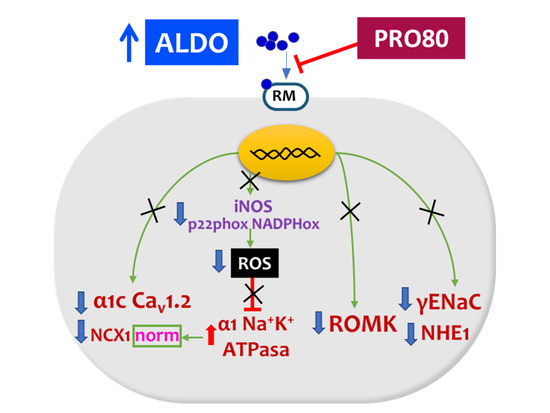
Proanthocyanidins Maintain Cardiac Ionic Homeostasis in Aldosterone-Induced Hypertension and Heart Failure
Abstract
:1. Introduction
2. Results
2.1. Hemodynamics, Cardiac Hypertrophy and Calcium Content
2.2. Cardiac Protein Expression in Channels
2.2.1. ROMK Protein Expression
2.2.2. Sodium Exchangers: NHE1, NKA and NCX1
2.2.3. Calcium Transient Mediators: Total, Phosphorylated and Oxidized CAMKII
2.2.4. Correlations between Cardiac Function Parameters and Ionic Exchangers Proteins
3. Discussion
3.1. PRO80 Maintains potassium Outflow Regulating ROMK Expression
3.2. PRO80 Prevents Massive Entry of Sodium; Role in NHE1, NKA and NCX1 Expression
3.3. PRO80 Controls Calcium Homeostasis by Regulating Cav1.2 and CAMKII Expression
4. Materials and Methods
4.1. Experimental Design and Animal Model
4.2. Hemodynamics
4.3. Histological Analysis
4.4. Western Inmunoblotting
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fuller, P.J.; Yao, Y.-Z.; Yang, J.; Young, M.J. Structural determinants of activation of the mineralocorticoid receptor: An evolutionary perspective. J. Hum. Hypertens. 2021, 35, 110–116. [Google Scholar] [CrossRef]
- Funder, J.W.; Carey, R.M.; Mantero, F.; Murad, M.H.; Reincke, M.; Shibata, H.; Stowasser, M.; Young, W.F. The Management of Primary Aldosteronism: Case Detection, Diagnosis, and Treatment: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2016, 101, 1889–1916. [Google Scholar] [CrossRef]
- Cannavo, A.; Liccardo, D.; Eguchi, A.; Elliott, K.J.; Traynham, C.J.; Ibetti, J.; Eguchi, S.; Leosco, D.; Ferrara, N.; Rengo, G.; et al. Myocardial pathology induced by aldosterone is dependent on non-canonical activities of G protein-coupled receptor kinases. Nat. Commun. 2016, 7, 10877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monticone, S.; D’Ascenzo, F.; Moretti, C.; Williams, T.A.; Veglio, F.; Gaita, F.; Mulatero, P. Cardiovascular events and target organ damage in primary aldosteronism compared with essential hypertension: A systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2018, 6, 41–50. [Google Scholar] [CrossRef]
- Quinkler, M.; Born-Frontsberg, E.; Fourkiotis, V.G. Comorbidities in Primary Aldosteronism. Horm. Metab. Res. 2010, 42, 429–434. [Google Scholar] [CrossRef]
- Menick, D.R.; Renaud, L.; Buchholz, A.; Müller, J.G.; Zhou, H.; Kappler, C.S.; Kubalak, S.W.; Conway, S.J.; Xu, L. Regulation of Ncx1 Gene Expression in the Normal and Hypertrophic Heart. Ann. N. Y. Acad. Sci. 2007, 1099, 195–203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mraiche, F.; Oka, T.; Gan, X.T.; Karmazyn, M.; Fliegel, L. Activated NHE1 is required to induce early cardiac hypertrophy in mice. Basic Res. Cardiol. 2011, 106, 603–616. [Google Scholar] [CrossRef] [PubMed]
- Moreau, A.; Gosselin-Badaroudine, P.; Mercier, A.; Burger, B.; Keller, D.I.; Chahine, M. A leaky voltage sensor domain of cardiac sodium channels causes arrhythmias associated with dilated cardiomyopathy. Sci. Rep. 2018, 8, 13804. [Google Scholar] [CrossRef]
- Wang, H.-S.; Arvanitis, D.; Dong, M.; Niklewski, P.J.; Zhao, W.; Lam, C.K.; Kranias, E.G.; Sanoudou, D. SERCA2a superinhibition by human phospholamban triggers electrical and structural remodeling in mouse hearts. Physiol. Genom. 2011, 43, 357–364. [Google Scholar] [CrossRef] [Green Version]
