-methylimidazolium thiocyanate ionic liquid |
| [C4mim][NTf2] | 1-butyl-3-methylimidazolium bis{(trifluoromethyl)sulfonyl}imide |
| [MMIM][DMP] | 1,3-dimethylimidazolium dimethyl-phosphate |
| ASHRAE | American Society of Heating, Refrigerating and Air-Conditioning Engineers |
| c | specific heat [J/(kgꞏK)] |
| CHF | critical heat flux [MW/m2] |
| CNT | carbon nanotubes |
| CSP | Concentrating Solar Power |
| Da | Damköhler numbers [–] |
| DPHE | double pipe heat exchanger |
| EG | ethylene glycol |
| EPCM | encapsulated phase change materials |
| f-ZnO NFs | functionalized ZnO nanofluids |
| GHE | geothermal heat exchanger |
| GNPs | graphene nanoparticles |
| GO | graphene oxide |
| GWP | Global Warming Potential |
| HE | heat exchanger |
| HTC | heat transfer coefficient [W/(m2ꞏK)] |
| HTF | heat transfer fluid |
| ILs | ionic liquids |
| INFs | ionanofluids |
| LDH | layered double hydroxides |
| MEPCM | micro-encapsulated phase change materials |
| MS | molten salts |
| MSNFs | molten salt nanofluids |
| MWCNT | multi-walled carbon nanotubes |
| NEPCM | nano-encapsulated phase change materials |
| NFs | nanofluids |
| NPs | nanoparticles |
| Nu | Nusselt number [–] |
| ORC | Organic Rankine Cycle |
| PCM | phase change materials |
| PG | propylene glycol |
| PMMA | polymethyl methacrylate |
| Pr | Prandtl number [–] |
| PS | polystyrene |
| PU | polyurethane |
| Ra | Rayleigh number [–] |
| Re | Reynolds number [–] |
| rGO | reduced graphene oxide |
| s-CO2 | supercritical CO2 |
| SWCNT | single-walled carbon nanotubes |
| TC | thermal conductivity [W/(mꞏK)] |
| TCE | trichloroethylene |
| TES | thermal energy storage |
| η | dynamic viscosity [mPaꞏs] |
| ρ | density [kg/m3] |
References
- Heller, L. Literature Review on Heat Transfer Fluids and Thermal Energy Storage Systems in CSP Plants; STERG: Stellenbosch, South Africa, 2013. [Google Scholar]
- Gong, J.; Sumathy, K. Active Solar Water Heating Systems; Elsevier Ltd.: Amsterdam, The Netherlands, 2016; ISBN 9780081003022. [Google Scholar]
- Asadi, A. A guideline towards easing the decision-making process in selecting an effective nanofluid as a heat transfer fluid. Energy Convers. Manag. 2018, 175, 1–10. [Google Scholar] [CrossRef]
- Kraichnan, R.H. Turbulent thermal convection at arbitrary prandtl number. Phys. Fluids 1962, 5, 1374–1389. [Google Scholar] [CrossRef]
- Chernikova, E.A.; Glukhov, L.M.; Krasovskiy, V.G.; Kustov, L.M.; Vorobyeva, M.G.; Koroteev, A.A. Ionic liquids as heat transfer fluids: Comparison with known systems, possible applications, advantages and disadvantages. Russ. Chem. Rev. 2015, 84, 875–890. [Google Scholar] [CrossRef]
- Mohapatra, S.C. Heat Transfer Fluids. In Encyclopedia of Chemical Processing; Lee, S., Ed.; Taylor & Francis: New York, NY, USA, 2006; pp. 1211–1220. [Google Scholar]
- Pacio, J.; Wetzel, T. Assessment of liquid metal technology status and research paths for their use as efficient heat transfer fluids in solar central receiver systems. Sol. Energy 2013, 93, 11–22. [Google Scholar] [CrossRef]
- Cordaro, J.G.; Rubin, N.C.; Bradshaw, R.W. Multicomponent molten salt mixtures based on nitrate/nitrite anions. J. Sol. Energy Eng. Trans. ASME 2011, 133, 1–5. [Google Scholar] [CrossRef]
- Mohapatra, S.C.; Loikits, D. Advances in liquid coolant technologies for electronics cooling. In Proceedings of the Semiconductor Thermal Measurement and Management IEEE Twenty First Annual IEEE Symposium, San Jose, CA, USA, 15–17 March 2005; pp. 354–360. [Google Scholar] [CrossRef]
- Minea, A.A. Overview of Ionic Liquids as Candidates for New Heat Transfer Fluids. Int. J. Thermophys. 2020, 41, 1–15. [Google Scholar] [CrossRef]
- Krishna, Y.; Faizal, M.; Saidur, R.; Ng, K.C.; Aslfattahi, N. State-of-the-art heat transfer fluids for parabolic trough collector. Int. J. Heat Mass Transf. 2020, 152, 119541. [Google Scholar] [CrossRef]
- Malviya, R.; Agrawal, A.; Baredar, P.V. A comprehensive review of different heat transfer working fluids for solar thermal parabolic trough concentrator. Mater. Today Proc. 2020. [Google Scholar] [CrossRef]
- Srivastva, U.; Malhotra, R.K.; Kaushik, S.C. Review of heat transport properties of solar heat transfer fluids. J. Therm. Anal. Calorim. 2017, 130, 605–621. [Google Scholar] [CrossRef]
- Rajendran, D.R.; Ganapathy Sundaram, E.; Jawahar, P.; Sivakumar, V.; Mahian, O.; Bellos, E. Review on influencing parameters in the performance of concentrated solar power collector based on materials, heat transfer fluids and design. J. Therm. Anal. Calorim. 2020, 140, 33–51. [Google Scholar] [CrossRef]
- Benoit, H.; Spreafico, L.; Gauthier, D.; Flamant, G. Review of heat transfer fluids in tube-receivers used in concentrating solar thermal systems: Properties and heat transfer coefficients. Renew. Sustain. Energy Rev. 2016, 55, 298–315. [Google Scholar] [CrossRef]
- Vignarooban, K.; Xu, X.; Arvay, A.; Hsu, K.; Kannan, A.M. Heat transfer fluids for concentrating solar power systems—A review. Appl. Energy 2015, 146, 383–396. [Google Scholar] [CrossRef]
- Bonk, A.; Sau, S.; Uranga, N.; Hernaiz, M.; Bauer, T. Advanced heat transfer fluids for direct molten salt line-focusing CSP plants. Prog. Energy Combust. Sci. 2018, 67, 69–87. [Google Scholar] [CrossRef]
- Hoffmann, J.F.; Vaitilingom, G.; Henry, J.F.; Chirtoc, M.; Olives, R.; Goetz, V.; Py, X. Temperature dependence of thermophysical and rheological properties of seven vegetable oils in view of their use as heat transfer fluids in concentrated solar plants. Sol. Energy Mater. Sol. Cells 2018, 178, 129–138. [Google Scholar] [CrossRef]
- Das, S.K.; Choi, S.U.S.; Patel, H.E. Heat transfer in nanofluids—A review. Heat Transf. Eng. 2006, 27, 3–19. [Google Scholar] [CrossRef]
- Trisaksri, V.; Wongwises, S. Critical review of heat transfer characteristics of nanofluids. Renew. Sustain. Energy Rev. 2007, 11, 512–523. [Google Scholar] [CrossRef]
- Kakaç, S.; Pramuanjaroenkij, A. Review of convective heat transfer enhancement with nanofluids. Int. J. Heat Mass Transf. 2009, 52, 3187–3196. [Google Scholar] [CrossRef]
- Sarkar, J.; Ghosh, P.; Adil, A. A review on hybrid nanofluids: Recent research, development and applications. Renew. Sustain. Energy Rev. 2015, 43, 164–177. [Google Scholar] [CrossRef]
- Nkurikiyimfura, I.; Wang, Y.; Pan, Z. Heat transfer enhancement by magnetic nanofluids—A review. Renew. Sustain. Energy Rev. 2013, 21, 548–561. [Google Scholar] [CrossRef]
- Huminic, G.; Huminic, A. Application of nanofluids in heat exchangers: A review. Renew. Sustain. Energy Rev. 2012, 16, 5625–5638. [Google Scholar] [CrossRef]
- Ahmadi, M.H.; Mirlohi, A.; Alhuyi Nazari, M.; Ghasempour, R. A review of thermal conductivity of various nanofluids. J. Mol. Liq. 2018, 265, 181–188. [Google Scholar] [CrossRef]
- Yang, L.; Ji, W.; Huang, J.N.; Xu, G. An updated review on the influential parameters on thermal conductivity of nano-fluids. J. Mol. Liq. 2019, 296. [Google Scholar] [CrossRef]
- Pinto, R.V.; Fiorelli, F.A.S. Review of the mechanisms responsible for heat transfer enhancement using nanofluids. Appl. Therm. Eng. 2016, 108, 720–739. [Google Scholar] [CrossRef]
- Mohammed, H.A.; Bhaskaran, G.; Shuaib, N.H.; Saidur, R. Heat transfer and fluid flow characteristics in microchannels heat exchanger using nanofluids: A review. Renew. Sustain. Energy Rev. 2011, 15, 1502–1512. [Google Scholar] [CrossRef]
- Sundar, L.S.; Sharma, K.V.; Singh, M.K.; Sousa, A.C.M. Hybrid nanofluids preparation, thermal properties, heat transfer and friction factor—A review. Renew. Sustain. Energy Rev. 2017, 68, 185–198. [Google Scholar] [CrossRef]
- Huminic, G.; Huminic, A. Heat transfer and flow characteristics of conventional fluids and nanofluids in curved tubes: A review. Renew. Sustain. Energy Rev. 2016, 58, 1327–1347. [Google Scholar] [CrossRef]
