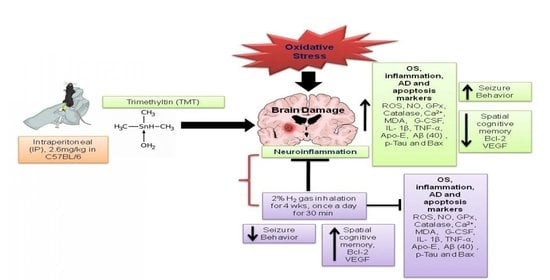
Therapeutic Effects of Hydrogen Gas Inhalation on Trimethyltin-Induced Neurotoxicity and Cognitive Impairment in the C57BL/6 Mice Model
Abstract
:1. Introduction
2. Results
2.1. Effects of H2 Gas Inhalation in TMT-Induced C57BL/6 Mice on Seizure Behavior and Body Weight Measurement
2.2. Effects of H2 Gas Inhalation on TMT-Induced Cognitive Dysfunction
2.3. Effects of H2 Gas Inhalation on TMT-Induced ROS and NO Levels in Mice Serum and Brain
2.4. Effects of H2 Gas Inhalation on TMT-Induced Ca2+ and Malondialdehyde Levels in Mice Serum and Brain
2.5. Effects of H2 Gas Inhalation on Antioxidative Enzyme Activities in Mice Serum and Brain under TMT-Induced Damage
2.6. Effects of H2 Gas Inhalation on Inflammatory Cytokines and Vascular Endothelial Growth Factor in Mice Serum and Brain under TMT-Induced Damage
2.7. Effects of H2 Gas Inhalation on Intracellular Apolipoprotein E Activities in Mice Serum and Brain under TMT-Induced Damage
2.8. Effects of H2 Gas Inhalation on AD-Related Biomarkers in Brain Tissue under TMT-Induced Damage
2.9. Effects of H2 Gas Inhalation on Bcl-2 and Bcl-Associated X Protein in Brain Tissue under TMT-Induced Damage
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Experimental Design
4.3. Seizure Scoring and Body Weight Measurement
4.4. Y-Maze for Behavioral Test
4.5. Detection of the ROS Level
4.6. Detection of the NO Level
4.7. Analysis of Antioxidant Enzyme Activities
4.8. Detection of Ca2+ Activity
4.9. Detection of MDA Activity
4.10. Detection of Inflammatory Cytokines and Vascular Endothelial Growth Factor by Multiplex Assay
4.11. Detection of Total Apo-E by ELISA
4.12. Western Blot Analysis
4.13. Data Management and Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tarozzi, A. Oxidative stress in neurodegenerative diseases: From preclinical studies to clinical applications. J. Clin. Med. 2020, 9, 1223. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, B. Oxidative stress and the pathogenesis of Alzheimer’s disease. Oxid. Med. Cell. Longev. 2013, 2013, 316523. [Google Scholar] [CrossRef] [Green Version]
- Rahman, M.H.; Bajgai, J.; Fadriquela, A.; Sharma, S.; Thi, T.T.; Akter, R.; Goh, S.H.; Kim, C.S.; Lee, K.J. Redox effects of molecular hydrogen and its therapeutic efficacy in the treatment of neurodegenerative diseases. Processes 2021, 9, 308. [Google Scholar] [CrossRef]
- Yu, H.; Wu, J. Amyloid-β: A double agent in Alzheimer’s disease? Biomed. Pharm. 2021, 139, 111575. [Google Scholar] [CrossRef] [PubMed]
- Ji, C.; Sigurdsson, E.M. Current Status of Clinical Trials on Tau Immunotherapies. Drugs 2021, 8, 1135–1152. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Prokop, S.; Giasson, B.I. “Don’t Phos Over Tau”: Recent developments in clinical biomarkers and therapies targeting tau phosphorylation in Alzheimer’s disease and other tauopathies. Mol. Neurodegener. 2021, 16, 37. [Google Scholar] [CrossRef] [PubMed]
- Cenini, G.; Lloret, A.; Cascella, R. Oxidative stress in neurodegenerative diseases: From a mitochondrial point of view. Oxid. Med. Cell. Longev. 2019, 2019, 2105607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sbodio, J.I.; Snyder, S.H.; Paul, B.D. Redox mechanisms in neurodegeneration: From disease outcomes to therapeutic opportunities. Antioxid. Redox. Signal. 2019, 30, 1450–1499. [Google Scholar] [CrossRef]
- Arimon, M.; Takeda, S.; Post, K.; Svirsky, S.; Hyman, B.T.; Berezovska, O. Oxidative stress and lipid peroxidation are upstream of amyloid pathology. Neurobiol. Dis. 2015, 84, 109–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.C.; Han, S.H.; Mook-Jung, I. Peripheral inflammatory biomarkers in Alzheimer’s disease: A brief review. BMB Rep. 2020, 53, 10–19. [Google Scholar] [CrossRef]