- Auguste, G.; Hurtado, R.; Thaboulet, G.; Viengchareun, S.; Gomez, A.; Morel, E.; Lombès, M.; Benitah, J. Molecular mechanism involved in MR upregulation of the cardiac L-type Ca2+ channels: PM2-107. Fundam. Clin. Pharmacol. 2014, 28. [Google Scholar]
- Benitah, J.-P.; Perrier, E.; Gómez, A.M.; Vassort, G. Effects of aldosterone on transient outward K+ current density in rat ventricular myocytes. J. Physiol. 2001, 537, 151–160. [Google Scholar] [CrossRef]
- Martin-Fernandez, B.; Miana, M.; Heras, N.D.L.; Ruiz-Hurtado, G.; Fernández-Velasco, M.; Bas, M.; Ballesteros, S.; Lahera, V.; Cachofeiro, V.; Delgado, C. Cardiac L-type calcium current is increased in a model of hyperaldosteronism in the rat. Exp. Physiol. 2009, 94, 675–683. [Google Scholar] [CrossRef] [PubMed]
- Martín-Fernández, B.; Valero-Munoz, M.; Heras, N.D.L.; Ballesteros, S.; Lahera, V. Relevance of SGK1 in structural, functional and molecular alterations produced by aldosterone in heart. Horm. Mol. Biol. Clin. Investig. 2014, 18, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Aiba, T.; Rosenberg, M.; Hessler, K.; Xiao, C.; Quintero, P.A.; Ottaviano, F.G.; Knight, A.C.; Graham, E.L.; Boström, P.; et al. Pathological Role of Serum- and Glucocorticoid-Regulated Kinase 1 in Adverse Ventricular Remodeling. Circulation 2012, 126, 2208–2219. [Google Scholar] [CrossRef]
- Galiana-Simal, A.; Olivares-Álvaro, E.; Klett-Mingo, M.; Ruiz-Roso, M.B.; Ballesteros, S.; Heras, N.D.L.; Fuller, P.J.; Lahera, V.; Martín-Fernández, B. Proanthocyanidins block aldosterone-dependent up-regulation of cardiac gamma ENaC and Nedd4-2 inactivation via SGK1. J. Nutr. Biochem. 2016, 37, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Lang, F.; Artunc, F.; Vallon, V. The physiological impact of the serum and glucocorticoid-inducible kinase SGK1. Curr. Opin. Nephrol. Hypertens. 2009, 18, 439–448. [Google Scholar] [CrossRef]
- Lang, F.; Voelkl, J. Therapeutic potential of serum and glucocorticoid inducible kinase inhibition. Expert Opin. Investig. Drugs 2013, 22, 701–714. [Google Scholar] [CrossRef]
- Shattock, M.J.; Ottolia, M.; Bers, D.; Blaustein, M.P.; Boguslavskyi, A.; Bossuyt, J.; Bridge, J.H.B.; Chen-Izu, Y.; Clancy, C.E.; Edwards, A.; et al. Na+/Ca2+ exchange and Na+/K+-ATPase in the heart. J. Physiol. 2015, 593, 1361–1382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parker, M.D.; Myers, E.J.; Schelling, J.R. Na+–H+ exchanger−1 (NHE1) regulation in kidney proximal tubule. Cell. Mol. Life Sci. 2015, 72, 2061–2074. [Google Scholar] [CrossRef] [Green Version]
- Slepkov, E.; Fliegel, L. Structure and function of the NHE1 isoform of the Na+/H+ exchanger. Biochem. Cell Biol. 2002, 80, 499–508. [Google Scholar] [CrossRef]
- Wakabayashi, S.; Hisamitsu, T.; Nakamura, T.Y. Regulation of the cardiac Na+/H+ exchanger in health and disease. J. Mol. Cell. Cardiol. 2013, 61, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Bers, D.M.; Barry, W.H.; Despa, S. Intracellular Na+ regulation in cardiac myocytes. Cardiovasc. Res. 2003, 57, 897–912. [Google Scholar] [CrossRef] [Green Version]