- Paul, T.C.; Tikadar, A.; Mahamud, R.; Salman, A.S.; Morshed, A.K.M.M.; Khan, J.A. A Critical Review on the Development of Ionic Liquids-Based Nanofluids as Heat Transfer Fluids for Solar Thermal Energy. Processes 2021, 9, 858. [Google Scholar] [CrossRef]
- Minea, A.A.; Sohel Murshed, S.M. Ionic liquids-based nanocolloids—A review of progress and prospects in convective heat transfer applications. Nanomaterials 2021, 11, 39. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, F.; Zhang, L.; Fang, X.; Zhang, Z. Thermodynamic properties and thermal stability of ionic liquid-based nanofluids containing graphene as advanced heat transfer fluids for medium-to-high-temperature applications. Renew. Energy 2014, 63, 519–523. [Google Scholar] [CrossRef]
- Wadekar, V.V. Ionic liquids as heat transfer fluids—An assessment using industrial exchanger geometries. Appl. Therm. Eng. 2017, 111, 1581–1587. [Google Scholar] [CrossRef]
- Looser, R.; Vivar, M.; Everett, V. Spectral characterisation and long-term performance analysis of various commercial Heat Transfer Fluids (HTF) as Direct-Absorption Filters for CPV-T beam-splitting applications. Appl. Energy 2014, 113, 1496–1511. [Google Scholar] [CrossRef]
- López-González, D.; Valverde, J.L.; Sánchez, P.; Sanchez-Silva, L. Characterization of different heat transfer fluids and degradation study by using a pilot plant device operating at real conditions. Energy 2013, 54, 240–250. [Google Scholar] [CrossRef]
- Oyekunle, L.O.; Susu, A.A. Characteristic properties of a locally produced paraffinic oil and its suitability as a heat-transfer fluid. Pet. Sci. Technol. 2005, 23, 1499–1509. [Google Scholar] [CrossRef]
- Bradshaw, R.W.; Cordaro, J.G.; Siegel, N.P. Molten Nitrate Salt Development for Thermal Energy Storage in Parabolic Trough Solar Power Systems. In Proceedings of the ASME 2009 3rd International Conference on Energy Sustainability, San Francisco, CA, USA, 19–23 July 2009; Volume 2, pp. 615–624. [Google Scholar] [CrossRef]
- Chen, W.; Zou, C.; Li, X. An investigation into the thermophysical and optical properties of SiC/ionic liquid nanofluid for direct absorption solar collector. Sol. Energy Mater. Sol. Cells 2017, 163, 157–163. [Google Scholar] [CrossRef]
- Blake, D.M.; Jane, M.; Price, H.; Kearney, D.; Herrmann, U. New Heat Transfer and Storage Fluids for Parabolic Trough Solar Thermal Electric Plants. In Proceedings of the 11th SolarPACES International Symposium on Concentrating Solar Power and Chemical Energy Technologies, Zurich, Switzerland, 4–6 September 2002; pp. 4–8. [Google Scholar]
- Good, P.; Zanganeh, G.; Ambrosetti, G.; Barbato, M.C.; Pedretti, A.; Steinfeld, A. Towards a commercial parabolic trough CSP system using air as heat transfer fluid. Energy Procedia 2014, 49, 381–385. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Bai, F.; Yang, B.; Wang, Y.; Xu, L.; Chang, Z.; Wang, Z.; El Hefni, B.; Yang, Z.; Kubo, S.; et al. Dynamic simulations of a honeycomb ceramic thermal energy storage in a solar thermal power plant using air as the heat transfer fluid. Appl. Therm. Eng. 2018, 129, 636–645. [Google Scholar] [CrossRef]
- Liu, M.; Belusko, M.; Steven Tay, N.H.; Bruno, F. Impact of the heat transfer fluid in a flat plate phase change thermal storage unit for concentrated solar power plants. Sol. Energy 2014, 101, 220–231. [Google Scholar] [CrossRef]
- Zunft, S.; Hänel, M.; Krüger, M.; Dreißigacker, V.; Göhring, F.; Wahl, E. Jlich solar power tower-experimental evaluation of the storage subsystem and performance calculation. J. Sol. Energy Eng. Trans. ASME 2011, 133, 1–5. [Google Scholar] [CrossRef]
- Good, P.; Ambrosetti, G.; Pedretti, A.; Steinfeld, A. A 1.2 MWth solar parabolic trough system based on air as heat transfer fluid at 500 °C—Engineering design, modelling, construction, and testing. Sol. Energy 2016, 139, 398–411. [Google Scholar] [CrossRef]
- Cinocca, A.; Cipollone, R.; Carapellucci, R.; Iampieri, V.; Rivo, M. CSP-PT gas plant using air as Heat Transfer Fluid with a packed-bed storage section. Energy Procedia 2018, 148, 852–859. [Google Scholar] [CrossRef]
- Toro, C.; Rocco, M.V.; Colombo, E. Exergy and thermoeconomic analyses of central receiver concentrated solar plants using air as heat transfer fluid. Energies 2016, 9, 885. [Google Scholar] [CrossRef] [Green Version]
- Orbey, H.; Sandler, S.I. Equation of State Modeling of Refrigerant Mixtures. Ind. Eng. Chem. Res. 1995, 34, 2520–2525. [Google Scholar] [CrossRef]
- Smith, G.F. Trichlorethylene: A Review. Occup. Environ. Med. 1966, 23, 249–262. [Google Scholar] [CrossRef] [Green Version]
- Cao, W.; Huang, W.; Jiang, F. Numerical study on variable thermophysical properties of heat transfer fluid affecting EGS heat extraction. Int. J. Heat Mass Transf. 2016, 92, 1205–1217. [Google Scholar] [CrossRef]
- Modi, A.; Haglind, F. Performance analysis of a Kalina cycle for a central receiver solar thermal power plant with direct steam generation. Appl. Therm. Eng. 2014, 65, 201–208. [Google Scholar] [CrossRef] [Green Version]
- Birnbaum, J.; Feldhoff, J.F.; Fichtner, M.; Hirsch, T.; Jöcker, M.; Pitz-Paal, R.; Zimmermann, G. Steam temperature stability in a direct steam generation solar power plant. Sol. Energy 2011, 85, 660–668. [Google Scholar] [CrossRef] [Green Version]
- Grigoraş, C.G.; Muntianu, G.; Gavrilă, L. Mathematical modelling of CaCl2 aqueous solutions thermophysical properties. Sci. Study Res. Chem. Chem. Eng. Biotechnol. Food Ind. 2016, 17, 417–426. [Google Scholar]
- N’Tsoukpoe, K.E.; Rammelberg, H.U.; Lele, A.F.; Korhammer, K.; Watts, B.A.; Schmidt, T.; Ruck, W.K.L. A review on the use of calcium chloride in applied thermal engineering. Appl. Therm. Eng. 2015, 75, 513–531. [Google Scholar] [CrossRef]
- Montes, M.J.; Abánades, A.; Martínez-Val, J.M. Thermofluidynamic model and comparative analysis of parabolic trough collectors using oil, water/steam, or molten salt as heat transfer fluids. J. Sol. Energy Eng. Trans. ASME 2010, 132, 0210011–0210017. [Google Scholar] [CrossRef]
- Edwards, J.; Bindra, H. An experimental study on storing thermal energy in packed beds with saturated steam as heat transfer fluid. Sol. Energy 2017, 157, 456–461. [Google Scholar] [CrossRef]
- Valkenburg, M.E.V.; Vaughn, R.L.; Williams, M.; Wilkes, J.S. Thermochemistry of ionic liquid heat-transfer fluids. Thermochim. Acta 2005, 425, 181–188. [Google Scholar] [CrossRef]
- Merrouni, A.A.; Ouali, H.A.L.; Moussaoui, M.A.; Mezrhab, A. Analysis and comparaison of different Heat Transfer Fluids for a 1MWe Parabolic Trough Collector. In Proceedings of the 2016 International Conference on Electrical and Information Technologies (ICEIT), Tangiers, Morocco, 4–7 May 2016; pp. 510–515. [Google Scholar] [CrossRef]
- Chang, Y.S.; Kim, M.S.; Ro, S.T. Performance and heat transfer characteristics of hydrocarbon refrigerants in a heat pump system. Int. J. Refrig. 2000, 23, 232–242. [Google Scholar] [CrossRef]
- Heilig, M.L. United States Patent Office. ACM SIGGRAPH Comput. Graph. 1994, 28, 131–134. [Google Scholar] [CrossRef]
- Sun, J. D-limonene: Safety and clinical applications. Altern. Med. Rev. 2007, 12, 259–264. [Google Scholar]
- Kim, Y.W.; Kim, M.J.; Chung, B.Y.; Bang, D.Y.; Lim, S.K.; Choi, S.M.; Lim, D.S.; Cho, M.C.; Yoon, K.; Kim, H.S.; et al. Safety evaluation and risk assessment of D-limonene. J. Toxicol. Environ. Heal. Part B Crit. Rev. 2013, 16, 17–38. [Google Scholar] [CrossRef] [PubMed]
- Tao, P.; Shu, L.; Zhang, J.; Lee, C.; Ye, Q.; Guo, H.; Deng, T. Silicone oil-based solar-thermal fluids dispersed with PDMS-modified Fe3O4@graphene hybrid nanoparticles. Prog. Nat. Sci. Mater. Int. 2018, 28, 554–562. [Google Scholar] [CrossRef]
- Sarkar, J.; Bhattacharyya, S. Application of graphene and graphene-based materials in clean energy-related devices Minghui. Arch. Thermodyn. 2012, 33, 23–40. [Google Scholar] [CrossRef]
