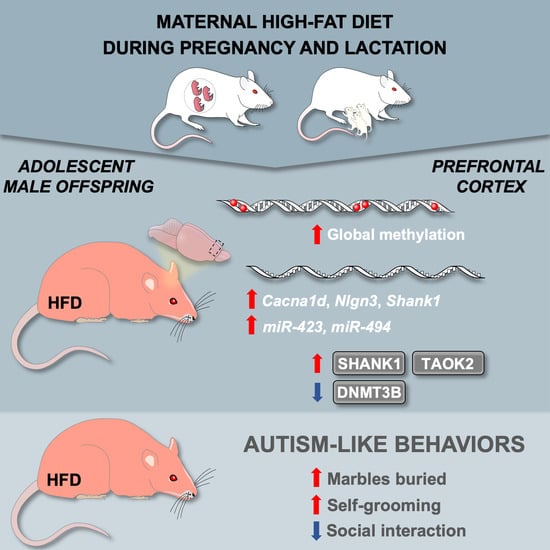
Alteration of the Early Development Environment by Maternal Diet and the Occurrence of Autistic-like Phenotypes in Rat Offspring
Abstract
:1. Introduction
2. Results
2.1. A Maternal Modified Diet Disrupts the Expression of ASD-Related Genes in the Offspring PFCx
2.2. Maternal HFD during Pregnancy and Lactation Promotes Autistic-like Behaviors in Adolescent Male Offspring
2.3. Maternal HFD Alters Global DNA Methylation and the DNA Methylating Enzymatic Machinery in Male Adolescent Offspring PFCx
2.4. Maternal HFD Alters the Levels of ASD-Related Proteins and the Expression of Mirnas in Adolescent Offspring PFCx
3. Discussion
4. Materials and Methods
4.1. Animals and Diets
4.2. Open Field Test
4.3. Marble Burying Task
4.4. Self-Grooming
4.5. Social Interaction Test
4.6. Brain Tissue Collection
4.7. Taqman Gene Expression Array Cards
4.8. Analysis of Gene Expression by RT-qPCR
4.9. Analysis of miRNA Expression by RT-qPCR
4.10. Enzyme-Linked Immunosorbent Assay (ELISA)
4.11. Quantify Global DNA Methylation
4.12. Assessment of DNA Methylation Level of the CpG Islands by Pyrosequencing
4.13. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chu, D.M.; Meyer, K.M.; Prince, A.L.; Aagaard, K.M. Impact of maternal nutrition in pregnancy and lactation on offspring gut microbial composition and function. Gut Microbes 2016, 7, 459–470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paradis, J.; Boureau, P.; Moyon, T.; Nicklaus, S.; Parnet, P.; Paillé, V. Perinatal Western Diet Consumption Leads to Profound Plasticity and GABAergic Phenotype Changes within Hypothalamus and Reward Pathway from Birth to Sexual Maturity in Rat. Front. Endocrinol. Lausanne 2017, 8, 216. [Google Scholar] [CrossRef] [Green Version]
- Edlow, A.G.; Guedj, F.; Sverdlov, D.; Pennings, J.L.A.; Bianchi, D.W. Significant Effects of Maternal Diet During Pregnancy on the Murine Fetal Brain Transcriptome and Offspring Behavior. Front. Neurosci. 2019, 13, 1335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gawlińska, K.; Gawliński, D.; Filip, M.; Przegaliński, E. Relationship of maternal high-fat diet during pregnancy and lactation to offspring health. Nutr. Rev. 2021, 79, 709–725. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, E.; Nousen, E.K.; Chamlou, K.A.; Grove, K.L. The impact of maternal high-fat diet consumption on neural development and behavior of offspring. Int. J. Obes. Suppl. 2012, 2, S7–S13. [Google Scholar] [CrossRef]
- Buffington, S.A.; Di Prisco, G.V.; Auchtung, T.; Ajami, N.J.; Petrosino, J.F.; Costa-Mattioli, M. Microbial Reconstitution Reverses Maternal Diet-Induced Social and Synaptic Deficits in Offspring. Cell 2016, 165, 1762–1775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sgritta, M.; Dooling, S.; Buffington, S.A.; Momin, E.N.; Francis, M.B.; Britton, R.A.; Costa-Mattioli, M. Mechanisms Underlying Microbial-Mediated Changes in Social Behavior in Mouse Models of Autism Spectrum Disorder. Neuron 2019, 101, 246–259.e6. [Google Scholar] [CrossRef] [Green Version]
