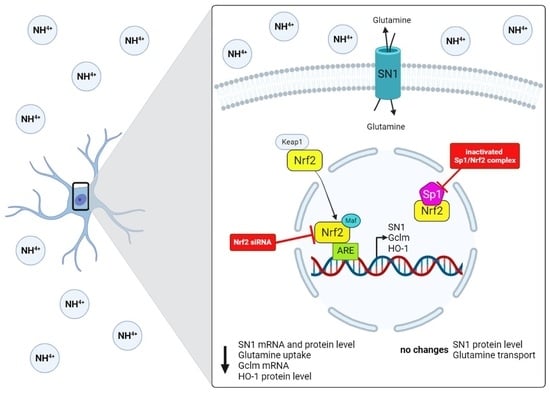
The Role of Nrf2 Transcription Factor and Sp1-Nrf2 Protein Complex in Glutamine Transporter SN1 Regulation in Mouse Cortical Astrocytes Exposed to Ammonia
Abstract
:1. Introduction
2. Results
2.1. Ammonia Increases Nrf2 Nuclear Protein Level and Its Activity in Mouse Cultured Astrocytes
2.2. Ammonia Enhances Nrf2 Binding to the Slc38a3 Promoter Region
2.3. Ammonia Decreases SN1 Expression in Astrocytes upon Nrf2 siRNA Transfection
2.4. Ammonia Decreases [3H]glutamine Uptake in Cultured Astrocytes upon Nrf2 siRNA Transfection
2.5. Nrf2 Silencing Decreases Expression of Nrf2 Target Genes in Mouse Cultured Astrocytes
2.6. Sp1-Nrf2 Complex Formation
2.7. Ammonia Does Not Affect SN1 Protein Level and [3H]glutamine Transport in Astrocytes with Inactivated Sp1-Nrf2 Complex
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture and Treatment
4.3. Nrf2 Transcription Factor Silencing
4.4. Inactivation of the Sp1-Nrf2 Protein Complex
4.5. RNA Isolation and Real-Time qPCR Analysis
4.6. Nuclear Protein Extraction
4.7. Protein Isolation and Western Blot
4.8. Nrf2 Activity Assay
4.9. [3H]glutamine Uptake
4.10. Chromatin Immunoprecipitation (ChIP)
4.11. Immunocytochemistry
4.12. Co-Immunoprecipitation
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ARE | Anti-oxidative response elements |
| ChIP | Chromatin immunoprecipitation |
| CNS | Central nervous system |
| DMEM | Dulbecco’s Modified Eagle’s Medium |
| FBS | Fetal bovine serum |
| GABA | Gamma-aminobutyric acid |
| Gcl | Glutamate-cysteine ligase |
| GAPDH | Glyceraldehyde 3-phosphate dehydrogenase |
| Gclc | Glutamate-cysteine ligase catalytic subunit |
| Gclm | Glutamate-cysteine ligase regulatory subunit |
| HO-1 | Heme-oxygenase 1 |
| Keap1 | Kelch like-ECH-associated protein 1 |
| MAPK | Mitogen-activated protein kinase |
| MEK 1/2 | Mitogen-activated protein kinase kinase |
| NQO1 | NAD(P)H quinone dehydrogenase |
| Nrf2 | Nuclear factor erythroid-related 2-like 2 |
| ONS | Oxidative/nitrosative stress |
| OS | Oxidative stress |
| PBS | Phosphate-buffered saline |
| pSp1 | Phosphorylated specificity protein 1 |
| RONS | Reactive oxygen-nitrogen species |
| ROS | Reactive oxygen species |
| RT | Room temperature |
| SN1 | SNAT3, solute carrier family 38 member 3 |
| Sp1 | Specificity protein 1 |
| TCA | Tricarboxylic acid |
| WT | Wild-type |
References
- Verkhratsky, A.; Nedergaard, M. Physiology of Astroglia. Physiol. Rev. 2018, 98, 239–389. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, J.; Sidoryk-Wegrzynowicz, M.; Zielińska, M.; Aschner, M. Roles of glutamine in neurotransmission. Neuron Glia Biol. 2010, 6, 263–276. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, J. Glutamine in the central nervous system: Function and dysfunction. Front. Biosci. 2007, 12, 332–343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albrecht, J.; Zielińska, M.; Norenberg, M.D. Glutamine as a mediator of ammonia neurotoxicity: A critical appraisal. Biochem. Pharmacol. 2010, 80, 1303–1308. [Google Scholar] [CrossRef] [Green Version]