- Yuliani, S.; Mustofa; Partadiredja, G. The neuroprotective effects of an ethanolic turmeric (Curcuma longa L.) extract against trimethyltin-induced oxidative stress in rats. Nutr. Neurosci. 2019, 22, 797–804. [Google Scholar] [CrossRef] [PubMed]
- Kandlur, A.; Satyamoorthy, K.; Gangadharan, G. Oxidative stress in cognitive and epigenetic aging: A retrospective glance. Front. Mol. Neurosci. 2020, 13, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geloso, M.C.; Corvino, V.; Michetti, F. Trimethyltin-induced hippocampal degeneration as a tool to investigate neurodegenerative processes. Neurochem. Int. 2011, 58, 729–738. [Google Scholar] [CrossRef] [PubMed]
- Kreyberg, S.; Torvik, A.; Bjorneboe, A.; Wiik-Larsen, W.; Jacobsen, D. Trimethyltin poisoning: Report of a case with postmortem examination. Clin. Neuropathol. 1992, 11, 256–259. [Google Scholar]
- Piacentini, R.; Gangitano, C.; Ceccariglia, S.; del Fa, A.; Azzena, G.B.; Michetti, F.; Grassi, C. Dysregulation of intracellular calcium homeostasis is responsible for neuronal death in an experimental model of selective hippocampal degeneration induced by trimethyltin. J. Neurochem. 2008, 105, 2109–2121. [Google Scholar] [CrossRef]
- Lattanzi, W.; Corvino, V.; di Maria, V.; Michetti, F.; Geloso, M.C. Gene expression profiling as a tool to investigate the molecular machinery activated during hippocampal neurodegeneration induced by trimethyltin (TMT) administration. Int. J. Mol. Sci. 2013, 14, 16817–16835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.S. Magnolol protects against trimethyltin-induced neuronal damage and glial activation in vitro and in vivo. Neurotoxicology 2016, 53, 173–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaur, S.; Nehru, B. Alteration in glutathione homeostasis and oxidative stress during the sequelae of trimethyltin syndrome in rat brain. Biol. Trace Elem. Res. 2013, 153, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Nilsberth, C.; Kostyszyn, B.; Luthman, J. Changes in app, ps1 and other factors related to Alzheimer’s disease pathophysiology after trimethyltin-induced brain lesion in the rat. Neurotox. Res. 2002, 4, 625–636. [Google Scholar] [CrossRef] [PubMed]
- Onaka, Y.; Wada, S.; Yamaguchi, T.; Yoneyama, M.; Ogita, K. Preventive effect of olanzapine on trimethyltin neurotoxicity in mice: Evaluation of hippocampal neuronal loss, microglial activation, and cognitive dysfunction. Glob. Drugs Ther. 2018, 3, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Ogita, K.; Nitta, Y.; Watanabe, M.; Nakatani, Y.; Nishiyama, N.; Sugiyama, C.; Yoneda, Y. In vivo activation of c-jun n-terminal kinase signaling cascade prior to granule cell death induced by trimethyltin in the dentate gyrus of mice. Neuropharmacology 2004, 47, 619–630. [Google Scholar] [CrossRef]
- Harry, G.J.; Tyler, K.; d’Hellencourt, C.L.; Tilson, H.A.; Maier, W.E. Morphological alterations and elevations in tumor necrosis factor-alpha, interleukin (il)-1alpha, and il-6 in mixed glia cultures following exposure to trimethyltin: Modulation by proinflammatory cytokine recombinant proteins and neutralizing antibodies. Toxicol. Appl. Pharmacol. 2002, 180, 205–218. [Google Scholar] [CrossRef]
- Kwon, O.Y.; Lee, S.H. Ishige okamurae suppresses trimethyltin-induced neurodegeneration and glutamate-mediated excitotoxicity by regulating mapks/nrf2/ho-1 antioxidant pathways. Antioxidants 2021, 10, 440. [Google Scholar] [CrossRef] [PubMed]