- Sanchez, C.E.; Barry, C.; Sabhlok, A.; Russell, K.; Majors, A.; Kollins, S.; Fuemmeler, B.F. Maternal pre-pregnancy obesity and child neurodevelopmental outcomes: A meta-analysis. Obes. Rev. 2018, 19, 464–484. [Google Scholar] [CrossRef]
- Contu, L.; Hawkes, C.A. A Review of the Impact of Maternal Obesity on the Cognitive Function and Mental Health of the Offspring. Int. J. Mol. Sci. 2017, 18, 1093. [Google Scholar] [CrossRef] [Green Version]
- Lyall, K.; Munger, K.L.; O’Reilly, J.; Santangelo, S.L.; Ascherio, A. Maternal Dietary Fat Intake in Association with Autism Spectrum Disorders. Am. J. Epidemiol. 2013, 178, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Jonsson, U.; Alaie, I.; Wilteus, A.L.; Zander, E.; Marschik, P.B.; Coghill, D.; Bölte, S. Annual Research Review: Quality of life and childhood mental and behavioural disorders—A critical review of the research. J. Child Psychol. Psychiatry Allied Discip. 2017, 58, 439–469. [Google Scholar] [CrossRef] [PubMed]
- Eggebrecht, A.T.; Dworetsky, A.; Hawks, Z.; Coalson, R.; Adeyemo, B.; Davis, S.; Gray, D.; McMichael, A.; Petersen, S.E.; Constantino, J.N.; et al. Brain function distinguishes female carriers and non-carriers of familial risk for autism. Mol. Autism 2020, 11, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Godfrey, K.M.; Reynolds, R.; Prescott, S.L.; Nyirenda, M.; Jaddoe, V.W.V.; Eriksson, J.G.; Broekman, B.F.P. Influence of maternal obesity on the long-term health of offspring. Lancet Diabetes Endocrinol. 2017, 5, 53–64. [Google Scholar] [CrossRef] [Green Version]
- Pauwels, S.; Ghosh, M.; Duca, R.C.; Bekaert, B.; Freson, K.; Huybrechts, I.; Langie, S.; Koppen, G.; Devlieger, R.; Godderis, L. Dietary and supplemental maternal methyl-group donor intake and cord blood DNA methylation. Epigenetics 2017, 12, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keleher, M.R.; Zaidi, S.; Shah, S.; Oakley, M.E.; Pavlatos, C.; El Idrissi, S.; Xing, X.; Li, D.; Wang, T.; Cheverud, J.M. Maternal high-fat diet associated with altered gene expression, DNA methylation, and obesity risk in mouse offspring. PLoS ONE 2018, 13, e0192606. [Google Scholar] [CrossRef] [Green Version]
- Tremblay, M.W.; Jiang, Y.-H. DNA Methylation and Susceptibility to Autism Spectrum Disorder. Annu. Rev. Med. 2019, 70, 151–166. [Google Scholar] [CrossRef]
- Courchesne, E.; Mouton, P.R.; Calhoun, M.E.; Semendeferi, K.; Ahrens-Barbeau, C.; Hallet, M.J.; Barnes, C.C.; Pierce, K. Neuron Number and Size in Prefrontal Cortex of Children with Autism. JAMA J. Am. Med. Assoc. 2011, 306, 2001–2010. [Google Scholar] [CrossRef]
- Cauvet, E.; Westeinde, A.V.; Toro, R.; Kuja-Halkola, R.; Neufeld, J.; Mevel, K.; Bölte, S. Sex Differences Along the Autism Continuum: A Twin Study of Brain Structure. Cereb. Cortex 2019, 29, 1342–1350. [Google Scholar] [CrossRef] [Green Version]
- Lillycrop, K.; Burdge, G.C. Maternal diet as a modifier of offspring epigenetics. J. Dev. Orig. Health Dis. 2015, 6, 88–95. [Google Scholar] [CrossRef] [Green Version]