- Schousboe, A.; Bak, L.K.; Waagepetersen, H.S. Astrocytic Control of Biosynthesis and Turnover of the Neurotransmitters Glutamate and GABA. Front. Endocrinol. 2013, 4, 102. [Google Scholar] [CrossRef] [Green Version]
- Butterworth, R.F. Pathophysiology of brain dysfunction in hyperammonemic syndromes: The many faces of glutamine. Mol. Genet. Metab. 2014, 113, 113–117. [Google Scholar] [CrossRef]
- Zielinska, M.; Dabrowska, K.; Hadera, M.G.; Sonnewald, U.; Albrecht, J. System N transporters are critical for glutamine release and modulate metabolic fluxes of glucose and acetate in cultured cortical astrocytes: Changes induced by ammonia. J. Neurochem. 2015, 136, 329–338. [Google Scholar] [CrossRef] [Green Version]
- Hamdani, E.H.; Popek, M.; Frontczak-Baniewicz, M.; Utheim, T.P.; Albrecht, J.; Zielińska, M.; Chaudhry, F.A. Perturbation of astroglial Slc38 glutamine transporters by NH 4 + contributes to neurophysiologic manifestations in acute liver failure. FASEB J. 2021, 35, e21588. [Google Scholar] [CrossRef]
- Schliess, F.; Görg, B.; Fischer, R.; Desjardins, P.; Bidmon, H.J.; Herrmann, A.; Butterworth, R.F.; Zilles, K.; Häussinger, D. Ammonia induces MK-801-sensitive nitration and phosphorylation of protein tyrosine residues in rat astrocytes. FASEB J. 2002, 16, 739–741. [Google Scholar] [CrossRef]
- Jayakumar, A.R.; Panickar, K.S.; Murthy, C.R.K.; Norenberg, M.D. Oxidative Stress and Mitogen-Activated Protein Kinase Phosphorylation Mediate Ammonia-Induced Cell Swelling and Glutamate Uptake Inhibition in Cultured Astrocytes. J. Neurosci. 2006, 26, 4774–4784. [Google Scholar] [CrossRef]
- Tonelli, C.; Chio, I.I.C.; Tuveson, D.A. Transcriptional Regulation by Nrf2. Antioxid. Redox Signal. 2018, 29, 1727–1745. [Google Scholar] [CrossRef] [Green Version]
- Mitsuishi, Y.; Taguchi, K.; Kawatani, Y.; Shibata, T.; Nukiwa, T.; Aburatani, H.; Yamamoto, M.; Motohashi, H. Nrf2 Redirects Glucose and Glutamine into Anabolic Pathways in Metabolic Reprogramming. Cancer Cell 2012, 22, 66–79. [Google Scholar] [CrossRef] [Green Version]
- Lee, E.; Yin, Z.; Sidoryk-Węgrzynowicz, M.; Jiang, H.; Aschner, M. 15-Deoxy-Δ12,14-prostaglandin J2 modulates manganese-induced activation of the NF-κB, Nrf2, and PI3K pathways in astrocytes. Free. Radic. Biol. Med. 2012, 52, 1067–1074. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.-C.; Yang, C.-C.; Chen, Y.-W.; Hsiao, L.-D.; Yang, C.-M. Arachidonic Acid Induces ARE/Nrf2-Dependent Heme Oxygenase-1 Transcription in Rat Brain Astrocytes. Mol. Neurobiol. 2017, 55, 3328–3343. [Google Scholar] [CrossRef]
- Dai, H.; Song, D.; Xu, J.; Li, B.; Hertz, L.; Peng, L. Ammonia-induced Na,K-ATPase/ouabain-mediated EGF receptor transactivation, MAPK/ERK and PI3K/AKT signaling and ROS formation cause astrocyte swelling. Neurochem. Int. 2013, 63, 610–625. [Google Scholar] [CrossRef]