- Anyanwu, E.E. Review of solid adsorption solar refrigerator I: An overview of the refrigeration cycle. Energy Convers. Manag. 2003, 44, 301–312. [Google Scholar] [CrossRef]
- Mastrullo, R.; Mauro, A.W.; Revellin, R.; Viscito, L. Flow boiling heat transfer and pressure drop of pure ethanol (99.8%) in a horizontal stainless steel tube at low reduced pressures. Appl. Therm. Eng. 2018, 145, 251–263. [Google Scholar] [CrossRef]
- Wajs, J.; Mikielewicz, D.; Jakubowska, B. Performance of the domestic micro ORC equipped with the shell-and-tube condenser with minichannels. Energy 2018, 157, 853–861. [Google Scholar] [CrossRef]
- Colangelo, G.; Favale, E.; Miglietta, P.; De Risi, A. Innovation in flat solar thermal collectors: A review of the last ten years experimental results. Renew. Sustain. Energy Rev. 2016, 57, 1141–1159. [Google Scholar] [CrossRef]
- Hayduk, W.; Malik, V.K. Density, Viscosity, and Carbon Dioxide Solubility and Diffusivity in Aqueous Ethylene Glycol Solutions. J. Chem. Eng. Data 1971, 16, 143–146. [Google Scholar] [CrossRef]
- Cordray, D.R.; Kaplan, L.R.; Woyciesjes, P.M.; Kozak, T.F. Solid-liquid phase diagram for ethylene glycol + water. Fluid Phase Equilib. 1996, 117, 146–152. [Google Scholar] [CrossRef]
- Duell, A.K.; Pankow, J.F.; Gillette, S.M.; Peyton, D.H. Boiling points of the propylene glycol + glycerol system at 1 atmosphere pressure: 188.6–292 °C without and with added water or nicotine. Chem. Eng. Commun. 2018, 205, 1691–1700. [Google Scholar] [CrossRef] [PubMed]
- Morrison, L.R. Glycerol. Kirk-Othmer Encycl. Chem. Technol. 2000, 11. [Google Scholar] [CrossRef]
- Colorado, D.; Ali, M.E.; García-Valladares, O.; Hernández, J.A. Heat transfer using a correlation by neural network for natural convection from vertical helical coil in oil and glycerol/water solution. Energy 2011, 36, 854–863. [Google Scholar] [CrossRef]
- Segur, J.B.; Oderstar, H.E. Viscosity of Glycerol and Its Aqueous Solutions. Ind. Eng. Chem. 1951, 43, 2117–2120. [Google Scholar] [CrossRef]
- Takamura, K.; Fischer, H.; Morrow, N.R. Physical properties of aqueous glycerol solutions. J. Pet. Sci. Eng. 2012, 98–99, 50–60. [Google Scholar] [CrossRef]
- Spangler, J.A.; Davies, E.C.H. Freezing Points, Densities, and Refractive Indexes of the System Glycerol-Ethylene Glycol-Water. Ind. Eng. Chem. Anal. Ed. 1943, 15, 96–99. [Google Scholar] [CrossRef]
- Trejo González, J.A.; Longinotti, M.P.; Corti, H.R. The viscosity of glycerol-water mixtures including the supercooled region. J. Chem. Eng. Data 2011, 56, 1397–1406. [Google Scholar] [CrossRef]
- Hafeez, M.U.; Hayat, T.; Alsaedi, A.; Khan, M.I. Numerical simulation for electrical conducting rotating flow of Au (Gold)-Zn (Zinc)/EG (Ethylene glycol) hybrid nanofluid. Int. Commun. Heat Mass Transf. 2021, 124, 105234. [Google Scholar] [CrossRef]
- Rashid, U.; Liang, H.; Ahmad, H.; Abbas, M.; Iqbal, A.; Hamed, Y.S. Study of (Ag and TiO2)/water nanoparticles shape effect on heat transfer and hybrid nanofluid flow toward stretching shrinking horizontal cylinder. Results Phys. 2021, 21, 103812. [Google Scholar] [CrossRef]
- Sarafraz, M.M.; Yang, B.; Pourmehran, O.; Arjomandi, M.; Ghomashchi, R. Fluid and heat transfer characteristics of aqueous graphene nanoplatelet (GNP) nanofluid in a microchannel. Int. Commun. Heat Mass Transf. 2019, 107, 24–33. [Google Scholar] [CrossRef]
- Choi, T.J.; Park, M.S.; Kim, S.H.; Jang, S.P. Experimental Study on the Effect of Nanoparticle Migration on the Convective Heat Transfer Coefficient of EG/Water-based Al2O3 Nanofluids. Int. J. Heat Mass Transf. 2021, 169, 120903. [Google Scholar] [CrossRef]
- Iacobazzi, F.; Milanese, M.; Colangelo, G.; Lomascolo, M.; Risi, A. De An explanation of the Al2O3 nano-fluid thermal conductivity based on the phonon theory of liquid. Energy 2016, 116, 786–794. [Google Scholar] [CrossRef]
- Milanese, M.; Iacobazzi, F.; Colangelo, G.; Risi, A. De International Journal of Heat and Mass Transfer An investigation of layering phenomenon at the liquid—Solid interface in Cu and CuO based nanofluids. Int. J. Heat Mass Transf. 2016, 103, 564–571. [Google Scholar] [CrossRef]
- Colangelo, G.; Favale, E.; Milanese, M.; De Risi, A.; Laforgia, D. Cooling of electronic devices: Nanofluids contribution. Appl. Therm. Eng. 2017, 127, 421–435. [Google Scholar] [CrossRef]
- Measurement and control system for thermo- solar plant and performance comparison between traditional and nanofluid solar thermal collectors. Int. J. Smart Sens. Intell. Syst. 2017, 9. [CrossRef] [Green Version]
- Khanlari, A.; Sözen, A.; Variyenli, H.İ. Simulation and experimental analysis of heat transfer characteristics in the plate type heat exchangers using TiO2/water nanofluid. Int. J. Numer. Methods Heat Fluid Flow 2019, 29, 1343–1362. [Google Scholar] [CrossRef]
- Chakraborty, S.; Sengupta, I.; Sarkar, I.; Pal, S.K.; Chakraborty, S. Effect of surfactant on thermo-physical properties and spray cooling heat transfer performance of Cu-Zn-Al LDH nanofluid. Appl. Clay Sci. 2019, 168, 43–55. [Google Scholar] [CrossRef]
- Ilyas, S.U.; Narahari, M.; Theng, J.T.Y.; Pendyala, R. Experimental evaluation of dispersion behavior, rheology and thermal analysis of functionalized zinc oxide-paraffin oil nanofluids. J. Mol. Liq. 2019, 294, 111613. [Google Scholar] [CrossRef]
- Zayed, M.E.; Zhao, J.; Du, Y.; Kabeel, A.E.; Shalaby, S.M. Factors affecting the thermal performance of the flat plate solar collector using nanofluids: A review. Sol. Energy 2019, 182, 382–396. [Google Scholar] [CrossRef]
- Sharaf, O.Z.; Taylor, R.A.; Abu-Nada, E. On the colloidal and chemical stability of solar nanofluids: From nanoscale interactions to recent advances. Phys. Rep. 2020, 867, 1–84. [Google Scholar] [CrossRef]
- Qamar, A.; Anwar, Z.; Ali, H.; Shaukat, R.; Imran, S.; Arshad, A.; Ali, H.M.; Korakianitis, T. Preparation and dispersion stability of aqueous metal oxide nanofluids for potential heat transfer applications: A review of experimental studies. J. Therm. Anal. Calorim. 2020, 1–24. [Google Scholar] [CrossRef]
- Noori, T.; Ghangrekar, M.M.; Mitra, A.; Mukherjee, C.K. Conference Proceedings of the Second International Conference on Recent Advances in Bioenergy Research; Springer: Berlin/Heidelberg, Germany, 2018; pp. 285–294. [Google Scholar] [CrossRef]
- Said, Z.; Saidur, R. Thermophysical Properties of Metal Oxides Nanofluids. Nanofluid Heat Mass Transf. Eng. Probl. 2017. [Google Scholar] [CrossRef] [Green Version]
- Saidina, D.S.; Abdullah, M.Z.; Hussin, M. Metal oxide nanofluids in electronic cooling: A review. J. Mater. Sci. Mater. Electron. 2020, 31, 4381–4398. [Google Scholar] [CrossRef]
- Hekmatipour, F.; Jalali, M. Application of copper oxide–thermal oil (CuO-HTO) nanofluid on convective heat transfer enhancement in inclined circular tube. J. Therm. Anal. Calorim. 2019, 136, 2449–2459. [Google Scholar] [CrossRef]
- Li, Z.; Sarafraz, M.M.; Mazinani, A.; Hayat, T.; Alsulami, H.; Goodarzi, M. Pool boiling heat transfer to CuO-H2O nanofluid on finned surfaces. Int. J. Heat Mass Transf. 2020, 156, 119780. [Google Scholar] [CrossRef]
- Maddah, H.; Ghazvini, M.; Ahmadi, M.H. Predicting the efficiency of CuO/water nanofluid in heat pipe heat exchanger using neural network. Int. Commun. Heat Mass Transf. 2019, 104, 33–40. [Google Scholar] [CrossRef]
- Said, Z.; Rahman, S.M.A.; El Haj Assad, M.; Alami, A.H. Heat transfer enhancement and life cycle analysis of a Shell-and-Tube Heat Exchanger using stable CuO/water nanofluid. Sustain. Energy Technol. Assessments 2019, 31, 306–317. [Google Scholar] [CrossRef]
- Pourfattah, F.; Motamedian, M.; Sheikhzadeh, G.; Toghraie, D.; Ali Akbari, O. The numerical investigation of angle of attack of inclined rectangular rib on the turbulent heat transfer of Water-Al2O3 nanofluid in a tube. Int. J. Mech. Sci. 2017, 131–132, 1106–1116. [Google Scholar] [CrossRef]