- Rylaarsdam, L.E.; Guemez-Gamboa, A. Genetic Causes and Modifiers of Autism Spectrum Disorder. Front. Cell. Neurosci. 2019, 13, 385. [Google Scholar] [CrossRef]
- Courchesne, E.; Pramparo, T.; Gazestani, V.H.; Lombardo, M.V.; Pierce, K.; Lewis, N.E. The ASD Living Biology: From cell proliferation to clinical phenotype. Mol. Psychiatry 2019, 24, 88–107. [Google Scholar] [CrossRef] [Green Version]
- Courchesne, E.; Gazestani, V.H.; Lewis, N.E. Prenatal Origins of ASD: The When, What, and How of ASD Development. Trends Neurosci. 2020, 43, 326–342. [Google Scholar] [CrossRef]
- Herrero, M.J.; Velmeshev, D.; Hernandez-Pineda, D.; Sethi, S.; Sorrells, S.; Banerjee, P.; Sullivan, C.; Gupta, A.R.; Kriegstein, A.R.; Corbin, J.G. Identification of amygdala-expressed genes associated with autism spectrum disorder. Mol. Autism 2020, 11, 1–14. [Google Scholar] [CrossRef]
- Hu, C.; Feng, P.; Yang, Q.; Xiao, L. Clinical and Neurobiological Aspects of TAO Kinase Family in Neurodevelopmental Disorders. Front. Mol. Neurosci. 2021, 14. [Google Scholar] [CrossRef]
- Richter, M.; Murtaza, N.; Scharrenberg, R.; White, S.H.; Johanns, O.; Walker, S.; Yuen, R.K.C.; Schwanke, B.; Bedürftig, B.; Henis, M.; et al. Altered TAOK2 activity causes autism-related neurodevelopmental and cognitive abnormalities through RhoA signaling. Mol. Psychiatry 2019, 24, 1329–1350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sato, D.; Lionel, A.C.; Leblond, C.S.; Prasad, A.; Pinto, D.; Walker, S.; O’Connor, I.; Russell, C.; Drmic, I.E.; Hamdan, F.F.; et al. SHANK1 Deletions in Males with Autism Spectrum Disorder. Am. J. Hum. Genet. 2012, 90, 879–887. [Google Scholar] [CrossRef] [Green Version]
- Ansel, A.; Rosenzweig, J.P.; Zisman, P.D.; Melamed, M.; Gesundheit, B. Variation in Gene Expression in Autism Spectrum Disorders: An Extensive Review of Transcriptomic Studies. Front. Neurosci. 2017, 10, 601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chow, M.L.; Pramparo, T.; Winn, M.E.; Barnes, C.C.; Li, H.-R.; Weiss, L.; Fan, J.-B.; Murray, S.; April, C.; Belinson, H.; et al. Age-Dependent Brain Gene Expression and Copy Number Anomalies in Autism Suggest Distinct Pathological Processes at Young Versus Mature Ages. PLoS Genet. 2012, 8, e1002592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gawlinska, K.; Gawlinski, D.; Przegalinski, E.; Filip, M. Maternal high-fat diet during pregnancy and lactation provokes depressive-like behavior and influences the irisin/brain-derived neurotrophic factor axis and inflammatory factors in male and female offspring in rats. J. Physiol. Pharmacol. 2019, 70, 407–411. [Google Scholar] [CrossRef]
- Gawlińska, K.; Gawliński, D.; Korostyński, M.; Borczyk, M.; Frankowska, M.; Piechota, M.; Filip, M.; Przegaliński, E. Maternal dietary patterns are associated with susceptibility to a depressive-like phenotype in rat offspring. Dev. Cogn. Neurosci. 2021, 47, 100879. [Google Scholar] [CrossRef]