- Chi, P.-L.; Lin, C.-C.; Chen, Y.-W.; Hsiao, L.-D.; Yang, C.-M. CO Induces Nrf2-Dependent Heme Oxygenase-1 Transcription by Cooperating with Sp1 and c-Jun in Rat Brain Astrocytes. Mol. Neurobiol. 2014, 52, 277–292. [Google Scholar] [CrossRef]
- Warskulat, U.; Görg, B.; Bidmon, H.-J.; Müller, H.W.; Schliess, F.; Häussinger, D. Ammonia-induced heme oxygenase-1 expression in cultured rat astrocytes and rat brain in vivo. Glia 2002, 40, 324–336. [Google Scholar] [CrossRef]
- Görg, B.; Karababa, A.; Häussinger, D. Hepatic Encephalopathy and Astrocyte Senescence. J. Clin. Exp. Hepatol. 2018, 8, 294–300. [Google Scholar] [CrossRef]
- Görg, B.; Karababa, A.; Schütz, E.; Paluschinski, M.; Schrimpf, A.; Shafigullina, A.; Castoldi, M.; Bidmon, H.J.; Häussinger, D. O-GlcNAcylation-dependent upregulation of HO1 triggers ammonia-induced oxidative stress and senescence in hepatic encephalopathy. J. Hepatol. 2019, 71, 930–941. [Google Scholar] [CrossRef]
- Lister, A.; Bourgeois, S.; Silva, P.H.I.; Rubio-Aliaga, I.; Marbet, P.; Walsh, J.; Shelton, L.M.; Keller, B.; Verrey, F.; Devuyst, O.; et al. NRF2 regulates the glutamine transporter Slc38a3 (SNAT3) in kidney in response to metabolic acidosis. Sci. Rep. 2018, 8, 5629. [Google Scholar] [CrossRef]
- Lee, C.B.; Lee, Y.S.; Lee, J.Y.; Lee, S.E.; Ahn, K.Y. Nrf2 and Sp family synergistically enhance the expression of ion transporters in potassium-depleted conditions. J. Nephrol. 2011, 25, 225–232. [Google Scholar] [CrossRef]
- Ikawa, T.; Sato, M.; Oh-Hashi, K.; Furuta, K.; Hirata, Y. Oxindole–curcumin hybrid compound enhances the transcription of γ-glutamylcysteine ligase. Eur. J. Pharmacol. 2021, 896, 173898. [Google Scholar] [CrossRef]
- Balkrishna, S.; Bröer, A.; Welford, S.M.; Hatzoglou, M.; Bröer, S. Expression of Glutamine Transporter Slc38a3 (SNAT3) During Acidosis is Mediated by a Different Mechanism than Tissue-Specific Expression. Cell. Physiol. Biochem. 2014, 33, 1591–1606. [Google Scholar] [CrossRef]
- Dąbrowska, K.; Zielińska, M. Silencing of Transcription Factor Sp1 Promotes SN1 Transporter Regulation by Ammonia in Mouse Cortical Astrocytes. Int. J. Mol. Sci. 2019, 20, 234. [Google Scholar] [CrossRef] [Green Version]
- Bémeur, C.; Desjardins, P.; Butterworth, R.F. Evidence for oxidative/nitrosative stress in the pathogenesis of hepatic encephalopathy. Metab. Brain Dis. 2010, 25, 3–9. [Google Scholar] [CrossRef]
- Häussinger, D.; Görg, B. Interaction of oxidative stress, astrocyte swelling and cerebral ammonia toxicity. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 87–92. [Google Scholar] [CrossRef]
- Matés, J.M.; Gomez, F.J.M.; de Castro, I.N.; Asenjo, M.; Márquez, J. Glutamine and its relationship with intracellular redox status, oxidative stress and cell proliferation/death. Int. J. Biochem. Cell Biol. 2001, 34, 439–458. [Google Scholar] [CrossRef]