- Ohsawa, I.; Ishikawa, M.; Takahashi, K.; Watanabe, M.; Nishimaki, K.; Yamagata, K.; Katsura, K.I.; Katayama, Y.; Asoh, S.; Ohta, S.; et al. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat. Med. 2007, 13, 688–694. [Google Scholar] [CrossRef]
- Ichihara, M.; Sobue, S.; Ito, M.; Ito, M.; Hirayama, M.; Ohno, K. Beneficial biological effects and the underlying mechanisms of molecular hydrogen—Comprehensive review of 321 original articles. Med. Gas Res. 2015, 5, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iketani, M.; Ohsawa, I. Molecular hydrogen as a neuroprotective agent. Curr. Neuropharmacol. 2017, 15, 324–331. [Google Scholar] [CrossRef] [Green Version]
- Huang, L. Molecular hydrogen: A therapeutic antioxidant and beyond. Med. Gas Res. 2016, 6, 219–222. [Google Scholar] [CrossRef] [Green Version]
- Guo, Q.; Yin, X.; Qiao, M.; Jia, Y.; Chen, D.; Shao, J.; Lebaron, T.W.; Gao, Y.; Shi, H.; Jia, B. Hydrogen-rich water ameliorates autistic-like behavioral abnormalities in valproic acid-treated adolescent mice offspring. Front. Behav. Neurosci. 2018, 12, 170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bajgai, J.; Lee, K.J.; Rahman, M.H.; Fadriquela, A.; Kim, C.S. Role of molecular hydrogen in skin diseases and its impact in beauty. Curr. Pharm. Des. 2021, 27, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Imai, K.; Kotani, T.; Tsuda, H.; Mano, Y.; Nakano, T.; Ushida, T.; Li, H.; Miki, R.; Sumigama, S.; Iwase, A. Neuroprotective potential of molecular hydrogen against perinatal brain injury via suppression of activated microglia. Free Radic. Biol. Med. 2016, 91, 154–163. [Google Scholar] [CrossRef]
- Ono, H.; Nishijima, Y.; Ohta, S.; Sakamoto, M.; Kinone, K.; Horikosi, T.; Takanami, H. Hydrogen gas inhalation treatment in acute cerebral infarction: A randomized controlled clinical study on safety and neuroprotection. J. Stroke Cerebrovasc. Dis. 2017, 26, 2587–2594. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Li, J.; Liu, Q.; Yang, R.; Zhang, J.H.; Cao, Y.P.; Sun, X.J. Hydrogen-rich saline reduces oxidative stress and inflammation by inhibit of jnk and nf-kappab activation in a rat model of amyloid-beta-induced Alzheimer’s disease. Neurosci. Lett. 2011, 491, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Huang, C.S.; Inoue, T.; Yamashita, T.; Ishida, T.; Kang, K.M.; Nakao, A. Drinking hydrogen water ameliorated cognitive impairment in senescence-accelerated mice. J. Clin. Biochem. Nutr. 2010, 46, 269–276. [Google Scholar] [CrossRef] [Green Version]
- Yoritaka, A.; Takanashi, M.; Hirayama, M.; Nakahara, T.; Ohta, S.; Hattori, N. Pilot study of h2 therapy in Parkinson’s disease: A randomized double-blind placebo-controlled trial. Mov. Disord. 2013, 28, 836–839. [Google Scholar] [CrossRef] [PubMed]
- Iuchi, K.; Imoto, A.; Kamimura, N.; Nishimaki, K.; Ichimiya, H.; Yokota, T.; Ohta, S. Molecular hydrogen regulates gene expression by modifying the free radical chain reaction-dependent generation of oxidized phospholipid mediators. Sci. Rep. 2016, 6, 18971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, T.; Zhang, M.; Du, H.; Ablimit, A.; Ye, R.; Lǚ, M.; Chang, X.; Zhao, Q.; Wang, Y.; Qin, Q. Hydrogen rich water ameliorate Trimethyltin induced spatial learning and memory impairment by regulation of Siah-1. Res. Square. 2020, 1–8. [Google Scholar] [CrossRef]
- Husain, M.A.; Laurent, B.; Plourde, M. Apoe and Alzheimer’s disease: From lipid transport to physiopathology and therapeutics. Front. Neurosci. 2021, 15, 630502. [Google Scholar] [CrossRef] [PubMed]