- Kang, S.S.; Kurti, A.; Fair, D.A.; Fryer, J.D. Dietary intervention rescues maternal obesity induced behavior deficits and neuroinflammation in offspring. J. Neuroinflamm. 2014, 11, 156. [Google Scholar] [CrossRef]
- Thomas, A.; Burant, A.; Bui, N.; Graham, D.; Yuva-Paylor, L.A.; Paylor, R. Marble burying reflects a repetitive and perseverative behavior more than novelty-induced anxiety. Psychopharmacol. Berl. 2009, 204, 361–373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meyza, K.; Blanchard, D. The BTBR mouse model of idiopathic autism—Current view on mechanisms. Neurosci. Biobehav. Rev. 2017, 76, 99–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McFarlane, H.G.; Kusek, G.K.; Yang, M.; Phoenix, J.L.; Bolivar, V.; Crawley, J.N. Autism-like behavioral phenotypes in BTBR T+tf/J mice. Genes Brain Behav. 2008, 7, 152–163. [Google Scholar] [CrossRef] [PubMed]
- Crawley, J.N. Mouse Behavioral Assays Relevant to the Symptoms of Autism. Brain Pathol. 2007, 17, 448–459. [Google Scholar] [CrossRef]
- Bellisario, V.; Berry, A.; Capoccia, S.; Raggi, C.; Panetta, P.; Branchi, I.; Piccaro, G.; Giorgio, M.; Pelicci, P.G.; Cirulli, F. Gender-dependent resiliency to stressful and metabolic challenges following prenatal exposure to high-fat diet in the p66Shc-/-mouse. Front. Behav. Neurosci. 2014, 8, 285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chao, O.Y.; Yunger, R.; Yang, Y.-M. Behavioral assessments of BTBR T+Itpr3tf/J mice by tests of object attention and elevated open platform: Implications for an animal model of psychiatric comorbidity in autism. Behav. Brain Res. 2018, 347, 140–147. [Google Scholar] [CrossRef]
- Mei, T.; Llera, A.; Floris, D.L.; Forde, N.J.; Tillmann, J.; Durston, S.; Moessnang, C.; Banaschewski, T.; Holt, R.J.; Baron-Cohen, S.; et al. Gray matter covariations and core symptoms of autism: The EU-AIMS Longitudinal European Autism Project. Mol. Autism 2020, 11, 86. [Google Scholar] [CrossRef]
- Ruskin, D.N.; Svedova, J.; Cote, J.L.; Sandau, U.; Rho, J.M.; Kawamura Jr, M.K.; Boison, D.; Masino, S.A. Ketogenic diet improves core symptoms of autism in BTBR mice. PLoS ONE 2013, 8, e65021. [Google Scholar] [CrossRef] [Green Version]
- Erickson, C.; Srivorakiat, L.; Wink, L.; Pedapati, E.; Fitzpatrick, S. Aggression in autism spectrum disorder: Presentation and treatment options. Neuropsychiatr. Dis. Treat. 2016, 12, 1525–1538. [Google Scholar] [CrossRef] [Green Version]
- Hill, A.P.; Zuckerman, K.E.; Hagen, A.D.; Kriz, D.J.; Duvall, S.W.; van Santen, J.; Nigg, J.; Fair, D.; Fombonne, E. Aggressive behavior problems in children with autism spectrum disorders: Prevalence and correlates in a large clinical sample. Res. Autism Spectr. Disord. 2014, 8, 1121–1133. [Google Scholar] [CrossRef] [Green Version]
- Sullivan, E.L.; Riper, K.M.; Lockard, R.; Valleau, J.C. Maternal high-fat diet programming of the neuroendocrine system and behavior. Horm. Behav. 2015, 76, 153–161. [Google Scholar] [CrossRef] [Green Version]