- Bodega, G.; Suarez, I.; Paniagua, C.; Vacas, E.; Fernández, B. Effect of ammonia, glutamine, and serum on calcineurin, p38MAPK-diP, GADD153/CHOP10, and CNTF in primary rat astrocyte cultures. Brain Res. 2007, 1175, 126–133. [Google Scholar] [CrossRef]
- Copple, I.M.; Goldring, C.E.; Kitteringham, N.R.; Park, B.K. The Keap1-Nrf2 Cellular Defense Pathway: Mechanisms of Regulation and Role in Protection Against Drug-Induced Toxicity. Adverse Drug React. 2009, 233–266. [Google Scholar] [CrossRef]
- Kobayashi, M.; Yamamoto, M. Nrf2–Keap1 regulation of cellular defense mechanisms against electrophiles and reactive oxygen species. Adv. Enzym. Regul. 2006, 46, 113–140. [Google Scholar] [CrossRef]
- Sidoryk-Wegrzynowicz, M.; Lee, E.; Mingwei, N.; Aschner, M. Disruption of astrocytic glutamine turnover by manganese is mediated by the protein kinase C pathway. Glia 2011, 59, 1732–1743. [Google Scholar] [CrossRef] [PubMed]
- Bodega, G.; Segura, B.; Ciordia, S.; Mena, M.D.C.; López-Fernández, L.A.; García, M.I.; Trabado, I.; Suárez, I. Ammonia Affects Astroglial Proliferation in Culture. PLoS ONE 2015, 10, e0139619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dąbrowska, K.; Albrecht, J.; Zielińska, M. Protein kinase C-mediated impairment of glutamine outward transport and SN1 transporter distribution by ammonia in mouse cortical astrocytes. Neurochem. Int. 2018, 118, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, F.; Reimer, R.J.; Krizaj, D.; Barber, D.; Storm-Mathisen, J.; Copenhagen, D.R.; Edwards, R.H. Molecular Analysis of System N Suggests Novel Physiological Roles in Nitrogen Metabolism and Synaptic Transmission. Cell 1999, 99, 769–780. [Google Scholar] [CrossRef] [Green Version]
- Murthy, C. Elevation of glutathione levels by ammonium ions in primary cultures of rat astrocytes. Neurochem. Int. 2000, 37, 255–268. [Google Scholar] [CrossRef]
- Węgrzynowicz, M.; Hilgier, W.; Dybel, A.; Oja, S.S.; Saransaari, P.; Albrecht, J. Upregulation of cerebral cortical glutathione synthesis by ammonia in vivo and in cultured glial cells: The role of cystine uptake. Neurochem. Int. 2007, 50, 883–889. [Google Scholar] [CrossRef]
- Hilgier, W.; Węgrzynowicz, M.; Ruszkiewicz, J.; Oja, S.S.; Saransaari, P.; Albrecht, J. Direct Exposure to Ammonia and Hyperammonemia Increase the Extracellular Accumulation and Degradation of Astroglia-Derived Glutathione in the Rat Prefrontal Cortex. Toxicol. Sci. 2010, 117, 163–168. [Google Scholar] [CrossRef] [Green Version]
- Shih, A.Y.; Johnson, D.A.; Wong, G.; Kraft, A.D.; Jiang, L.; Erb, H.; Johnson, J.A.; Murphy, T. Coordinate Regulation of Glutathione Biosynthesis and Release by Nrf2-Expressing Glia Potently Protects Neurons from Oxidative Stress. J. Neurosci. 2003, 23, 3394–3406. [Google Scholar] [CrossRef]