- Manetti, L.L.; Stephen, M.T.; Beck, P.A.; Cardoso, E.M. Evaluation of the heat transfer enhancement during pool boiling using low concentrations of Al2O3-water based nanofluid. Exp. Therm. Fluid Sci. 2017, 87, 191–200. [Google Scholar] [CrossRef] [Green Version]
- Mansoury, D.; Doshmanziari, F.I.; Kiani, A.; Chamkha, A.J.; Sharifpur, M. Heat Transfer and Flow Characteristics of Al2O3/Water Nanofluid in Various Heat Exchangers: Experiments on Counter Flow. Heat Transf. Eng. 2020, 41, 220–234. [Google Scholar] [CrossRef]
- Yasinskiy, A.; Navas, J.; Aguilar, T.; Alcántara, R.; Gallardo, J.J.; Sánchez-Coronilla, A.; Martín, E.I.; De Los Santos, D.; Fernández-Lorenzo, C. Dramatically enhanced thermal properties for TiO2-based nanofluids for being used as heat transfer fluids in concentrating solar power plants. Renew. Energy 2018, 119, 809–819. [Google Scholar] [CrossRef]
- Salimi-Yasar, H.; Zeinali Heris, S.; Shanbedi, M. Influence of soluble oil-based TiO2 nanofluid on heat transfer performance of cutting fluid. Tribol. Int. 2017, 112, 147–154. [Google Scholar] [CrossRef]
- Ahmed, W.; Chowdhury, Z.Z.; Kazi, S.N.; Johan, M.R.; Akram, N.; Oon, C.S. Effect of ZnO-water based nanofluids from sonochemical synthesis method on heat transfer in a circular flow passage. Int. Commun. Heat Mass Transf. 2020, 114, 104591. [Google Scholar] [CrossRef]
- Subhedar, D.G.; Ramani, B.M.; Gupta, A. Experimental investigation of heat transfer potential of Al2O3/Water-Mono Ethylene Glycol nanofluids as a car radiator coolant. Case Stud. Therm. Eng. 2018, 11, 26–34. [Google Scholar] [CrossRef]
- Goudarzi, K.; Jamali, H. Heat transfer enhancement of Al2O3-EG nanofluid in a car radiator with wire coil inserts. Appl. Therm. Eng. 2017, 118, 510–517. [Google Scholar] [CrossRef] [Green Version]
- Eid, M.R.; Al-Hossainy, A.F. Synthesis, DFT calculations, and heat transfer performance large-surface TiO2: Ethylene glycol nanofluid and coolant applications. Eur. Phys. J. Plus 2020, 135, 1–19. [Google Scholar] [CrossRef]
- Krishnakumar, T.S.; Sheeba, A.; Mahesh, V.; Jose Prakash, M. Heat transfer studies on ethylene glycol/water nanofluid containing TiO2 nanoparticles. Int. J. Refrig. 2019, 102, 55–61. [Google Scholar] [CrossRef]
- Islam, M.R.; Shabani, B.; Rosengarten, G. Electrical and Thermal Conductivities of 50/50 Water-ethylene Glycol Based TiO2 Nanofluids to be Used as Coolants in PEM Fuel Cells. Energy Procedia 2017, 110, 101–108. [Google Scholar] [CrossRef]
- Colangelo, G.; Raho, B.; Milanese, M.; Risi, A. De Numerical Evaluation of a HVAC System Based on a High-Performance Heat Transfer Fluid. Energies 2021, 14, 3298. [Google Scholar] [CrossRef]
- Iacobazzi, F. A critical analysis of clustering phenomenon in Al2O3 nanofluids. J. Therm. Anal. Calorim. 2019, 9, 371–377. [Google Scholar] [CrossRef]
- Javed, M.; Shaik, A.H.; Khan, T.A.; Imran, M.; Aziz, A.; Ansari, A.R.; Chandan, M.R. Synthesis of stable waste palm oil based CuO nanofluid for heat transfer applications. Heat Mass Transf. Stoffuebertragung 2018, 54, 3739–3745. [Google Scholar] [CrossRef]
- Gkountas, A.A.; Benos, L.T.; Sofiadis, G.N.; Sarris, I.E. A printed-circuit heat exchanger consideration by exploiting an Al2O3-water nanofluid: Effect of the nanoparticles interfacial layer on heat transfer. Therm. Sci. Eng. Prog. 2021, 22, 100818. [Google Scholar] [CrossRef]
- Du, R.; Jiang, D.D.; Wang, Y.; Wei Shah, K. An experimental investigation of CuO/water nanofluid heat transfer in geothermal heat exchanger. Energy Build. 2020, 227, 110402. [Google Scholar] [CrossRef]
- Zhong, D.; Zhong, H.; Wen, T. Investigation on the thermal properties, heat transfer and flow performance of a highly self-dispersion TiO2 nanofluid in a multiport mini channel. Int. Commun. Heat Mass Transf. 2020, 117, 104783. [Google Scholar] [CrossRef]
- Wen, T.; Lu, L.; Zhong, H.; Shen, B. Thermal properties measurement and performance evaluation of water/ZnO nanofluid in a mini channel with offset fins. Int. J. Heat Mass Transf. 2020, 162, 120361. [Google Scholar] [CrossRef]
- Shahrestani, M.I.; Maleki, A.; Shadloo, M.S.; Tlili, I. Numerical Investigation of Forced Convective Heat Transfer and Performance Evaluation Criterion of Al2O3/Water Nanofluid Flow inside an Axisymmetric Microchannel. Symmetry. 2020, 12, 120. [Google Scholar] [CrossRef] [Green Version]
- Zhu, D.; Wang, L.; Yu, W.; Xie, H. Intriguingly high thermal conductivity increment for CuO nanowires contained nanofluids with low viscosity. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Ahmadi, M.A.; Ahmadi, M.H.; Fahim Alavi, M.; Nazemzadegan, M.R.; Ghasempour, R.; Shamshirband, S. Determination of thermal conductivity ratio of CuO/ethylene glycol nanofluid by connectionist approach. J. Taiwan Inst. Chem. Eng. 2018, 91, 383–395. [Google Scholar] [CrossRef]
- Hemmat Esfe, M.; Nadooshan, A.A.; Arshi, A.; Alirezaie, A. Convective heat transfer and pressure drop of aqua based TiO2 nanofluids at different diameters of nanoparticles: Data analysis and modeling with artificial neural network. Phys. E Low-Dimensional Syst. Nanostructures 2018, 97, 155–161. [Google Scholar] [CrossRef]
- Ding, M.; Liu, C.; Rao, Z. Experimental investigation on heat transfer characteristic of TiO2-H2O nanofluid in microchannel for thermal energy storage. Appl. Therm. Eng. 2019, 160, 114024. [Google Scholar] [CrossRef]
- Milanese, M.; Colangelo, G.; Cretì, A.; Lomascolo, M.; Iacobazzi, F.; Risi, A. De Solar Energy Materials & Solar Cells Optical absorption measurements of oxide nanoparticles for application as nano fl uid in direct absorption solar power systems—Part I: Water-based nano fl uids behavior. Sol. Energy Mater. Sol. Cells 2016, 147, 315–320. [Google Scholar] [CrossRef]
- Milanese, M.; Colangelo, G.; Cretì, A.; Lomascolo, M.; Iacobazzi, F.; Risi, A. De Solar Energy Materials & Solar Cells Optical absorption measurements of oxide nanoparticles for application as nano fl uid in direct absorption solar power systems—Part II: ZnO, CeO2, Fe2O3 nanoparticles behavior. Sol. Energy Mater. Sol. Cells 2016, 147, 321–326. [Google Scholar] [CrossRef]
- Potenza, M.; Milanese, M.; Colangelo, G.; Risi, A. De Experimental investigation of transparent parabolic trough collector based on gas-phase nanofluid. Appl. Energy 2017, 203, 560–570. [Google Scholar] [CrossRef]
- Wen, D.; Lin, G.; Vafaei, S.; Zhang, K. Review of nanofluids for heat transfer applications. Particuology 2009, 7, 141–150. [Google Scholar] [CrossRef]
- Nakhchi, M.E.; Esfahani, J.A. Numerical investigation of turbulent Cu-water nanofluid in heat exchanger tube equipped with perforated conical rings. Adv. Powder Technol. 2019, 30, 1338–1347. [Google Scholar] [CrossRef]
- Mebarek-Oudina, F.; Bessaïh, R. Numerical simulation of natural convection heat transfer of copper-water nanofluid in a vertical cylindrical annulus with heat sources. Thermophys. Aeromechanics 2019, 26, 325–334. [Google Scholar] [CrossRef]
- Gholamalipour, P.; Siavashi, M.; Doranehgard, M.H. Eccentricity effects of heat source inside a porous annulus on the natural convection heat transfer and entropy generation of Cu-water nanofluid. Int. Commun. Heat Mass Transf. 2019, 109, 104367. [Google Scholar] [CrossRef]
- Saleem, S.; Qasin, M.; Alderremy, A.A.; Noreen, S. Heat transfer enhancement using different shapes of Cu nanoparticles in the flow of water based nanofluid. Phys. Scr. 2020, 95, 055209. [Google Scholar] [CrossRef]
- Hadavand, M.; Yousefzadeh, S.; Akbari, O.A.; Pourfattah, F.; Nguyen, H.M.; Asadi, A. A numerical investigation on the effects of mixed convection of Ag-water nanofluid inside a sim-circular lid-driven cavity on the temperature of an electronic silicon chip. Appl. Therm. Eng. 2019, 162, 114298. [Google Scholar] [CrossRef]
- Mir, S.; Akbari, O.A.; Toghraie, D.; Sheikhzadeh, G.; Marzban, A.; Mir, S.; Talebizadehsardari, P. A comprehensive study of two-phase flow and heat transfer of water/Ag nanofluid in an elliptical curved minichannel. Chin. J. Chem. Eng. 2020, 28, 383–402. [Google Scholar] [CrossRef]