- Giriko, C.; Andreoli, C.A.; Mennitti, L.V.; Hosoume, L.F.; Souto, T.D.S.; Da Silva, A.V.; Mendes-Da-Silva, C. Delayed physical and neurobehavioral development and increased aggressive and depression-like behaviors in the rat offspring of dams fed a high-fat diet. Int. J. Dev. Neurosci. 2013, 31, 731–739. [Google Scholar] [CrossRef]
- Raygada, M.; Cho, E.; Hilakivi-Clarke, L. High maternal intake of polyunsaturated fatty acids during pregnancy in mice alters offsprings’ aggressive behavior, immobility in the swim test, locomotor activity and brain protein kinase C activity. J. Nutr. 1998, 128, 2505–2511. [Google Scholar] [CrossRef]
- Chaliha, D.; Albrecht, M.; Vaccarezza, M.; Takechi, R.; Lam, V.; Al-Salami, H.; Mamo, J. A Systematic Review of the Valproic-Acid-Induced Rodent Model of Autism. Dev. Neurosci. 2020, 42, 12–48. [Google Scholar] [CrossRef]
- Schneider, T.; Przewłocki, R. Behavioral Alterations in Rats Prenatally Exposed to Valproic Acid: Animal Model of Autism. Neuropsychopharmacology 2005, 30, 80–89. [Google Scholar] [CrossRef]
- Fernandes, D.J.; Spring, S.; Roy, A.R.; Qiu, L.R.; Yee, Y.; Nieman, B.J.; Lerch, J.P.; Palmert, M.R. Exposure to maternal high-fat diet induces extensive changes in the brain of adult offspring. Transl. Psychiatry 2021, 11, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Robinson, E.B.; Lichtenstein, P.; Anckarsäter, H.; Happé, F.; Ronald, A. Examining and interpreting the female protective effect against autistic behavior. Proc. Natl. Acad. Sci. USA 2013, 110, 5258–5262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Werling, D.; Geschwind, D.H. Sex differences in autism spectrum disorders. Curr. Opin. Neurol. 2013, 26, 146–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Li, N.; Li, C.; Zhang, Z.; Teng, H.; Wang, Y.; Zhao, T.; Shi, L.; Zhang, K.; Xia, K.; et al. Genetic evidence of gender difference in autism spectrum disorder supports the female-protective effect. Transl. Psychiatry 2020, 10, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Gartstein, M.; Skinner, M.K. Prenatal influences on temperament development: The role of environmental epigenetics. Dev. Psychopathol. 2018, 30, 1269–1303. [Google Scholar] [CrossRef] [Green Version]
- Kundakovic, M.; Jaric, I. The Epigenetic Link between Prenatal Adverse Environments and Neurodevelopmental Disorders. Genes Basel 2017, 8, 104. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Xiao, X.; Zheng, J.; Li, M.; Yu, M.; Ping, F.; Wang, T.; Wang, X. A Maternal High-Fat Diet Induces DNA Methylation Changes That Contribute to Glucose Intolerance in Offspring. Front. Endocrinol. Lausanne 2019, 10, 871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, C.C.Y.; Smith, R.G.; Hannon, E.; Ramaswami, G.; Parikshak, N.N.; Assary, E.; Troakes, C.; Poschmann, J.; Schalkwyk, L.C.; Sun, W.; et al. Genome-wide DNA methylation profiling identifies convergent molecular signatures associated with idiopathic and syndromic autism in post-mortem human brain tissue. Hum. Mol. Genet. 2019, 28, 2201–2211. [Google Scholar] [CrossRef] [Green Version]