- Barnham, K.J.; Masters, C.L.; Bush, A.I. Neurodegenerative diseases and oxidative stress. Nat. Rev. Drug Discov. 2004, 3, 205–214. [Google Scholar] [CrossRef]
- Dohi, K.; Satoh, K.; Miyamoto, K.; Momma, S.; Fukuda, K.; Higuchi, R.; Banks, W.A. Molecular hydrogen in the treatment of acute and chronic neurological conditions: Mechanisms of protection and routes of administration. J. Clin. Biochem. Nutr. 2017, 61, 1–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, B.; Xie, Y.; Chen, J.; Xue, J.; Zhang, X.; Zhao, M.; Qin, S. Protective effect of molecular hydrogen following different routes of administration on D-Galactose-induced aging mice. J. Inflamm. Res. 2021, 14, 5541. [Google Scholar] [CrossRef]
- Manaenko, A.; Lekic, T.; Ma, Q.; Zhang, J.H.; Tang, J. Hydrogen inhalation ameliorated mast cell mediated brain injury after ICH in mice. Crit. Care. Med. 2013, 41, 1266–1275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, B.; Li, Y.; Dai, W.; Wu, A.; Wu, H.; Mao, D. Hydrogen-rich saline alleviates early brain injury through regulating of ER stress and autophagy after experimental subarachnoid hemorrhage. Acta Cir. Bras. 2021, 36, e360804. [Google Scholar] [CrossRef]
- Ning, K.; Liu, W.W.; Huang, J.L.; Lu, H.T.; Sun, X.J. Effects of hydrogen on polarization of macrophages and microglia in a stroke model. Med. Gas. Res. 2018, 8, 154–159. [Google Scholar]
- Kumagai, K.; Toyooka, T.; Takeuchi, S.; Otani, N.; Wada, K.; Tomiyama, A.; Mori, K. Hydrogen gas inhalation improves delayed brain injury by alleviating early brain injury after experimental subarachnoid hemorrhage. Sci Rep. 2020, 10, 12319. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Feng, J.; Lian, N.; Yang, M.; Xie, K.; Wang, G.; Yu, Y. Hydrogen gas alleviates blood-brain barrier impairment and cognitive dysfunction of septic mice in an Nrf2-dependent pathway. Int. Immunopharmacol. 2020, 85, 106585. [Google Scholar] [CrossRef]
- Nagata, K.; Nakashima-Kamimura, N.; Mikami, T.; Ohsawa, I.; Ohta, S. Consumption of molecular hydrogen prevents the stressinduced impairments in hippocampus-dependent learning tasks during chronic physical restraint in mice. Neuropsychopharmacology 2009, 34, 501–508. [Google Scholar] [CrossRef] [Green Version]
- Shao, A.; Wu, H.; Hong, Y.; Tu, S.; Sun, X.; Wu, Q.; Sheng, J. Hydrogen-rich saline attenuated subarachnoid hemorrhage-induced early brain injury in rats by suppressing inflammatory response: Possible involvement of NF-κB pathway and NLRP3 inflammasome. Mol. Neurobiol. 2016, 53, 3462–3476. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Chen, P.; Zhou, S.; Yan, X.; Zhang, J.; Sun, X.; Yu, W. Hydrogen-rich saline attenuated neuropathic pain by reducing oxidative stress. Can. J. Neurol. Sci. 2013, 40, 857–863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, C.; Kim, S.R. Linking oxidative stress and proteinopathy in Alzheimer’s disease. Antioxidants 2021, 10, 1231. [Google Scholar] [CrossRef] [PubMed]
- Kent, S.A.; Spires-Jones, T.L.; Durrant, C.S. The physiological roles of tau and Aβ: Implications for Alzheimer’s disease pathology and therapeutics. Acta Neuropathol. 