- Saleh, B.; Sundar, L.S. Experimental study on heat transfer, friction factor, entropy and exergy efficiency analyses of a corrugated plate heat exchanger using Ni/water nanofluids. Int. J. Therm. Sci. 2021, 165, 106935. [Google Scholar] [CrossRef]
- Hemmat Esfe, M.; Saedodin, S.; Wongwises, S.; Toghraie, D. An experimental study on the effect of diameter on thermal conductivity and dynamic viscosity of Fe/water nanofluids. J. Therm. Anal. Calorim. 2015, 119, 1817–1824. [Google Scholar] [CrossRef]
- Khoshvaght-Aliabadi, M.; Davoudi, S.; Dibaei, M.H. Performance of agitated-vessel U tube heat exchanger using spiky twisted tapes and water based metallic nanofluids. Chem. Eng. Res. Des. 2018, 133, 26–39. [Google Scholar] [CrossRef]
- Mahmoudi, A.H.; Shahi, M.; Raouf, A.H.; Ghasemian, A. Numerical study of natural convection cooling of horizontal heat source mounted in a square cavity filled with nanofluid. Int. Commun. Heat Mass Transf. 2010, 37, 1135–1141. [Google Scholar] [CrossRef]
- Ashorynejad, H.R.; Mohamad, A.A.; Sheikholeslami, M. Magnetic field effects on natural convection flow of a nanofluid in a horizontal cylindrical annulus using Lattice Boltzmann method. Int. J. Therm. Sci. 2013, 64, 240–250. [Google Scholar] [CrossRef]
- Roszko, A.; Fornalik-Wajs, E.; Donizak, J.; Wajs, J.; Kraszewska, A.; Pleskacz, L.; Kenjeres, S. Magneto-thermal convection of low concentration nanofluids. MATEC Web Conf. 2014, 18, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Fornalik-Wajs, E.; Roszko, A.; Donizak, J. Nanofluid flow driven by thermal and magnetic forces—Experimental and numerical studies. Energy 2020, 201. [Google Scholar] [CrossRef]
- Mishra, G.; Dash, B.; Pandey, S. Layered double hydroxides: A brief review from fundamentals to application as evolving biomaterials. Appl. Clay Sci. 2018, 153, 172–186. [Google Scholar] [CrossRef]
- Chakraborty, S.; Sarkar, I.; Ashok, A.; Sengupta, I.; Pal, S.K.; Chakraborty, S. Synthesis of Cu-Al LDH nanofluid and its application in spray cooling heat transfer of a hot steel plate. Powder Technol. 2018, 335, 285–300. [Google Scholar] [CrossRef]
- Tiara, A.M.; Chakraborty, S.; Sarkar, I.; Pal, S.K.; Chakraborty, S. Heat transfer in jet impingement on a hot steel surface using surfactant based Cu-Al layered double hydroxide nanofluid. Int. J. Heat Mass Transf. 2016, 101, 825–833. [Google Scholar] [CrossRef]
- Chakraborty, S.; Sarkar, I.; Ashok, A.; Sengupta, I.; Pal, S.K.; Chakraborty, S. Thermo-physical properties of Cu-Zn-Al LDH nanofluid and its application in spray cooling. Appl. Therm. Eng. 2018, 141, 339–351. [Google Scholar] [CrossRef]
- Ponnada, S.; Subrahmanyam, T.; Naidu, S.V. An experimental investigation on heat transfer and friction factor of Silicon Carbide/water nanofluids in a circular tube. Energy Procedia 2019, 158, 5156–5161. [Google Scholar] [CrossRef]
- Al-Waeli, A.H.A.; Sopian, K.; Chaichan, M.T.; Kazem, H.A.; Hasan, H.A.; Al-Shamani, A.N. An experimental investigation of SiC nanofluid as a base-fluid for a photovoltaic thermal PV/T system. Energy Convers. Manag. 2017, 142, 547–558. [Google Scholar] [CrossRef]
- Verrilli, F.; Srinivasan, S.; Gambino, G.; Canelli, M.; Himanka, M.; Del Vecchio, C.; Sasso, M.; Glielmo, L. Model Predictive Control-Based Optimal Operations of District Heating System with Thermal Energy Storage and Flexible Loads. IEEE Trans. Autom. Sci. Eng. 2017, 14, 547–557. [Google Scholar] [CrossRef]
- Jóźwiak, B.; Dzido, G.; Kolanowska, A.; Jędrysiak, R.G.; Zorębski, E.; Greer, H.F.; Dzida, M.; Boncel, S. From lab and up: Superior and economic heat transfer performance of ionanofluids containing long carbon nanotubes and 1-ethyl-3-methylimidazolium thiocyanate. Int. J. Heat Mass Transf. 2021, 172. [Google Scholar] [CrossRef]
- Moradi, A.; Toghraie, D.; Isfahani, A.H.M.; Hosseinian, A. An experimental study on MWCNT–water nanofluids flow and heat transfer in double-pipe heat exchanger using porous media. J. Therm. Anal. Calorim. 2019, 137, 1797–1807. [Google Scholar] [CrossRef]
- Sarafraz, M.M.; Nikkhah, V.; Nakhjavani, M.; Arya, A. Fouling formation and thermal performance of aqueous carbon nanotube nanofluid in a heat sink with rectangular parallel microchannel. Appl. Therm. Eng. 2017, 123, 29–39. [Google Scholar] [CrossRef]
- Fan, L.W.; Li, J.Q.; Wu, Y.Z.; Zhang, L.; Yu, Z.T. Pool boiling heat transfer during quenching in carbon nanotube (CNT)-based aqueous nanofluids: Effects of length and diameter of the CNTs. Appl. Therm. Eng. 2017, 122, 555–565. [Google Scholar] [CrossRef]
- Abdeen, D.H.; Atieh, M.A.; Merzougui, B.; Khalfaoui, W. Corrosion evaluation of 316L stainless steel in CNT-water nanofluid: Effect of CNTs loading. Materials 2019, 12, 1634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selvam, C.; Mohan Lal, D.; Harish, S. Enhanced heat transfer performance of an automobile radiator with graphene based suspensions. Appl. Therm. Eng. 2017, 123, 50–60. [Google Scholar] [CrossRef]
- Sadri, R.; Hosseini, M.; Kazi, S.N.; Bagheri, S.; Zubir, N.; Ahmadi, G.; Dahari, M.; Zaharinie, T. A novel, eco-friendly technique for covalent functionalization of graphene nanoplatelets and the potential of their nanofluids for heat transfer applications. Chem. Phys. Lett. 2017, 675, 92–97. [Google Scholar] [CrossRef]
- Sözen, A.; Filiz, Ç.; Aytaç, İ.; Martin, K.; Ali, H.M.; Boran, K.; Yetişken, Y. Upgrading of the Performance of an Air-to-Air Heat Exchanger Using Graphene/Water Nanofluid. Int. J. Thermophys. 2021, 42, 1–15. [Google Scholar] [CrossRef]
- Selvam, C.; Solaimalai Raja, R.; Mohan Lal, D.; Harish, S. Overall heat transfer coefficient improvement of an automobile radiator with graphene based suspensions. Int. J. Heat Mass Transf. 2017, 115, 580–588. [Google Scholar] [CrossRef]
- Zhan, X.; Si, C.; Zhou, J.; Sun, Z. MXene and MXene-based composites: Synthesis, properties and environment-related applications. Nanoscale Horizons 2020, 5, 235–258. [Google Scholar] [CrossRef]
- Bao, Z.; Bing, N.; Zhu, X.; Xie, H.; Yu, W. Ti3C2Tx MXene contained nanofluids with high thermal conductivity, super colloidal stability and low viscosity. Chem. Eng. J. 2021, 406, 126390. [Google Scholar] [CrossRef]
- Aslfattahi, N.; Samylingam, L.; Abdelrazik, A.S.; Arifutzzaman, A.; Saidur, R. MXene based new class of silicone oil nanofluids for the performance improvement of concentrated photovoltaic thermal collector. Sol. Energy Mater. Sol. Cells 2020, 211, 110526. [Google Scholar] [CrossRef]
- Samylingam, L.; Aslfattahi, N.; Saidur, R.; Yahya, S.M.; Afzal, A.; Arifutzzaman, A.; Tan, K.H.; Kadirgama, K. Thermal and energy performance improvement of hybrid PV/T system by using olein palm oil with MXene as a new class of heat transfer fluid. Sol. Energy Mater. Sol. Cells 2020, 218, 110754. [Google Scholar] [CrossRef]
- Rubbi, F.; Habib, K.; Saidur, R.; Aslfattahi, N.; Yahya, S.M.; Das, L. Performance optimization of a hybrid PV/T solar system using Soybean oil/MXene nanofluids as A new class of heat transfer fluids. Sol. Energy 2020, 208, 124–138. [Google Scholar] [CrossRef]
- Hassan, M.; Marin, M.; Ellahi, R.; Alamri, S.Z. Exploration of convecti ve heat transfer and flow characteristics synthesis by Cu-Ag/Water hybrid-nanofluids. Heat Transf. Res. 2018, 49, 1837–1848. [Google Scholar] [CrossRef]
- Akram, J.; Akbar, N.S.; Tripathi, D. A Theoretical Investigation on the Heat Transfer Ability of Water-Based Hybrid (Ag–Au) Nanofluids and Ag Nanofluids Flow Driven by Electroosmotic Pumping Through a Microchannel. Arab. J. Sci. Eng. 2021, 46, 2911–2927. [Google Scholar] [CrossRef]
- Hayat, T.; Nadeem, S. Heat transfer enhancement with Ag–CuO/water hybrid nanofluid. Results Phys. 2017, 7, 2317–2324. [Google Scholar] [CrossRef]