- Pop, S.; Enciu, A.M.; Tarcomnicu, I.; Gille, E.; Tanase, C. Phytochemicals in cancer prevention: Modulating epigenetic alterations of DNA methylation. Phytochem. Rev. 2019, 18, 1005–1024. [Google Scholar] [CrossRef] [Green Version]
- McKee, S.E.; Zhang, S.; Chen, L.; Rabinowitz, J.D.; Reyes, T.M. Perinatal high fat diet and early life methyl donor supplementation alter one carbon metabolism and DNA methylation in the brain. J. Neurochem. 2018, 145, 362–373. [Google Scholar] [CrossRef] [PubMed]
- Carlin, J.; George, R.; Reyes, T.M. Methyl Donor Supplementation Blocks the Adverse Effects of Maternal High Fat Diet on Offspring Physiology. PLoS ONE 2013, 8, e63549. [Google Scholar] [CrossRef] [PubMed]
- Marco, A.; Kisliouk, T.; Tabachnik, T.; Meiri, N.; Weller, A. Overweight and CpG methylation of the Pomc promoter in offspring of high-fat-diet-fed dams are not “reprogrammed” by regular chow diet in rats. FASEB J. 2014, 28, 4148–4157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monteiro, P.; Feng, G. SHANK proteins: Roles at the synapse and in autism spectrum disorder. Nat. Rev. Neurosci. 2017, 18, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Bruining, H.; Hardstone, R.; Juarez-Martinez, E.L.; Sprengers, J.; Avramiea, A.-E.; Simpraga, S.; Houtman, S.J.; Poil, S.-S.; Dallares, E.; Palva, S.; et al. Measurement of excitation-inhibition ratio in autism spectrum disorder using critical brain dynamics. Sci. Rep. 2020, 10, 1–15. [Google Scholar] [CrossRef]
- Culotta, L.; Penzes, P. Exploring the mechanisms underlying excitation/inhibition imbalance in human iPSC-derived models of ASD. Mol. Autism 2020, 11, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Bautista, J.; Liu, E.; Zikopoulos, B. Imbalance of laminar-specific excitatory and inhibitory circuits of the orbitofrontal cortex in autism. Mol. Autism 2020, 11, 1–19. [Google Scholar] [CrossRef]
- Fang, C.-Y.; Lai, T.-C.; Hsiao, M.; Chang, Y.-C. The Diverse Roles of TAO Kinases in Health and Diseases. Int. J. Mol. Sci. 2020, 21, 7463. [Google Scholar] [CrossRef] [PubMed]
- De Anda, F.C.; Rosario, A.L.R.D.; Durak, O.; Tran, T.; Gräff, J.; Meletis, K.; Rei, D.; Soda, T.; Madabhushi, R.; Ginty, D.D.; et al. Autism spectrum disorder susceptibility gene TAOK2 affects basal dendrite formation in the neocortex. Nat. Neurosci. 2012, 15, 1022–1031. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Oses-Prieto, J.A.; Peters, C.; Zhou, J.; Pleasure, S.J.; Burlingame, A.L.; Jan, L.; Jan, Y.-N. TAOK2 Kinase Mediates PSD95 Stability and Dendritic Spine Maturation through Septin7 Phosphorylation. Neuron 2017, 93, 379–393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sungur, A.; Schwarting, R.K.; Wöhr, M. Early communication deficits in theShank1knockout mouse model for autism spectrum disorder: Developmental aspects and effects of social context. Autism Res. 2016, 9, 696–709. [Google Scholar] [CrossRef]