2020, 140, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Shin, E.J.; Suh, S.K.; Lim, Y.K.; Jhoo, W.K.; Hjelle, O.P.; Ottersen, O.P.; Shin, C.Y.; Ko, K.H.; Kim, W.K.; Kim, D.S.; et al. Ascorbate attenuates trimethyltin-induced oxidative burden and neuronal degeneration in the rat hippocampus by maintaining glutathione homeostasis. Neuroscience 2005, 133, 715–727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huong, N.Q.; Nakamura, Y.; Kuramoto, N.; Yoneyama, M.; Nagashima, R.; Shiba, T.; Yamaguchi, T.; Hasebe, S.; Ogita, K. Indomethacin ameliorates trimethyltin-induced neuronal damage in vivo by attenuating oxidative stress in the dentate gyrus of mice. Biol. Pharm. Bull. 2011, 34, 1856–1863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qu, M.; Zhou, Z.; Chen, C.; Li, M.; Pei, L.; Chu, F.; Yang, J.; Wang, Y.; Li, L.; Liu, C.; et al. Lycopene protects against trimethyltin-induced neurotoxicity in primary cultured rat hippocampal neurons by inhibiting the mitochondrial apoptotic pathway. Neurochem. Int. 2011, 59, 1095–1103. [Google Scholar] [CrossRef]
- Ohno, K.; Ito, M.; Ichihara, M.; Ito, M. Molecular hydrogen as an emerging therapeutic medical gas for neurodegenerative and other diseases. Oxid. Med. Cell. Longev. 2012, 2012, 353152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magi, S.; Castaldo, P.; Macri, M.L.; Maiolino, M.; Matteucci, A.; Bastioli, G.; Gratteri, S.; Amoroso, S.; Lariccia, V. Intracellular calcium dysregulation: Implications for Alzheimer’s disease. Biomed. Res. Int. 2016, 2016, 6701324. [Google Scholar] [CrossRef] [Green Version]
- Berridge, M.J. Dysregulation of neural calcium signaling in Alzheimer disease, bipolar disorder and schizophrenia. Prion 2013, 7, 2–13. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Zhu, Y.; Xi, X. Anti-inflammatory and antitumor action of hydrogen via reactive oxygen species. Oncol. Lett. 2018, 16, 2771–2776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du Yihong, S.Y.; Yang, R.; Wang, L.; Cai, M. Mechanisms of neuroinflammation in mild cognitive impairment. Chin. J. Tissue Eng. Res. 2021, 25, 4743. [Google Scholar]
- Kinney, J.W.; Bemiller, S.M.; Murtishaw, A.S.; Leisgang, A.M.; Salazar, A.M.; Lamb, B.T. Inflammation as a central mechanism in Alzheimer’s disease. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2018, 4, 575–590. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.J.; Kim, Y.S. Trimethyltin-induced microglial activation via nadph oxidase and mapks pathway in bv-2 microglial cells. Mediat. Inflamm. 2015, 2015, 729509. [Google Scholar] [CrossRef] [Green Version]
- Taipa, R.; das Neves, S.P.; Sousa, A.L.; Fernandes, J.; Pinto, C.; Correia, A.P.; Santos, E.; Pinto, P.S.; Carneiro, P.; Costa, P.; et al. Proinflammatory and anti-inflammatory cytokines in the csf of patients with Alzheimer’s disease and their correlation with cognitive decline. Neurobiol. Aging 2019, 76, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Garcia, K.O.; Ornellas, F.L.; Martin, P.K.; Patti, C.L.; Mello, L.E.; Frussa-Filho, R.; Han, S.W.; Longo, B.M. Therapeutic effects of the transplantation of vegf overexpressing bone marrow mesenchymal stem cells in the hippocampus of murine model of Alzheimer’s disease. Front. Aging Neurosci. 2014, 6, 30. [Google Scholar] [CrossRef] [Green Version]
- Kura, B.; Bagchi, A.K.; Singal, P.K.; Barancik, M.; LeBaron, T.W.; Valachova, K.; Slezák, J. Molecular hydrogen: Potential in mitigating oxidative-stress-induced radiation injury. Can. J. Physiol. Pharmacol. 2019, 97, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.T.; Shi, Q.Q.; Zhang, L.; Yue, C.P.; He, Z.J.; Li, X.X.; He, Q.J.; Liu, Q.; Du, X.B. Hydrogen-rich water ameliorates neuropathological impairments in a mouse model of Alzheimer’s disease through reducing neuroinflammation and modulating intestinal microbiota. Neural Regen. Res. 2021, 17, 409–417. [Google Scholar]