- Sonalker, P.A.; Tofovic, S.P.; Jackson, E.K. Increased Expression of the Sodium Transporter BSC-1 in Spontaneously Hypertensive Rats. J. Pharmacol. Exp. Ther. 2004, 311, 1052–1061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, L.; Li, D.; Welling, P.A. Hypertension resistance polymorphisms in ROMK (Kir1.1) alter channel function by different mechanisms. Am. J. Physiol. Physiol. 2010, 299, F1359–F1364. [Google Scholar] [CrossRef] [Green Version]
- Garcia, M.L.; Kaczorowski, G.J. Targeting the inward-rectifier potassium channel ROMK in cardiovascular disease. Curr. Opin. Pharmacol. 2014, 15, 1–6. [Google Scholar] [CrossRef]
- He, J.; Cai, Y.; Luo, L.-M.; Wang, R. Expression of Wnt and NCX1 and its correlation with cardiomyocyte apoptosis in mouse with myocardial hypertrophy. Asian Pac. J. Trop. Med. 2015, 8, 930–936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rueda, J.O.V.; Palomeque, J.; Mattiazzi, A. Early apoptosis in different models of cardiac hypertrophy induced by high renin-angiotensin system activity involves CaMKII. J. Appl. Physiol. 2012, 112, 2110–2120. [Google Scholar] [CrossRef]
- Savarese, G.; Lund, L.H. Global Public Health Burden of Heart Failure. Card. Fail. Rev. 2017, 3, 7–11. [Google Scholar] [CrossRef]
- Martín-Fernandez, B.; Heras, N.D.L.; Valero-Muñoz, M.; Ballesteros, S.; Yao, Y.-Z.; Stanton, P.G.; Fuller, P.J.; Lahera, V. Beneficial Effects of Proanthocyanidins in the Cardiac Alterations Induced by Aldosterone in Rat Heart through Mineralocorticoid Receptor Blockade. PLoS ONE 2014, 9, e111104. [Google Scholar] [CrossRef]
- Beesley, A.H.; Hornby, D.; White, S.J. Regulation of distal nephron K+ channels (ROMK) mRNA expression by aldosterone in rat kidney. J. Physiol. 1998, 509, 629–634. [Google Scholar] [CrossRef]
- Wald, H. Regulation of the ROMK Potassium Channel in the Kidney. Nephron Exp. Nephrol. 1999, 7, 201–206. [Google Scholar] [CrossRef]
- Lang, F.; Böhmer, C.; Palmada, M.; Seebohm, G.; Strutz-Seebohm, N.; Vallon, V. (Patho)physiological Significance of the Serum- and Glucocorticoid-Inducible Kinase Isoforms. Physiol. Rev. 2006, 86, 1151–1178. [Google Scholar] [CrossRef] [PubMed]
- Welling, P.A.; Ho, K. A comprehensive guide to the ROMK potassium channel: Form and function in health and disease. Am. J. Physiol.-Ren. Physiol. 2009, 297, F849–F863. [Google Scholar] [CrossRef] [Green Version]
- Yoo, D.; Kim, B.Y.; Campo, C.; Nance, L.; King, A.; Maouyo, D.; Welling, P.A. Cell Surface Expression of the ROMK (Kir 1.1) Channel Is Regulated by the Aldosterone-induced Kinase, SGK-1, and Protein Kinase A. J. Biol. Chem. 2003, 278, 23066–23075. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hattori, T.; Makiyama, T.; Akao, M.; Ehara, E.; Ohno, S.; Iguchi, M.; Nishio, Y.; Sasaki, K.; Itoh, H.; Yokode, M.; et al. A novel gain-of-function KCNJ2 mutation associated with short-QT syndrome impairs inward rectification of Kir2.1 currents. Cardiovasc. Res. 2011, 93, 666–673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ortega, A.; Tarazón, E.; Roselló-Lletí, E.; Gil-Cayuela, C.; Lago, F.; Juanatey, J.R.G.; Cinca, J.; Jorge, E.; Martinez-Dolz, L.; Portoles, M.; et al. Patients with Dilated Cardiomyopathy and Sustained Monomorphic Ventricular Tachycardia Show Up-Regulation of KCNN3 and KCNJ2 Genes and CACNG8-Linked Left Ventricular Dysfunction. PLoS ONE 2015, 10, e0145518. [Google Scholar] [CrossRef] [PubMed]
