Polygodial and Ophiobolin A Analogues for Covalent Crosslinking of Anticancer Targets
Abstract
:1. Introduction
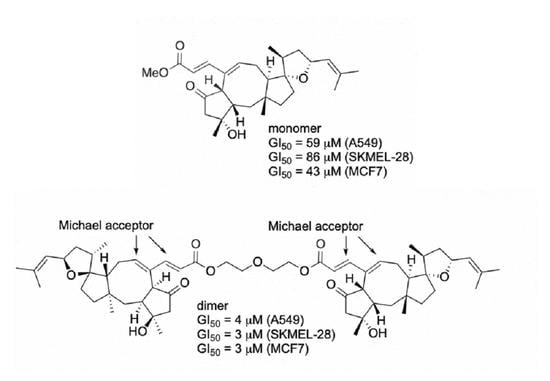
2. Results and Discussion
3. Materials and Methods
3.1. Human Cell Lines and Antiproliferative Effects
3.2. Natural Product Isolation
3.3. Selected Procedure for the Preparation of Polygodial-Based Cross-Linking Agent 16
3.4. Selected Procedure for the Preparation of Ophiobolin A-Based Cross-Linking Agent 23
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brenner, H. Long-Term Survival Rates of Cancer Patients Achieved by the End of the 20th Century: A Period Analysis. Lancet 2002, 360, 1131–1135. [Google Scholar] [CrossRef]
- Agnihotri, S.; Burrell, K.E.; Wolf, A.; Jalali, S.; Hawkins, C.; Rutka, J.T.; Zadeh, G. Glioblastoma, a Brief Review of History, Molecular Genetics, Animal Models and Novel Therapeutic Strategies. Arch. Immunol. Ther. Exp. 2013, 61, 25–41. [Google Scholar] [CrossRef] [PubMed]
- Lefranc, F.; Sadeghi, N.; Camby, I.; Metens, T.; Dewitte, O.; Kiss, R. Present and Potential Future Issues in Glioblastoma Treatment. Expert Rev. Anticancer Ther. 2006, 6, 719–732. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Hegi, M.E.; Mason, W.P.; Van Den Bent, M.J.; Taphoorn, M.J.B.; Janzer, R.C.; Ludwin, S.K.; Allgeier, A.; Fisher, B.; Belanger, K. Effects of Radiotherapy with Concomitant and Adjuvant Temozolomide versus Radiotherapy Alone on Survival in Glioblastoma in a Randomised Phase III Study: 5-Year Analysis of the EORTC-NCIC Trial. Lancet Oncol. 2009, 10, 459–466. [Google Scholar] [CrossRef]
- Simpson, C.D.; Anyiwe, K.; Schimmer, A.D. Anoikis Resistance and Tumor Metastasis. Cancer Lett. 2008, 272, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Savage, P.; Stebbing, J.; Bower, M.; Crook, T. Why Does Cytotoxic Chemotherapy Cure Only Some Cancers? Nat. Clin. Pract. Oncol. 2009, 6, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Dasari, R.; Banuls, L.M.Y.; Masi, M.; Pelly, S.C.; Mathieu, V.; Green, I.R.; van Otterlo, W.A.L.; Evidente, A.; Kiss, R.; Kornienko, A. C1, C2-Ether Derivatives of the Amaryllidaceae Alkaloid Lycorine: Retention of Activity of Highly Lipophilic Analogues against Cancer Cells. Bioorg. Med. Chem. Lett. 2014, 24, 923–927. [Google Scholar] [CrossRef]
- Kornienko, A.; Mathieu, V.; Rastogi, S.K.; Lefranc, F.; Kiss, R. Therapeutic Agents Triggering Nonapoptotic Cancer Cell Death. J. Med. Chem. 2013, 56, 4823–4839. [Google Scholar] [CrossRef]
- Bury, M.; Girault, A.; Megalizzi, V.; Spiegl-Kreinecker, S.; Mathieu, V.; Berger, W.; Evidente, A.; Kornienko, A.; Gailly, P.; Vandier, C. Ophiobolin A Induces Paraptosis-like Cell Death in Human Glioblastoma Cells by Decreasing BKCa Channel Activity. Cell Death Dis. 2013, 4, e561. [Google Scholar] [CrossRef] [Green Version]
- Mathieu, V.; Chantôme, A.; Lefranc, F.; Cimmino, A.; Miklos, W.; Paulitschke, V.; Mohr, T.; Maddau, L.; Kornienko, A.; Berger, W. Sphaeropsidin A Shows Promising Activity against Drug-Resistant Cancer Cells by Targeting Regulatory Volume Increase. Cell. Mol. Life Sci. 2015, 72, 3731–3746. [Google Scholar] [CrossRef] [Green Version]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the 30 Years from 1981 to 2010. J. Nat. Prod. 2012, 75, 311–335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Safarzadeh, E.; Shotorbani, S.S.; Baradaran, B. Herbal Medicine as Inducers of Apoptosis in Cancer Treatment. Adv. Pharm. Bull. 2014, 4 (Suppl. 1), 421. [Google Scholar] [PubMed]