- Smith, G.E.; Bohac, D.L.; Waring, S.C.; Kokmen, E.; Tangalos, E.G.; Ivnik, R.J.; Petersen, R.C. Apolipoprotein e genotype influences cognitive ‘phenotype’ in patients with Alzheimer’s disease but not in healthy control subjects. Neurology 1998, 50, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Yamada, K.; Liddelow, S.A.; Smith, S.T.; Zhao, L.; Luo, W.; Tsai, R.M.; Spina, S.; Grinberg, L.T.; Rojas, J.C.; et al. Apoe4 markedly exacerbates tau-mediated neurodegeneration in a mouse model of tauopathy. Nature 2017, 549, 523–527. [Google Scholar] [CrossRef]
- Park, S.K.; Kang, J.Y.; Kim, J.M.; Yoo, S.K.; Han, H.J.; Chung, D.H.; Kim, D.O.; Kim, G.H.; Heo, H.J. Fucoidan-rich substances from ecklonia cava improve trimethyltin-induced cognitive dysfunction via down-regulation of amyloid beta production/tau hyperphosphorylation. Mar. Drugs 2019, 17, 591. [Google Scholar] [CrossRef] [Green Version]
- Zhuang, Z.; Zhou, M.L.; You, W.C.; Zhu, L.; Ma, C.Y.; Sun, X.J.; Shi, J.X. Hydrogen-rich saline alleviates early brain injury via reducing oxidative stress and brain edema following experimental subarachnoid hemorrhage in rabbits. BMC Neurosci. 2012, 13, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wen, D.; Hui, R.; Wang, J.; Shen, X.; Xie, B.; Gong, M.; Ma, C. Effects of molecular hydrogen on methamphetamine-induced neurotoxicity and spatial memory impairment. Front. Pharmacol. 2019, 10, 823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.; Yang, M.; Kim, S.H.; Kim, J.C.; Wang, H.; Shin, T.; Moon, C. Possible role of the glycogen synthase kinase-3 signaling pathway in trimethyltin-induced hippocampal neurodegeneration in mice. PLoS ONE 2013, 8, e70356. [Google Scholar] [CrossRef] [PubMed]
- Fabrizi, C.; Pompili, E.; Somma, F.; de Vito, S.; Ciraci, V.; Artico, M.; Fumagalli, L. Lithium limits trimethyltin-induced cytotoxicity and proinflammatory response in microglia without affecting the concurrent autophagy impairment. J. Appl. Toxicol. 2017, 37, 207–213. [Google Scholar] [CrossRef] [PubMed] [Green Version]











Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, E.-S.; Bajgai, J.; You, I.-S.; Rahman, M.H.; Fadriquela, A.; Sharma, S.; Kwon, H.-U.; Lee, S.-Y.; Kim, C.-S.; Lee, K.-J. Therapeutic Effects of Hydrogen Gas Inhalation on Trimethyltin-Induced Neurotoxicity and Cognitive Impairment in the C57BL/6 Mice Model. Int. J. Mol. Sci. 2021, 22, 13313. https://doi.org/10.3390/ijms222413313
Jeong E-S, Bajgai J, You I-S, Rahman MH, Fadriquela A, Sharma S, Kwon H-U, Lee S-Y, Kim C-S, Lee K-J. Therapeutic Effects of Hydrogen Gas Inhalation on Trimethyltin-Induced Neurotoxicity and Cognitive Impairment in the C57BL/6 Mice Model. International Journal of Molecular Sciences. 2021; 22(24):13313. https://doi.org/10.3390/ijms222413313
Chicago/Turabian StyleJeong, Eun-Sook, Johny Bajgai, In-Soo You, Md. Habibur Rahman, Ailyn Fadriquela, Subham Sharma, Hwang-Un Kwon, So-Yeon Lee, Cheol-Su Kim, and Kyu-Jae Lee. 2021. "Therapeutic Effects of Hydrogen Gas Inhalation on Trimethyltin-Induced Neurotoxicity and Cognitive Impairment in the C57BL/6 Mice Model" International Journal of Molecular Sciences 22, no. 24: 13313. https://doi.org/10.3390/ijms222413313
APA StyleJeong, E. -S., Bajgai, J., You, I. -S., Rahman, M. H., Fadriquela, A., Sharma, S., Kwon, H. -U., Lee, S. -Y., Kim, C. -S., & Lee, K. -J. (2021). Therapeutic Effects of Hydrogen Gas Inhalation on Trimethyltin-Induced Neurotoxicity and Cognitive Impairment in the C57BL/6 Mice Model. International Journal of Molecular Sciences, 22(24), 13313. https://doi.org/10.3390/ijms222413313