- Szuts, V.; Ménesi, D.; Varga-Orvos, Z.; Zvara, Á.; Houshmand, N.; Bitay, M.; Bogáts, G.; Virág, L.; Baczkó, I.; Szalontai, B.; et al. Altered expression of genes for Kir ion channels in dilated cardiomyopathy. Can. J. Physiol. Pharmacol. 2013, 91, 648–656. [Google Scholar] [CrossRef] [Green Version]
- Coppini, R.; Ferrantini, C.; Mazzoni, L.; Sartiani, L.; Olivotto, I.; Poggesi, C.; Cerbai, E.; Mugelli, A. Regulation of intracellular Na+in health and disease: Pathophysiological mechanisms and implications for treatment. Glob. Cardiol. Sci. Pract. 2013, 2013, 222–242. [Google Scholar] [CrossRef]
- Popov, S.; Venetsanou, K.; Chedrese, P.J.; Pinto, V.; Takemori, H.; Franco-Cereceda, A.; Eriksson, P.; Mochizuki, N.; Soares-Da-Silva, P.; Bertorello, A.M. Increases in intracellular sodium activate transcription and gene expression via the salt-inducible kinase 1 network in an atrial myocyte cell line. Am. J. Physiol. -Heart Circ. Physiol. 2012, 303, H57–H65. [Google Scholar] [CrossRef] [Green Version]
- Cingolani, H.E.; Ennis, I. Sodium-Hydrogen Exchanger, Cardiac Overload, and Myocardial Hypertrophy. Circulation 2007, 115, 1090–1100. [Google Scholar] [CrossRef]
- Karmazyn, M.; Liu, Q.; Gan, X.T.; Brix, B.J.; Fliegel, L. Aldosterone Increases NHE-1 Expression and Induces NHE-1-Dependent Hypertrophy in Neonatal Rat Ventricular Myocytes. Hypertension 2003, 42, 1171–1176. [Google Scholar] [CrossRef]
- Voelkl, J.; Lin, Y.; Alesutan, I.S.; Ahmed, M.S.E.; Pasham, V.; Mia, S.; Gu, S.; Feger, M.; Saxena, A.; Metzler, B.; et al. Sgk1 sensitivity of Na+/H+ exchanger activity and cardiac remodeling following pressure overload. Basic Res. Cardiol. 2012, 107, 236. [Google Scholar] [CrossRef] [PubMed]
- Voelkl, J.; Pasham, V.; Ahmed, M.S.E.; Walker, B.; Szteyn, K.; Kuhl, D.; Metzler, B.; Alesutan, I.; Lang, F. Sgk1-Dependent Stimulation of Cardiac Na+/H+ Exchanger Nhe1 by Dexamethasone. Cell. Physiol. Biochem. 2013, 32, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Rossier, B.C. Epithelial sodium channel (ENaC) and the control of blood pressure. Curr. Opin. Pharmacol. 2014, 15, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, U.; Hyman, R.; Smith, T.; Medford, R. Aldosterone-mediated regulation of Na+, K(+)-ATPase gene expression in adult and neonatal rat cardiocytes. J. Biol. Chem. 1991, 266, 12058–12066. [Google Scholar] [CrossRef]
- Marver, D. Influence of adrenalectomy and steroid replacement on heart citrate synthase levels. Am. J. Physiol. Endocrinol. Metab. 1984, 246, E452–E457. [Google Scholar] [CrossRef] [PubMed]
- Bers, D.M.; Despa, S. Na/K-ATPase—An Integral Player in the Adrenergic Fight-or-Flight Response. Trends Cardiovasc. Med. 2009, 19, 111–118. [Google Scholar] [CrossRef] [Green Version]
- Doshi, D.; Marx, S.O. Ion Channels, Transporters, and Pumps as Targets for Heart Failure Therapy. J. Cardiovasc. Pharmacol. 2009, 54, 273–278. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Li, J.; Liu, J.; Yuan, Z.; Pierre, S.V.; Qu, W.; Zhao, X.; Xie, Z. Involvement of Na+/K+-ATPase in hydrogen peroxide-induced hypertrophy in cardiac myocytes. Free. Radic. Biol. Med. 2006, 41, 1548–1556. [Google Scholar] [CrossRef]