- Wondrak, G.T. Redox-Directed Cancer Therapeutics: Molecular Mechanisms and Opportunities. Antioxid. Redox Signal. 2009, 11, 3013–3069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmad, A.; Sakr, W.A.; Rahman, K.M. Novel Targets for Detection of Cancer and Their Modulation by Chemopreventive Natural Compounds. Front. Biosci. 2012, 4, 410–425. [Google Scholar] [CrossRef]
- Lee, D.; Kim, I.Y.; Saha, S.; Choi, K.S. Paraptosis in the Anti-Cancer Arsenal of Natural Products. Pharmacol. Ther. 2016, 162, 120–133. [Google Scholar] [CrossRef]
- Wang, X.; Feng, Y.; Wang, N.; Cheung, F.; Tan, H.Y.; Zhong, S.; Li, C.; Kobayashi, S. Chinese Medicines Induce Cell Death: The Molecular and Cellular Mechanisms for Cancer Therapy. Biomed Res. Int. 2014, 2014. [Google Scholar] [CrossRef]
- Gali-Muhtasib, H.; Hmadi, R.; Kareh, M.; Tohme, R.; Darwiche, N. Cell Death Mechanisms of Plant-Derived Anticancer Drugs: Beyond Apoptosis. Apoptosis 2015, 20, 1531–1562. [Google Scholar] [CrossRef] [PubMed]
- Dasari, R.; De Carvalho, A.; Medellin, D.C.; Middleton, K.N.; Hague, F.; Volmar, M.N.M.; Frolova, L.V.; Rossato, M.F.; Jorge, J.; Dybdal-Hargreaves, N.F. Wittig Derivatization of Sesquiterpenoid Polygodial Leads to Cytostatic Agents with Activity against Drug Resistant Cancer Cells and Capable of Pyrrolylation of Primary Amines. Eur. J. Med. Chem. 2015, 103, 226–237. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Gavin, T.; DeCaprio, A.P.; LoPachin, R.M. γ-Diketone Axonopathy: Analyses of Cytoskeletal Motors and Highways in CNS Myelinated Axons. Toxicol. Sci. 2010, 117, 180–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Graham, D.G.; Anthony, D.C.; Boekelheide, K.; Maschmann, N.A.; Richards, R.G.; Wolfram, J.W.; Shaw, B.R. Studies of the Molecular Pathogenesis of Hexane Neuropathy: II. Evidence That Pyrrole Derivatization of Lysyl Residues Leads to Protein Crosslinking. Toxicol. Appl. Pharmacol. 1982, 64, 415–422. [Google Scholar] [CrossRef]
- Masi, M.; Dasari, R.; Evidente, A.; Mathieu, V.; Kornienko, A. Chemistry and Biology of Ophiobolin A and Its Congeners. Bioorg. Med. Chem. Lett. 2019, 29, 859–869. [Google Scholar] [CrossRef]
- Dasari, R.; Masi, M.; Lisy, R.; Ferdérin, M.; English, L.R.; Cimmino, A.; Mathieu, V.; Brenner, A.J.; Kuhn, J.G.; Whitten, S.T. Fungal Metabolite Ophiobolin A as a Promising Anti-Glioma Agent: In vivo Evaluation, Structure–Activity Relationship and Unique Pyrrolylation of Primary Amines. Bioorg. Med. Chem. Lett. 2015, 25, 4544–4548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chidley, C.; Trauger, S.A.; Birsoy, K.; O’Shea, E.K. The Anticancer Natural Product Ophiobolin A Induces Cytotoxicity by Covalent Modification of Phosphatidylethanolamine. Elife 2016, 5, e14601. [Google Scholar] [CrossRef] [PubMed]
- Janganati, V.; Ponder, J.; Jordan, C.T.; Borrelli, M.J.; Penthala, N.R.; Crooks, P.A. Dimers of Melampomagnolide B Exhibit Potent Anticancer Activity against Hematological and Solid Tumor Cells. J. Med. Chem. 2015, 58, 8896–8906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mantaj, J.; Jackson, P.J.M.; Rahman, K.M.; Thurston, D.E. From Anthramycin to Pyrrolobenzodiazepine (PBD)-containing Antibody–Drug Conjugates (ADCs). Angew. Chemie Int. Ed. 2017, 56, 462–488. [Google Scholar] [CrossRef] [Green Version]