- Ghadikolaei, S.S.; Yassari, M.; Sadeghi, H.; Hosseinzadeh, K.; Ganji, D.D. Investigation on thermophysical properties of TiO2–Cu/H2O hybrid nanofluid transport dependent on shape factor in MHD stagnation point flow. Powder Technol. 2017, 322, 428–438. [Google Scholar] [CrossRef]
- Gürbüz, E.Y.; Variyenli, H.İ.; Sözen, A.; Khanlari, A.; Ökten, M. Experimental and numerical analysis on using CuO-Al2O3/water hybrid nanofluid in a U-type tubular heat exchanger. Int. J. Numer. Methods Heat Fluid Flow 2021, 31, 519–540. [Google Scholar] [CrossRef]
- Minea, A.A. Hybrid nanofluids based on Al2O3, TiO2 and SiO2: Numerical evaluation of different approaches. Int. J. Heat Mass Transf. 2017, 104, 852–860. [Google Scholar] [CrossRef]
- Gupta, N.; Gupta, S.M.; Sharma, S.K. Preparation of stable metal/COOH-MWCNT hybrid nanofluid. Mater. Today Proc. 2019, 36, 649–656. [Google Scholar] [CrossRef]
- Kumar, V.; Sarkar, J. Particle ratio optimization of Al2O3-MWCNT hybrid nanofluid in minichannel heat sink for best hydrothermal performance. Appl. Therm. Eng. 2020, 165, 114546. [Google Scholar] [CrossRef]
- Hussien, A.A.; Abdullah, M.Z.; Yusop, N.M.; Al-Nimr, M.A.; Atieh, M.A.; Mehrali, M. Experiment on forced convective heat transfer enhancement using MWCNTs/GNPs hybrid nanofluid and mini-tube. Int. J. Heat Mass Transf. 2017, 115, 1121–1131. [Google Scholar] [CrossRef]
- Said, Z.; Abdelkareem, M.A.; Rezk, H.; Nassef, A.M.; Atwany, H.Z. Stability, thermophysical and electrical properties of synthesized carbon nanofiber and reduced-graphene oxide-based nanofluids and their hybrid along with fuzzy modeling approach. Powder Technol. 2020, 364, 795–809. [Google Scholar] [CrossRef]
- Wang, J.; Guan, Z.; Gurgenci, H.; Hooman, K.; Veeraragavan, A.; Kang, X. Computational investigations of heat transfer to supercritical CO2 in a large horizontal tube. Energy Convers. Manag. 2018, 157, 536–548. [Google Scholar] [CrossRef]
- Aguilar, R.; Valenzuela, L.; Avila-Marin, A.L.; Garcia-Ybarra, P.L. Simplified heat transfer model for parabolic trough solar collectors using supercritical CO2. Energy Convers. Manag. 2019, 196, 807–820. [Google Scholar] [CrossRef]
- Wang, K.Z.; Xu, X.X.; Liu, C.; Bai, W.J.; Dang, C. bin Experimental and numerical investigation on heat transfer characteristics of supercritical CO2 in the cooled helically coiled tube. Int. J. Heat Mass Transf. 2017, 108, 1645–1655. [Google Scholar] [CrossRef]
- Zhang, S.; Xu, X.; Liu, C.; Liu, X.; Ru, Z.; Dang, C. Experimental and numerical comparison of the heat transfer behaviors and buoyancy effects of supercritical CO2 in various heating tubes. Int. J. Heat Mass Transf. 2020, 149. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, Z.D.; Li, M.J.; Min, C.H. A coupled optical-thermal-fluid-mechanical analysis of parabolic trough solar receivers using supercritical CO2 as heat transfer fluid. Appl. Therm. Eng. 2021, 183, 116154. [Google Scholar] [CrossRef]
- Guo, P.; Liu, S.; Yan, J.; Wang, J.; Zhang, Q. Experimental study on heat transfer of supercritical CO2 flowing in a mini tube under heating conditions. Int. J. Heat Mass Transf. 2020, 153. [Google Scholar] [CrossRef]
- Zhang, L.; Jiang, P.; Wang, Z.; Xu, R. Convective heat transfer of supercritical CO2 in a rock fracture for enhanced geothermal systems. Appl. Therm. Eng. 2017, 115, 923–936. [Google Scholar] [CrossRef]
- Khalesi, J.; Sarunac, N.; Razzaghpanah, Z. Supercritical CO2 conjugate heat transfer and flow analysis in a rectangular microchannel subject to uniformly heated substrate wall. Therm. Sci. Eng. Prog. 2020, 19, 100596. [Google Scholar] [CrossRef]
- American National Standards Institute; ASHRAE. Designation and Safety Classification of Refrigerants; ANSI/ASHRAE Standard 34-2019; ANSI: Washington, DC, USA; ASHRAE: Atlanta, GA, USA, 2019; Volume 2019, pp. 1–52. [Google Scholar]
- Bellos, E.; Tzivanidis, C. A comparative study of CO2 refrigeration systems. Energy Convers. Manag. X 2019, 1, 100002. [Google Scholar] [CrossRef]
- Xu, X.; Wang, X.; Li, P.; Li, Y.; Hao, Q.; Xiao, B.; Elsentriecy, H.; Gervasio, D. Experimental Test of Properties of KCl-MgCl2 Eutectic Molten Salt for Heat Transfer and Thermal Storage Fluid in Concentrated Solar Power Systems. J. Sol. Energy Eng. Trans. ASME 2018, 140, 1–9. [Google Scholar] [CrossRef]
- Liu, T.; Xu, X.; Liu, W.; Zhuang, X. Corrosion of alloys in high temperature molten-salt heat transfer fluids with air as the cover gas. Sol. Energy 2019, 191, 435–448. [Google Scholar] [CrossRef]
- Fuqiang, W.; Huijian, J.; Hao, W.; Ziming, C.; Jianyu, T.; Yuan, Y.; Yuhang, S.; Wenjie, Z. Radiative, conductive and laminar convective coupled heat transfer analysis of molten salts based on finite element method. Appl. Therm. Eng. 2018, 131, 19–29. [Google Scholar] [CrossRef]
- Kuchibhotla, A.; Banerjee, D.; Dhir, V. Forced convection heat transfer of molten Salts: A review. Nucl. Eng. Des. 2020, 362, 110591. [Google Scholar] [CrossRef]
- Zou, L.L.; Chen, X.; Wu, Y.T.; Wang, X.; Ma, C.F. Experimental study of thermophysical properties and thermal stability of quaternary nitrate molten salts for thermal energy storage. Sol. Energy Mater. Sol. Cells 2019, 190, 12–19. [Google Scholar] [CrossRef]
- Vaka, M.; Walvekar, R.; Jagadish, P.; Khalid, M.; Mubarak, N.M.; Panchal, H. High-temperature molten salts optimisation using mixture design for energy storage application. J. Energy Storage 2020, 32, 101981. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Zhao, C.Y. Thermophysical properties of Ca(NO3)2-NaNO3-KNO3 mixtures for heat transfer and thermal storage. Sol. Energy 2017, 146, 172–179. [Google Scholar] [CrossRef]
- Trabelsi, S.E.; Qoaider, L.; Guizani, A. Investigation of using molten salt as heat transfer fluid for dry cooled solar parabolic trough power plants under desert conditions. Energy Convers. Manag. 2018, 156, 253–263. [Google Scholar] [CrossRef]
- Rizvi, S.M.M.; Shin, D. Mechanism of heat capacity enhancement in molten salt nanofluids. Int. J. Heat Mass Transf. 2020, 161, 120260. [Google Scholar] [CrossRef]
- Chen, X.; Wu, Y.T.; Zhang, L.D.; Wang, X.; Ma, C.F. Experimental study on thermophysical properties of molten salt nanofluids prepared by high-temperature melting. Sol. Energy Mater. Sol. Cells 2019, 191, 209–217. [Google Scholar] [CrossRef]
- Xiong, Y.; Sun, M.; Wu, Y.; Xu, P.; Xu, Q.; Li, C.; Ding, Y.; Ma, C. Effects of Synthesis Methods on Thermal Performance of Nitrate Salt Nanofluids for Concentrating Solar Power. Energy Fuels 2020, 34, 11606–11619. [Google Scholar] [CrossRef]
- Ying, Z.; He, B.; Su, L.; Kuang, Y.; He, D.; Lin, C. Convective heat transfer of molten salt-based nanofluid in a receiver tube with non-uniform heat flux. Appl. Therm. Eng. 2020, 181, 115922. [Google Scholar] [CrossRef]
- Wei, X.; Yin, Y.; Qin, B.; Wang, W.; Ding, J.; Lu, J. Preparation and enhanced thermal conductivity of molten salt nanofluids with nearly unaltered viscosity. Renew. Energy 2020, 145, 2435–2444. [Google Scholar] [CrossRef]
- Singh, S.K.; Savoy, A.W. Ionic liquids synthesis and applications: An overview. J. Mol. Liq. 2020, 297, 112038. [Google Scholar] [CrossRef]
- Shamshina, J.L.; Zavgorodnya, O.; Rogers, R.D. Ionic Liquids, 3rd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2019; ISBN 9780081019832. [Google Scholar]
- Oster, K.; Jacquemin, J.; Hardacre, C.; Ribeiro, A.P.C.; Elsinawi, A. Further development of the predictive models for physical properties of pure ionic liquids: Thermal conductivity and heat capacity. J. Chem. Thermodyn. 2018, 118, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Oster, K.; Goodrich, P.; Jacquemin, J.; Hardacre, C.; Ribeiro, A.P.C.; Elsinawi, A. A new insight into pure and water-saturated quaternary phosphonium-based carboxylate ionic liquids: Density, heat capacity, ionic conductivity, thermogravimetric analysis, thermal conductivity and viscosity. J. Chem. Thermodyn. 2018, 121, 97–111. [Google Scholar] [CrossRef]