- Mihailidou, A.S.; Bundgaard, H.; Mardini, M.; Hansen, P.S.; Kjeldsen, K.; Rasmussen, H.H. Hyperaldosteronemia in Rabbits Inhibits the Cardiac Sarcolemmal Na+-K+ Pump. Circ. Res. 2000, 86, 37–42. [Google Scholar] [CrossRef] [Green Version]
- Zhao, G.; Gao, H.; Qiu, J.; Lu, W.; Wei, X. The molecular mechanism of protective effects of grape seed proanthocyanidin extract on reperfusion arrhythmias in rats in vivo. Biol. Pharm. Bull. 2010, 33, 759–767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roos, K.P.; Jordan, M.C.; Fishbein, M.C.; Ritter, M.R.; Friedlander, M.; Chang, H.C.; Rahgozar, P.; Han, T.; Garcia, A.J.; MacLellan, W.R.; et al. Hypertrophy and Heart Failure in Mice Overexpressing the Cardiac Sodium-Calcium Exchanger. J. Card. Fail. 2007, 13, 318–329. [Google Scholar] [CrossRef] [Green Version]
- Hobai, I.A.; Maack, C.; O’Rourke, B. Partial Inhibition of Sodium/Calcium Exchange Restores Cellular Calcium Handling in Canine Heart Failure. Circ. Res. 2004, 95, 292–299. [Google Scholar] [CrossRef]
- Lee, C.; Dhalla, N.S.; Hryshko, L.V. Therapeutic potential of novel Na+-Ca2+ exchange inhibitors in attenuating ischemia-reperfusion injury. Can. J. Cardiol. 2005, 21, 509–516. [Google Scholar]
- Satoh, H.; Ginsburg, K.S.; Qing, K.; Terada, H.; Hayashi, H.; Bers, D.M. KB-R7943 block of Ca2+ influx via Na+/Ca2+ exchange does not alter twitches or glycoside inotropy but prevents Ca2+ overload in rat ventricular myocytes. Circulation 2000, 101, 1441–1446. [Google Scholar] [CrossRef] [Green Version]
- Seki, S.; Taniguchi, M.; Takeda, H.; Nagai, M.; Taniguchi, I.; Mochizuki, S. Inhibition by KB-R7943 of the Reverse Mode of the Na+/Ca2+ Exchanger Reduces Ca2+ Overload in Ischemic-Reperfused Rat Hearts. Circ. J. 2002, 66, 390–396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, G.-Z.; Zhou, J.-J.; Wang, B.; Wu, F.; Bi, H.; Wang, Y.-M.; Yi, D.-H.; Yu, S.-Q.; Pei, J.-M. Diastolic Ca2+ overload caused by Na+/Ca2+ exchanger during the first minutes of reperfusion results in continued myocardial stunning. Eur. J. Pharmacol. 2007, 572, 1–11. [Google Scholar] [CrossRef]
- Bénitah, J.-P.; Vassort, G. Aldosterone Upregulates Ca2+ Current in Adult Rat Cardiomyocytes. Circ. Res. 1999, 85, 1139–1145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, Q.; Wu, Z.; Li, X.; Yan, J.; Zhao, L.; Yang, C.; Lu, J.; Deng, J.; Chen, M. Resveratrol ameliorates cardiac dysfunction induced by pressure overload in rats via structural protection and modulation of Ca2+ cycling proteins. J. Transl. Med. 2014, 12, 323. [Google Scholar] [CrossRef] [Green Version]
- Santos, C.N.; Gomes, A.; Oudot, C.; Pedroso, D.P.M.D.; Rodriguez-Mateos, A.; Vieira, H.; Brenner, C. Pure Polyphenols Applications for Cardiac Health and Disease. Curr. Pharm. Des. 2018, 24, 2137–2156. [Google Scholar] [CrossRef] [Green Version]
- Hofmann, F.; Flockerzi, V.; Kahl, S.; Wegener, J. L-type CaV1.2 calcium channels: From in vitro findings to in vivo function. Physiol. Rev. 2014, 94, 303–326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maturana, A.; Lenglet, S.; Python, M.; Kuroda, S.; Rossier, M.F. Role of the T-Type Calcium Channel CaV3.2 in the Chronotropic Action of Corticosteroids in Isolated Rat Ventricular Myocytes. Endocrinology 2009, 150, 3726–3734. [Google Scholar] [CrossRef]