- Hartley, J.A. Antibody-Drug Conjugates (ADCs) Delivering Pyrrolobenzodiazepine (PBD) Dimers for Cancer Therapy. Expert Opin. Biol. Ther. 2021, 21, 931–943. [Google Scholar] [CrossRef]
- Gong, Y.; Gallis, B.M.; Goodlett, D.R.; Yang, Y.; Lu, H.; Lacoste, E.; Lai, H.; Sasaki, T. Effects of Transferrin Conjugates of Artemisinin and Artemisinin Dimer on Breast Cancer Cell Lines. Anticancer Res. 2013, 33, 123–132. [Google Scholar]
- Dury, L.; Nasr, R.; Lorendeau, D.; Comsa, E.; Wong, I.; Zhu, X.; Chan, K.-F.; Chan, T.-H.; Chow, L.; Falson, P. Flavonoid Dimers Are Highly Potent Killers of Multidrug Resistant Cancer Cells Overexpressing MRP1. Biochem. Pharmacol. 2017, 124, 10–18. [Google Scholar] [CrossRef]
- Wong, I.L.K.; Zhu, X.; Chan, K.-F.; Law, M.C.; Lo, A.M.Y.; Hu, X.; Chow, L.M.C.; Chan, T.H. Discovery of Novel Flavonoid Dimers To Reverse Multidrug Resistance Protein 1 (MRP1, ABCC1) Mediated Drug Resistance in Cancers Using a High Throughput Platform with “Click Chemistry”. J. Med. Chem. 2018, 61, 9931–9951. [Google Scholar] [CrossRef]
- Zhu, X.; Wong, I.L.K.; Chan, K.-F.; Cui, J.; Law, M.C.; Chong, T.C.; Hu, X.; Chow, L.M.C.; Chan, T.H. Triazole Bridged Flavonoid Dimers as Potent, Nontoxic, and Highly Selective Breast Cancer Resistance Protein (BCRP/ABCG2) Inhibitors. J. Med. Chem. 2019, 62, 8578–8608. [Google Scholar] [CrossRef]
- Kung Sutherland, M.S.; Walter, R.B.; Jeffrey, S.C.; Burke, P.J.; Yu, C.; Kostner, H.; Stone, I.; Ryan, M.C.; Sussman, D.; Lyon, R.P. SGN-CD33A: A Novel CD33-Targeting Antibody–Drug Conjugate Using a Pyrrolobenzodiazepine Dimer Is Active in Models of Drug-Resistant AML. Blood J. Am. Soc. Hematol. 2013, 122, 1455–1463. [Google Scholar] [CrossRef]
- Just, J.; Jordan, T.B.; Paull, B.; Bissember, A.C.; Smith, J.A. Practical Isolation of Polygodial from Tasmannia Lanceolata: A Viable Scaffold for Synthesis. Org. Biomol. Chem. 2015, 13, 11200–11207. [Google Scholar] [CrossRef] [Green Version]
- Bury, M.; Novo-Uzal, E.; Andolfi, A.; Cimini, S.; Wauthoz, N.; Heffeter, P.; Lallemand, B.; Avolio, F.; Delporte, C.; Cimmino, A. Ophiobolin A, a Sesterterpenoid Fungal Phytotoxin, Displays Higher in vitro Growth-Inhibitory Effects in Mammalian than in Plant Cells and Displays in vivo Antitumor Activity. Int. J. Oncol. 2013, 43, 575–585. [Google Scholar] [CrossRef] [Green Version]
- Ingrassia, L.; Lefranc, F.; Dewelle, J.; Pottier, L.; Mathieu, V.; Spiegl-Kreinecker, S.; Sauvage, S.; El Yazidi, M.; Dehoux, M.; Berger, W. Structure− Activity Relationship Analysis of Novel Derivatives of Narciclasine (an Amaryllidaceae Isocarbostyril Derivative) as Potential Anticancer Agents. J. Med. Chem. 2009, 52, 1100–1114. [Google Scholar] [CrossRef]
- Le Calvé, B.; Rynkowski, M.; Le Mercier, M.; Bruyère, C.; Lonez, C.; Gras, T.; Haibe-Kains, B.; Bontempi, G.; Decaestecker, C.; Ruysschaert, J.-M. Long-Term in vitro Treatment of Human Glioblastoma Cells with Temozolomide Increases Resistance in vivo through up-Regulation of GLUT Transporter and Aldo-Keto Reductase Enzyme AKR1C Expression. Neoplasia 2010, 12, 727–739. [Google Scholar] [CrossRef]
- Branle, F.; Lefranc, F.; Camby, I.; Jeuken, J.; Geurts-Moespot, A.; Sprenger, S.; Sweep, F.; Kiss, R.; Salmon, I. Evaluation of the Efficiency of Chemotherapy in in vivo Orthotopic Models of Human Glioma Cells with and without 1p19q Deletions and in C6 Rat Orthotopic Allografts Serving for the Evaluation of Surgery Combined with Chemotherapy. Cancer Interdiscip. Int. J. Am. Cancer Soc. 2002, 95, 641–655. [Google Scholar] [CrossRef]