- Paul, T.C.; Morshed, A.K.M.M.; Fox, E.B.; Khan, J.A. Enhanced thermophysical properties of NEILs as heat transfer fluids for solar thermal applications. Appl. Therm. Eng. 2017, 110, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Hosseinghorbani, A.; Mozaffarian, M.; Pazuki, G. Application of graphene oxide IoNanofluid as a superior heat transfer fluid in concentrated solar power plants. Int. Commun. Heat Mass Transf. 2020, 111. [Google Scholar] [CrossRef]
- Das, L.; Habib, K.; Saidur, R.; Aslfattahi, N.; Yahya, S.M.; Rubbi, F. Improved thermophysical properties and energy efficiency of aqueous ionic liquid/mxene nanofluid in a hybrid pv/t solar system. Nanomaterials 2020, 10, 1732. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Zhang, L.; Guo, Y.; Jiang, Z.; He, F.; Xie, C.; Fan, J.; Wu, J.; Zhang, K. Novel segregated-structure phase change materials composed of paraffin-graphene microencapsules with high latent heat and thermal conductivity. J. Mater. Sci. 2018, 53, 2566–2575. [Google Scholar] [CrossRef]
- Du, K.; Calautit, J.; Wang, Z.; Wu, Y.; Liu, H. A review of the applications of phase change materials in cooling, heating and power generation in different temperature ranges. Appl. Energy 2018, 220, 242–273. [Google Scholar] [CrossRef]
- Ghalambaz, M.; Mehryan, S.A.M.; Hajjar, A.; Veismoradi, A. Unsteady natural convection flow of a suspension comprising Nano-Encapsulated Phase Change Materials (NEPCMs) in a porous medium. Adv. Powder Technol. 2020, 31, 954–966. [Google Scholar] [CrossRef]
- Chananipoor, A.; Azizi, Z.; Raei, B.; Tahmasebi, N. Optimization of the thermal performance of nano-encapsulated phase change material slurry in double pipe heat exchanger: Design of experiments using response surface methodology (RSM). J. Build. Eng. 2021, 34, 101929. [Google Scholar] [CrossRef]
- Ghalambaz, M.; Mehryan, S.A.M.; Zahmatkesh, I.; Chamkha, A. Free convection heat transfer analysis of a suspension of nano–encapsulated phase change materials (NEPCMs) in an inclined porous cavity. Int. J. Therm. Sci. 2020, 157, 106503. [Google Scholar] [CrossRef]
- Nomura, T.; Sheng, N.; Zhu, C.; Saito, G.; Hanzaki, D.; Hiraki, T.; Akiyama, T. Microencapsulated phase change materials with high heat capacity and high cyclic durability for high-temperature thermal energy storage and transportation. Appl. Energy 2017, 188, 9–18. [Google Scholar] [CrossRef]
- Khan, A.; Ali, H.M.; Nazir, R.; Ali, R.; Munir, A.; Ahmad, B.; Ahmad, Z. Experimental investigation of enhanced heat transfer of a car radiator using ZnO nanoparticles in H2O–ethylene glycol mixture. J. Therm. Anal. Calorim. 2019, 138, 3007–3021. [Google Scholar] [CrossRef]
- Fazeli, I.; Sarmasti Emami, M.R.; Rashidi, A. Investigation and optimization of the behavior of heat transfer and flow of MWCNT-CuO hybrid nanofluid in a brazed plate heat exchanger using response surface methodology. Int. Commun. Heat Mass Transf. 2021, 122, 105175. [Google Scholar] [CrossRef]
- Qiu, Y.; Li, M.J.; Li, M.J.; Zhang, H.H.; Ning, B. Numerical and experimental study on heat transfer and flow features of representative molten salts for energy applications in turbulent tube flow. Int. J. Heat Mass Transf. 2019, 135, 732–745. [Google Scholar] [CrossRef]
- Yee, R.P.; Hermes, C.J.L. A thermodynamic study of water-based nanosuspensions as secondary heat transfer fluids in refrigeration systems. Int. J. Refrig. 2018, 89, 104–111. [Google Scholar] [CrossRef]
- Mondejar, M.E.; Andreasen, J.G.; Regidor, M.; Riva, S.; Kontogeorgis, G.; Persico, G.; Haglind, F. Prospects of the use of nanofluids as working fluids for organic Rankine cycle power systems. Energy Procedia 2017, 129, 160–167. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Zhao, M.; Wang, G. Effects of heat transfer fluid and boundary conditions on temperature field of enhanced geothermal system. Petroleum 2021. [Google Scholar] [CrossRef]
- Gagne-Boisvert, L.; Bernier, M. Comparison of the Energy Use for Different Heat Transfer Fluids in Geothermal Systems. In Proceedings of the IGSHPA Technical/Research Conference and Expo, Denver, CO, USA, 14–16 March 2017. [Google Scholar] [CrossRef] [Green Version]
- Rane, M.V.; Tandale, M.S. Water-to-water heat transfer in tube-tube heat exchanger: Experimental and analytical study. Appl. Therm. Eng. 2005, 25, 2715–2729. [Google Scholar] [CrossRef]
- Fares, M.; AL-Mayyahi, M.; AL-Saad, M. Heat transfer analysis of a shell and tube heat exchanger operated with graphene nanofluids. Case Stud. Therm. Eng. 2020, 18, 100584. [Google Scholar] [CrossRef]
- Ahmed, M.S.; Elsaid, A.M. Effect of hybrid and single nanofluids on the performance characteristics of chilled water air conditioning system. Appl. Therm. Eng. 2019, 163, 114398. [Google Scholar] [CrossRef]
- Adyanshee Pattanayak, R.K.; Sahoo, N.; Mishra, P. Performance Analysis of a Domestic Refrigerator using Al2O3 Nanoparticles. IOSR J. Mech. Civ. Eng. Ver. IV 2016, 12, 1684–2278. [Google Scholar] [CrossRef]
- Sarkar, I.; Chakraborty, S.; Jha, J.M.; Pal, S.K.; Chakraborty, S. Ultrafast cooling of a hot steel plate using Cu-Al layered double hydroxide nanofluid jet. Int. J. Therm. Sci. 2017, 116, 52–62. [Google Scholar] [CrossRef]
- Jha, J.M.; Sarkar, I.; Chakraborty, S.; Pal, S.K.; Chakraborty, S. Heat transfer from a hot moving steel plate by using Cu-Al layered double hydroxide nanofluid based air atomized spray. Exp. Heat Transf. 2017, 30, 500–516. [Google Scholar] [CrossRef]
- Kwon, J.S.; Son, S.; Heo, J.Y.; Lee, J.I. Compact heat exchangers for supercritical CO2 power cycle application. Energy Convers. Manag. 2020, 209, 112666. [Google Scholar] [CrossRef]
- Lei, Y.; Chen, Z. Cooling heat transfer and pressure drop of supercritical CO2 in wavy microchannels with consistent and opposite crests and troughs. Int. J. Refrig. 2020, 109, 64–81. [Google Scholar] [CrossRef]
- Henchoz, S.; Favrat, D.; Girardin, L. District heating and cooling energy network using CO2 as a heat and mass transfer fluid. Heat Pump. Technol. Mag. 2018, 36, 19–21. [Google Scholar]
- Fritsch, A.; Frantz, C.; Uhlig, R. Techno-economic analysis of solar thermal power plants using liquid sodium as heat transfer fluid. Sol. Energy 2019, 177, 155–162. [Google Scholar] [CrossRef] [Green Version]
- Yu, Q.; Lu, Y.; Zhang, C.; Wu, Y.; Sunden, B. Experimental and numerical study of natural convection in bottom-heated cylindrical cavity filled with molten salt nanofluids. J. Therm. Anal. Calorim. 2020, 141, 1207–1219. [Google Scholar] [CrossRef]
- Yang, H.; Li, J.; Huang, Y.; Kwan, T.H.; Cao, J.; Pei, G. Feasibility research on a hybrid solar tower system using steam and molten salt as heat transfer fluid. Energy 2020, 205, 118094. [Google Scholar] [CrossRef]
- Muñoz-Sánchez, B.; Nieto-Maestre, J.; Iparraguirre-Torres, I.; García-Romero, A.; Sala-Lizarraga, J.M. Molten salt-based nanofluids as efficient heat transfer and storage materials at high temperatures. An overview of the literature. Renew. Sustain. Energy Rev. 2018, 82, 3924–3945. [Google Scholar] [CrossRef]
- Zahir, M.H.; Mohamed, S.A.; Saidur, R.; Al-Sulaiman, F.A. Supercooling of phase-change materials and the techniques used to mitigate the phenomenon. Appl. Energy 2019, 240, 793–817. [Google Scholar] [CrossRef]
- Sun, X.; Medina, M.A.; Lee, K.O.; Jin, X. Laboratory assessment of residential building walls containing pipe-encapsulated phase change materials for thermal management. Energy 2018, 163, 383–391. [Google Scholar] [CrossRef]
- Qiu, L.; Ouyang, Y.; Feng, Y.; Zhang, X. Review on micro/nano phase change materials for solar thermal applications. Renew. Energy 2019, 140, 513–538. [Google Scholar] [CrossRef]
- Shyam Prasad, S.; Deepak Selvakumar, R. Heat transfer performance of Al2O3-([C4mim][NTf2]) nano-suspension in a 2-D channel for application in a flat plate solar collector. IOP Conf. Ser. Mater. Sci. Eng. 2019, 577. [Google Scholar] [CrossRef]