- Rossier, M.F.; Python, M.; Maturana, A.D. Contribution of Mineralocorticoid and Glucocorticoid Receptors to the Chronotropic and Hypertrophic Actions of Aldosterone in Neonatal Rat Ventricular Myocytes. Endocrinology 2010, 151, 2777–2787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wagner, M.; Rudakova, E.; Volk, T. Aldosterone-induced changes in the cardiac L-type Ca2+ current can be prevented by antioxidants in vitro and are absent in rats on low salt diet. Pflügers Arch. -Eur. J. Physiol. 2008, 457, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Nagasawa, K.; Takahashi, K.; Matsuura, N.; Takatsu, M.; Hattori, T.; Watanabe, S.; Harada, E.; Niinuma, K.; Murohara, T.; Nagata, K. Comparative effects of valsartan in combination with cilnidipine or amlodipine on cardiac remodeling and diastolic dysfunction in Dahl salt-sensitive rats. Hypertens. Res. 2014, 38, 39–47. [Google Scholar] [CrossRef] [PubMed]






| CONTROL | ALDO | PRO80 | ALDO + PRO80 | |
|---|---|---|---|---|
| SBP (mmHg) | 108 ± 0.9 | 144 ± 4.3 * | 115 ± 2.6 | 128 ± 1.1 # |
| DBP (mmHg) | 76 ± 5.1 | 116 ±3.7 * | 71 ± 4.2 | 88 ± 2.9 # |
| LVSP (mmHg) | 113 ± 3.1 | 147 ± 5.1 * | 124 ± 7.1 | 124 ± 7.8 # |
| LVEDP (mmHg) | 3.8 ± 0.3 | 13 ± 0.9 * | 4.6 ± 0.2 | 4.5 ± 0.3 # |
| BW baseline (g) | 253 ± 2.5 | 248 ± 6.2 | 261 ± 7.5 | 246 ± 3.2 |
| BW final (g) | 323 ± 3.1 | 315 ± 4.3 | 330 ± 6.6 | 318 ± 5.2 |
| HW/BW (mg g−1) | 2.28 ± 0.06 | 3.20 ± 0.1 * | 2.32 ± 0.06 | 2.43 ± 0.08 # |
| Antibody | Ref. | Dilution |
|---|---|---|
| anti-ROMK | ab92285 | 1:250 |
| anti-NHE1 | ab67314 | 1:500 |
| anti-1α NKA | ab7671 | 1:1000 |
| anti-α1C Cav1.2 | ab58552 | 1:500 |
| anti-NCX1 | ab6495 | 1:500 |
| anti-CaMKII | ab52476 | 1:500 |
| anti-pCaMKII | ab32678 | 1:500 |
| anti-oxCaMKII | mp07-1387 | 1:500 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de las Heras, N.; Galiana, A.; Ballesteros, S.; Olivares-Álvaro, E.; Fuller, P.J.; Lahera, V.; Martín-Fernández, B. Proanthocyanidins Maintain Cardiac Ionic Homeostasis in Aldosterone-Induced Hypertension and Heart Failure. Int. J. Mol. Sci. 2021, 22, 9602. https://doi.org/10.3390/ijms22179602
de las Heras N, Galiana A, Ballesteros S, Olivares-Álvaro E, Fuller PJ, Lahera V, Martín-Fernández B. Proanthocyanidins Maintain Cardiac Ionic Homeostasis in Aldosterone-Induced Hypertension and Heart Failure. International Journal of Molecular Sciences. 2021; 22(17):9602. https://doi.org/10.3390/ijms22179602
Chicago/Turabian Stylede las Heras, Natalia, Adrián Galiana, Sandra Ballesteros, Elena Olivares-Álvaro, Peter J. Fuller, Vicente Lahera, and Beatriz Martín-Fernández. 2021. "Proanthocyanidins Maintain Cardiac Ionic Homeostasis in Aldosterone-Induced Hypertension and Heart Failure" International Journal of Molecular Sciences 22, no. 17: 9602. https://doi.org/10.3390/ijms22179602
APA Stylede las Heras, N., Galiana, A., Ballesteros, S., Olivares-Álvaro, E., Fuller, P. J., Lahera, V., & Martín-Fernández, B. (2021). Proanthocyanidins Maintain Cardiac Ionic Homeostasis in Aldosterone-Induced Hypertension and Heart Failure. International Journal of Molecular Sciences, 22(17), 9602. https://doi.org/10.3390/ijms22179602