- Mathieu, V.; Pirker, C.; de Lassalle, E.M.; Vernier, M.; Mijatovic, T.; DeNeve, N.; Gaussin, J.; Dehoux, M.; Lefranc, F.; Berger, W. The Sodium Pump A1 Sub-unit: A Disease Progression–Related Target for Metastatic Melanoma Treatment. J. Cell. Mol. Med. 2009, 13, 3960–3972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Medellin, D.C.; Zhou, Q.; Scott, R.; Hill, R.M.; Frail, S.K.; Dasari, R.; Ontiveros, S.J.; Pelly, S.C.; van Otterlo, W.A.L.; Betancourt, T. Novel Microtubule-Targeting 7-Deazahypoxanthines Derived from Marine Alkaloid Rigidins with Potent in vitro and in vivo Anticancer Activities. J. Med. Chem. 2016, 59, 480–485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perez-Benito, L.; Henry, A.; Matsoukas, M.-T.; Lopez, L.; Pulido, D.; Royo, M.; Cordomi, A.; Tresdern, G.; Pardo, L. The size matters? A computational tool to design bivalent ligands. Bioinformatics 2018, 34, 3857–3863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knox, A.; Kalchschmid, C.; Schuster, D.; Gaggia, F.; Manzl, C.; Baecker, D.; Gust, R. Development of bivalent trialkene- and cyclofenil-derived dual estrogen receptor antagonist and downregulators. Eur. J. Med. Chem. 2020, 192, 112191. [Google Scholar] [CrossRef]
- Paquin, A.; Reyes-Moreno, C.; Berube, G. Recent advances in the use of the dimerization strategy as a means to increase the biological potential of natural or synthetic molecules. Molecules 2021, 26, 2340. [Google Scholar] [CrossRef] [PubMed]







| Compound | GI50 In Vitro Values (µM) 1 | |||||
|---|---|---|---|---|---|---|
| Resistant to Apoptosis | Sensitive to Apoptosis | |||||
| A549 | SKMEL-28 | U373 | MCF7 | Hs683 | B16F10 | |
| 1 | 30 | 42 | 41 | 36 | 29 | 7 |
| 15 | 7 | 14 | 9 | 4 | 4 | 5 |
| 16 | 17 | 22 | 28 | 9 | 8 | 9 |
| 17 | 55 | 29 | 79 | 36 | 50 | 24 |
| 21 | 25 | 24 | 28 | 23 | 17 | 6 |
| 42 | 59 | 86 | 86 | 43 | 59 | ND |
| 22 | 5 | 8 | 32 | 8 | 10 | 4 |
| 23 | 4 | 3 | 5 | 3 | 3 | 3 |
| cisplatin | 7 | 26 | ND | 35 | 7 | 8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maslivetc, V.; Laguera, B.; Chandra, S.; Dasari, R.; Olivier, W.J.; Smith, J.A.; Bissember, A.C.; Masi, M.; Evidente, A.; Mathieu, V.; et al. Polygodial and Ophiobolin A Analogues for Covalent Crosslinking of Anticancer Targets. Int. J. Mol. Sci. 2021, 22, 11256. https://doi.org/10.3390/ijms222011256
Maslivetc V, Laguera B, Chandra S, Dasari R, Olivier WJ, Smith JA, Bissember AC, Masi M, Evidente A, Mathieu V, et al. Polygodial and Ophiobolin A Analogues for Covalent Crosslinking of Anticancer Targets. International Journal of Molecular Sciences. 2021; 22(20):11256. https://doi.org/10.3390/ijms222011256
Chicago/Turabian StyleMaslivetc, Vladimir, Breana Laguera, Sunena Chandra, Ramesh Dasari, Wesley J. Olivier, Jason A. Smith, Alex C. Bissember, Marco Masi, Antonio Evidente, Veronique Mathieu, and et al. 2021. "Polygodial and Ophiobolin A Analogues for Covalent Crosslinking of Anticancer Targets" International Journal of Molecular Sciences 22, no. 20: 11256. https://doi.org/10.3390/ijms222011256
APA StyleMaslivetc, V., Laguera, B., Chandra, S., Dasari, R., Olivier, W. J., Smith, J. A., Bissember, A. C., Masi, M., Evidente, A., Mathieu, V., & Kornienko, A. (2021). Polygodial and Ophiobolin A Analogues for Covalent Crosslinking of Anticancer Targets. International Journal of Molecular Sciences, 22(20), 11256. https://doi.org/10.3390/ijms222011256