- Kraft, A.D.; Johnson, D.A.; Johnson, J.A. Nuclear Factor E2-Related Factor 2-Dependent Antioxidant Response Element Activation by tert-Butylhydroquinone and Sulforaphane Occurring Preferentially in Astrocytes Conditions Neurons against Oxidative Insult. J. Neurosci. 2004, 24, 1101–1112. [Google Scholar] [CrossRef] [Green Version]
- Hertz, L.; Juurlink, B.H.J.; Hertz, E.; Fosmark, H.; Schousboe, A. Preparation of primary cultures of mouse (rat) astrocytes. In A Dissection and Tissue Culture, Manual of the Nervous System; Alan R. Liss, Inc.: New York, NY, USA, 1989; pp. 105–108. [Google Scholar]
- Skowrońska, K.; Obara-Michlewska, M.; Czarnecka, A.M.; Dąbrowska, K.; Zielińska, M.; Albrecht, J. Persistent Overexposure to N-Methyl-d-Aspartate (NMDA) Calcium-Dependently Downregulates Glutamine Synthetase, Aquaporin 4, and Kir4.1 Channel in Mouse Cortical Astrocytes. Neurotox. Res. 2018, 35, 271–280. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2 − ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Sidoryk-Wecgrzynowicz, M.; Lee, E.-S.; Ni, M.; Aschner, M.; Sidoryk-Wȩgrzynowicz, M. Manganese-induced downregulation of astroglial glutamine transporter SNAT3 involves ubiquitin-mediated proteolytic system. Glia 2010, 58, 1905–1912. [Google Scholar] [CrossRef]






| [3H]glutamine Uptake [Fold of Control Total None] | ||
|---|---|---|
| None | Nrf2- | |
| Total | ||
| Control | 1.00 ± 0.17 | 1.01 ± 0.22 |
| Ammonia | 0.82 ± 0.15 | 0.62 ± 0.19 + |
| System N | ||
| Control | 0.29 ± 0.14 * | 0.24 ± 0.06 + |
| Ammonia | 0.21 ± 0.10 # | 0.12 ± 0.06 =,^ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dąbrowska, K.; Skowrońska, K.; Popek, M.; Albrecht, J.; Zielińska, M. The Role of Nrf2 Transcription Factor and Sp1-Nrf2 Protein Complex in Glutamine Transporter SN1 Regulation in Mouse Cortical Astrocytes Exposed to Ammonia. Int. J. Mol. Sci. 2021, 22, 11233. https://doi.org/10.3390/ijms222011233
Dąbrowska K, Skowrońska K, Popek M, Albrecht J, Zielińska M. The Role of Nrf2 Transcription Factor and Sp1-Nrf2 Protein Complex in Glutamine Transporter SN1 Regulation in Mouse Cortical Astrocytes Exposed to Ammonia. International Journal of Molecular Sciences. 2021; 22(20):11233. https://doi.org/10.3390/ijms222011233
Chicago/Turabian StyleDąbrowska, Katarzyna, Katarzyna Skowrońska, Mariusz Popek, Jan Albrecht, and Magdalena Zielińska. 2021. "The Role of Nrf2 Transcription Factor and Sp1-Nrf2 Protein Complex in Glutamine Transporter SN1 Regulation in Mouse Cortical Astrocytes Exposed to Ammonia" International Journal of Molecular Sciences 22, no. 20: 11233. https://doi.org/10.3390/ijms222011233
APA StyleDąbrowska, K., Skowrońska, K., Popek, M., Albrecht, J., & Zielińska, M. (2021). The Role of Nrf2 Transcription Factor and Sp1-Nrf2 Protein Complex in Glutamine Transporter SN1 Regulation in Mouse Cortical Astrocytes Exposed to Ammonia. International Journal of Molecular Sciences, 22(20), 11233. https://doi.org/10.3390/ijms222011233