| Fluid | Environmental Aspects | Technical/Efficiency Aspects | Economic Aspects | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Corrosivity | Toxicity | Temperature Range (°C) | ρ (kg/m3) | c (J/(kg·K)) | TC (W/(m·K)) | η (mPa·s) | Pr (-) | Price ($/kg) | |
| air | No | No | +540 to 1090 | 1.293 (0 °C) 1.128 (40 °C) 1.000 (80 °C) | 1004 (0 °C) 1004 (40 °C) 1006 (80 °C) | 0.024 (0 °C) 0.028 (40 °C) 0.031 (80 °C) | 0.017 (0 °C) 0.019 (40 °C) 0.021 (80 °C) | 0.71 (0 °C) 0.68 (40 °C) 0.68 (80 °C) | 0 |
| fluoroalkanes | No | No | −100 to +150 | 1840 (0 °C) 1800 (20 °C) | 1000 (0 °C) 1050 (20 °C) | 0.06 (0 °C) 0.06 (20 °C) | 2 (0 °C) 1.3 (20 °C) | 33.3 (0 °C) 22.8 (20 °C) | 5 (TCE) |
| water | Yes | No | 0 to +100 | 1000 (0 °C) 992 (40 °C) 972 (80 °C) | 4222 (0 °C) 4175 (40 °C) 4195 (80 °C) | 0.558 (0 °C) 0.633 (40 °C) 0.673 (80 °C) | 1.792 (0 °C) 0.656 (40 °C) 0.357 (80 °C) | 13.56 (0 °C) 4.33 (40 °C) 2.23 (80 °C) | 0.01 |
| aliphatic hydrocarbons | Yes | No | −60 to +150 | 815 (−50 °C) | 1880 (−50 °C) | 0.115 (−50 °C) | 15 (−50 °C) | 245.2 (−50 °C) | 2–4 (isoparaffinic hydrocarbons) |
| aromatic hydrocarbons | Yes | Yes | −70 to +260 | 920 (−50 °C) | 1636 (−50 °C) | 0.143 (−50 °C) | 3.9 (−50 °C) | 44.6 (−50 °C) | 2.5–3 (diethylbenzene) |
| ethylene glycol (41 wt% aqueous solution) | Yes | Yes | −20 to +100 | 1069 (−20 °C) 1062 (0 °C) | 3320 (−20 °C) 3405 (0 °C) | 0.391 (−20 °C) 0.406 (0 °C) | 15.6 (−20 °C) 5.95 (0 °C) | 132.5 (−20 °C) 49.9 (0 °C) | 1.5 |
| propylene glycol (44 wt% aqueous solution) | Yes | No | −20 to +100 | 1053 (−20 °C) 1045 (0 °C) | 3620 (−20 °C) 3640 (0 °C) | 0.362 (−20 °C) 0.373 (0 °C) | 62 (−20 °C) 13.9 (0 °C) | 620 (−20 °C) 135.6 (0 °C) | 3 |
| silicone oil | No | No | −100 to +260 | 927 (−50 °C) 900 (0 °C) 890 (20 °C) | 1625 (−50 °C) 1700 (0 °C) 1750 (20 °C) | 0.125 (−50 °C) 0.110 (0 °C) 0.100 (20 °C) | 6.9 (−50 °C) 2 (0 °C) 1.2 (20 °C) | 89.7 (−50 °C) 30.9 (0 °C) 21 (20 °C) | 3–5 |
| paraffinic oil | No | No | +30 to +300 | 721 (300 °C) | 2436 (300 °C) | ~0.1 (300 °C) | 1.09 (300 °C) | 26.6 (300 °C) | 2–5 |
| eutectic mixture of biphenyl/diphenyl oxide | No | Yes | +12 to +400 | 849 (300 °C) | 1930 (300 °C) | ~0.01 (300 °C) | 0.59 (300 °C) | 113.9 (300 °C) | 100 |
| Fluid | Environmental Aspects | Technical Aspects | Economic Aspects | Ref. | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Corrosivity | Toxicity | Temperature Range (°C) | ρ (kg/m3) | c (J/kg·K) | TC (W/m·K) | η (mPa·s) | Pr (-) | Estimated Price($/kg) | ||
| 0.6 vol% Ni/water | Yes | No | 0 to +100 | 1045 (20 °C) 1035 (50 °C) | 4160 (20 °C) 4158 (50 °C) | 0.72 (20 °C) 0.84 (50 °C) | 1.5 (20 °C) 0.85 (50 °C) | 8.67 (20 °C) 4.21 (50 °C) | 0.13 | [132] |
| 0.04 vol% ZnO/H2O–EG(50:50 v/v) | Yes | Yes | −20 to +100 | 1002 (60 °C) | 4016 (60 °C) | 0.708 (60 °C) | 0.89 (60 °C) | 5.048 (60 °C) | 0.76 | [206] |
| 0.16% Cu-Al(4:1) LDH/water | Yes | No | 0 to +100 | n.d | n.d | 0.68 (30 °C) | 1.35 (30 °C) | n.d | n.d. | [140] |
| 0.05 vol% graphene/water | Yes | No | 0 to +100 | 998.5 (20 °C) 992.5 (45 °C) | 4060 (20 °C) 4075 (45 °C) | 0.675 (20 °C) 0.78 (45 °C) | 1.12 (20 °C) 0.72 (45 °C) | 6.736 (20 °C) 3.76 (45 °C) | 0.04 | [152] |
| 0.1 wt% MWCNT/CuO/water | Yes | No | 0 to +100 | 998.8 (20 °C) 992.8(40 °C) | 4178(20 °C) 4175 (40 °C) | 0.54 (20 °C) 0.76 (40 °C) | 1.02 (20 °C) 0.66 (40 °C) | 7.89 (20 °C) 3.63 (40 °C) | 0.07 | [207] |
| 0.04 vol% f-CNT/rGO/water | Yes | No | 0 to +100 | 997.5 (25 °C) 986.0 (55 °C) | n.d | 0.65 (25 °C) 0.80 (55 °C) | 1.1 (25 °C) 0.6 (55 °C) | n.d | n.d. | [169] |
| supercritical CO2 | Yes | No | −73 to +1000 | 800 (25 °C) 300 (50 °C) 190 (100 °C) p = 10 MPa | 3000 (25 °C) 4000 (50 °C) 1500 (100 °C) p = 10 MPa | 0.085 (25 °C) 0.04 (50 °C) 0.03 (100 °C) p = 10 MPa | 0.07 (25 °C) 0.025 (50 °C) 0.025 (100 °C) p = 10 MPa | 2.47 (25 °C) 2.5 (50 °C) 1.3 (100 °C) p = 10 MPa | n.d. | [174] |
| Solar Salt | Yes | No | +220 to +550 | 1900 (300 °C) 1775 (500 °C) | 1495 (300 °C) 1800 (500 °C) | 0.5 (300 °C) 0.54 (500 °C) | 3.0 (300 °C) 1.25 (500 °C) | 8.97 (300 °C) 4.17 (500 °C) | 0.49 | [208] |
| 0.5 wt% SiO2/Hitec | Yes | No | +142 to +450 | n.d | 1890 (200 °C) 2000 (300 °C) | 0.475 (200 °C) 0.525 (300 °C) | 2.25 (200 °C) 1.75 (300 °C) | 8.95 (200 °C) 6.67 (300 °C) | 0.93 | [189] |
| 20% n-dodecanol/PMMA/GO/water | No | No | 0 to +100 | 949.4 (20 °C) | 3650 (20 °C) | 0.5 (20 °C) | 2.49 (20 °C) | 18.18 (20 °C) | n.d. | [203] |
| Ionic liquid ([bmim][Tf2N]) | Yes | No | +25 to +200 | 1429 (25 °C) 1354 (100 °C) 1254 (200 °C) | 1252 (25 °C) 1430 (100 °C) 1667 (200 °C) | 0.1271 (25 °C) 0.1219 (100 °C) 0.1149 (200 °C) | 41.0 (25 °C) 8.1 (100 °C) 1.5 (200 °C) | 403.87 (25 °C) 95.02(100 °C) 21.76 (200 °C) | n.d. | [34] |
| Fluid | Applications | Advantages | Limitations | Ref. |
|---|---|---|---|---|
| air |
|
|
| [41,46,47] |
| water |
|
|
| [56,213] |
| nanofluids |
|
|
| [104,130,154,206,214,215,216,217,218] |
| supercritical CO2 |
|
|
| [113,176,219,220,221] |
| molten salts |
|
|
| [185,222,223,224,225] |
| EPCM |
|
|
| [201,203,226,227,228] |
| ionic liquids |
|
|
| [198,199,229] |
| Fluid | Temperature Range (°C) | Percentage Change in Parameter Compared to the Base Fluid | ||||
|---|---|---|---|---|---|---|
| ρ (kg/m3) | c (J/kg·K) | TC (W/m·K) | η (mPa·s) | Pr (-) | ||
| Water-based nanofluids | ||||||
| 0.6 vol% Ni/water | 0 to +100 | +4.7% (20 °C) +4.8% (50 °C) | −0.47% (20 °C) −0.47% (50 °C) | +20.6% (20 °C) +29.8% (50 °C) | +49.3% (20 °C) +54.8% (50 °C) | +23.2% (20 °C) +18.6% (50 °C) |
| 0.16% Cu-Al(4:1) LDH/water | 0 to +100 | n.d. | n.d. | +10.6% (30 °C) | +68.6% (30 °C) | n.d. |
| 0.05 vol% graphene/water | 0 to +100 | +0.05% (20 °C) +0.3% (45 °C) | −2.9% (20 °C) −2.4% (45 °C) | +13.1% (20 °C) +21.9% (45 °C) | +11.4% (20 °C) +19.4% (45 °C) | −4.3% (20 °C) −4.6% (45 °C) |
| 0.1 wt% MWCNT/CuO/water | 0 to +100 | +0.08% (20 °C) +0.08% (40 °C) | −0.05% (20 °C) 0% (40 °C) | −9.5% (20 °C) +20.1% (40 °C) | +1.5% (20 °C) +0.6% (40 °C) | +12.1% (20 °C) −16.2% (40 °C) |
| 0.04 vol% f-CNT/rGO/water | 0 to +100 | +0.05% (25 °C) +0.05% (55 °C) | n.d. | +7.3% (25 °C) +22.5% (55 °C) | +22.2% (25 °C) +17.9% (55 °C) | n.d. |
| 20% n-dodecanol/PMMA/GO/water | 0 to +100 | −4.9% (20 °C) | −12.7% (20 °C) | −16.2% (20 °C) | +147.8% (20 °C) | +158.2% (20 °C) |
| Water-EG-based nanofluids | ||||||
| 0.04 vol% ZnO/H2O–EG(50:50 v/v) | −20 to +100 | −8.4% (20 °C) | +14.9% (20 °C) | +68.2% (20 °C) | −88.8% (20 °C) | −92.4% (20 °C) |
| Approach | Advantages | Disadvantages |
|---|---|---|
| Environmental |
|
|
| Technical |
|
|
| Economical |
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Czaplicka, N.; Grzegórska, A.; Wajs, J.; Sobczak, J.; Rogala, A. Promising Nanoparticle-Based Heat Transfer Fluids—Environmental and Techno-Economic Analysis Compared to Conventional Fluids. Int. J. Mol. Sci. 2021, 22, 9201. https://doi.org/10.3390/ijms22179201
Czaplicka N, Grzegórska A, Wajs J, Sobczak J, Rogala A. Promising Nanoparticle-Based Heat Transfer Fluids—Environmental and Techno-Economic Analysis Compared to Conventional Fluids. International Journal of Molecular Sciences. 2021; 22(17):9201. https://doi.org/10.3390/ijms22179201
Chicago/Turabian StyleCzaplicka, Natalia, Anna Grzegórska, Jan Wajs, Joanna Sobczak, and Andrzej Rogala. 2021. "Promising Nanoparticle-Based Heat Transfer Fluids—Environmental and Techno-Economic Analysis Compared to Conventional Fluids" International Journal of Molecular Sciences 22, no. 17: 9201. https://doi.org/10.3390/ijms22179201
APA StyleCzaplicka, N., Grzegórska, A., Wajs, J., Sobczak, J., & Rogala, A. (2021). Promising Nanoparticle-Based Heat Transfer Fluids—Environmental and Techno-Economic Analysis Compared to Conventional Fluids. International Journal of Molecular Sciences, 22(17), 9201. https://doi.org/10.3390/ijms22179201