- Mor, M.; Nardone, S.; Sams, D.S.; Elliott, E. Hypomethylation of miR-142 promoter and upregulation of microRNAs that target the oxytocin receptor gene in the autism prefrontal cortex. Mol. Autism 2015, 6, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Tonacci, A.; Bagnato, G.; Pandolfo, G.; Billeci, L.; Sansone, F.; Conte, R.; Gangemi, S. MicroRNA Cross-Involvement in Autism Spectrum Disorders and Atopic Dermatitis: A Literature Review. J. Clin. Med. 2019, 8, 88. [Google Scholar] [CrossRef] [Green Version]
- Salloum-Asfar, S.; Satheesh, N.J.; Abdulla, S.A. Circulating miRNAs, Small but Promising Biomarkers for Autism Spectrum Disorder. Front. Mol. Neurosci. 2019, 12, 253. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.-S. Impact of Maternal Diet on the Epigenome during In Utero Life and the Developmental Programming of Diseases in Childhood and Adulthood. Nutrients 2015, 7, 9492–9507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bresnahan, M.; Hornig, M.; Schultz, A.F.; Gunnes, N.; Hirtz, D.; Lie, K.K.; Magnus, P.; Reichborn-Kjennerud, T.; Roth, C.; Schjølberg, S.; et al. Association of maternal report of infant and toddler gastrointestinal symptoms with autism: Evidence from a prospective birth cohort. JAMA Psychiatry 2015, 72, 466–474. [Google Scholar] [CrossRef] [Green Version]
- Martin, C.R.; Osadchiy, V.; Kalani, A.; Mayer, E.A. The Brain-Gut-Microbiome Axis. Cell. Mol. Gastroenterol. Hepatol. 2018, 6, 133–148. [Google Scholar] [CrossRef] [Green Version]
- Needham, B.; Tang, W.; Wu, W.-L. Searching for the gut microbial contributing factors to social behavior in rodent models of autism spectrum disorder. Dev. Neurobiol. 2018, 78, 474–499. [Google Scholar] [CrossRef]
- Saurman, V.; Margolis, K.G.; Luna, R.A. Autism Spectrum Disorder as a Brain-Gut-Microbiome Axis Disorder. Dig. Dis. Sci. 2020, 65, 818–828. [Google Scholar] [CrossRef] [Green Version]
- Kong, X.-J.; Liu, J.; Liu, K.; Koh, M.; Sherman, H.; Liu, S.; Tian, R.; Sukijthamapan, P.; Wang, J.; Fong, M.; et al. Probiotic and Oxytocin Combination Therapy in Patients with Autism Spectrum Disorder: A Randomized, Double-Blinded, Placebo-Controlled Pilot Trial. Nutrients 2021, 13, 1552. [Google Scholar] [CrossRef]
- Donovan, A.; Basson, M.A. The neuroanatomy of autism—A developmental perspective. J. Anat. 2017, 230, 4–15. [Google Scholar] [CrossRef] [Green Version]
- Reinhardt, V.; Iosif, A.-M.; Libero, L.; Heath, B.; Rogers, S.J.; Ferrer, E.; Nordahl, C.; Ghetti, S.; Amaral, D.; Solomon, M. Understanding hippocampal development in young children with autism spectrum disorder. J. Am. Acad. Child Adolesc. Psychiatry 2020, 59, 1069–1079. [Google Scholar] [CrossRef] [PubMed]
- Gawliński, D.; Gawlińska, K.; Frankowska, M.; Filip, M. Maternal Diet Influences the Reinstatement of Cocaine-Seeking Behavior and the Expression of Melanocortin-4 Receptors in Female Offspring of Rats. Nutrients 2020, 12, 1462. [Google Scholar] [CrossRef]
- Festing, M.F.W. Design and Statistical Methods in Studies Using Animal Models of Development. ILAR J. 2006, 47, 5–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zimcikova, E.; Simko, J.; Karesova, I.; Kremlacek, J.; Malakova, J. Behavioral effects of antiepileptic drugs in rats: Are the effects on mood and behavior detectable in open-field test? Seizure 2017, 52, 35–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, 4th ed.; Academic Press: San Diego, CA, USA, 1998. [Google Scholar]




| Gene | Main Effect of Diet | Diet × Sex Interaction |
|---|---|---|
| PND 28 | ||
| Ankrd11 | F(3, 72) = 3.06, p < 0.05 | F(3, 72) = 0.45, p = 0.72 |
| Cacna1d | F(3, 72) = 1.28, p = 0.29 | F(3, 72) = 3.17, p < 0.05 |
| En2 | F(3, 72) = 4.67, p < 0.01 | F(3, 72) = 0.10, p = 0.96 |
| Itgb3 | F(3, 72) = 4.29, p < 0.01 | F(3, 72) = 2.84, p < 0.05 |
| Nlgn3 | F(3, 72) = 0.96, p = 0.42 | F(3, 72) = 3.03, p < 0.05 |
| Shank1 | F(3, 72) = 0.92, p = 0.44 | F(3, 72) = 3.46, p < 0.05 |
| Slc6a4 | F(3, 72) = 2.96, p < 0.05 | F(3, 72) = 1.33, p = 0.27 |
| Setd1b | F(3, 72) = 5.10, p < 0.01 | F(3, 72) = 24.44, p < 0.001 |
| Taok2 | F(3, 72) = 4.71, p < 0.01 | F(3, 72) = 0.68, p = 0.57 |
| PND 63 | ||
| Fmr1 | F(3, 72) = 8.23, p < 0.001 | F(3, 72) = 2.80, p < 0.05 |
| Itgb3 | F(3, 72) = 5.07, p < 0.01 | F(3, 72) = 5.04, p < 0.01 |
| Mecp2 | F(3, 72) = 5.41, p < 0.01 | F(3, 72) = 2.51, p = 0.07 |
| Pten | F(3, 72) = 2.55, p = 0.06 | F(3, 72) = 3.02, p < 0.05 |
| Reln | F(3, 72) = 5.06, p < 0.01 | F(3, 72) = 0.29, p = 0.83 |
| Shank2 | F(3, 72) = 4.32, p < 0.01 | F(3, 72) = 0.24, p = 0.86 |
| Setd1b | F(3, 72) = 25.82, p < 0.001 | F(3, 72) = 7.81, p < 0.001 |
| Taok2 | F(3, 72) = 0.87, p = 0.97 | F(3, 72) = 9.89, p < 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gawlińska, K.; Gawliński, D.; Kowal-Wiśniewska, E.; Jarmuż-Szymczak, M.; Filip, M. Alteration of the Early Development Environment by Maternal Diet and the Occurrence of Autistic-like Phenotypes in Rat Offspring. Int. J. Mol. Sci. 2021, 22, 9662. https://doi.org/10.3390/ijms22189662
Gawlińska K, Gawliński D, Kowal-Wiśniewska E, Jarmuż-Szymczak M, Filip M. Alteration of the Early Development Environment by Maternal Diet and the Occurrence of Autistic-like Phenotypes in Rat Offspring. International Journal of Molecular Sciences. 2021; 22(18):9662. https://doi.org/10.3390/ijms22189662
Chicago/Turabian StyleGawlińska, Kinga, Dawid Gawliński, Ewelina Kowal-Wiśniewska, Małgorzata Jarmuż-Szymczak, and Małgorzata Filip. 2021. "Alteration of the Early Development Environment by Maternal Diet and the Occurrence of Autistic-like Phenotypes in Rat Offspring" International Journal of Molecular Sciences 22, no. 18: 9662. https://doi.org/10.3390/ijms22189662
APA StyleGawlińska, K., Gawliński, D., Kowal-Wiśniewska, E., Jarmuż-Szymczak, M., & Filip, M. (2021). Alteration of the Early Development Environment by Maternal Diet and the Occurrence of Autistic-like Phenotypes in Rat Offspring. International Journal of Molecular Sciences, 22(18), 9662. https://doi.org/10.3390/ijms22189662