NLRP3 Inflammasome: A New Pharmacological Target for Reducing Testicular Damage Associated with Varicocele
Abstract
:1. Introduction
2. Results
2.1. Effects of Se, PDRN and Their Association on Testis Weight
2.2. Effects of Se, PDRN and Their Association on Testosterone Levels
2.3. Effects of Se, PDRN and Their Association on Glutathione (GSH) and Glutathione Peroxidase (GPx)
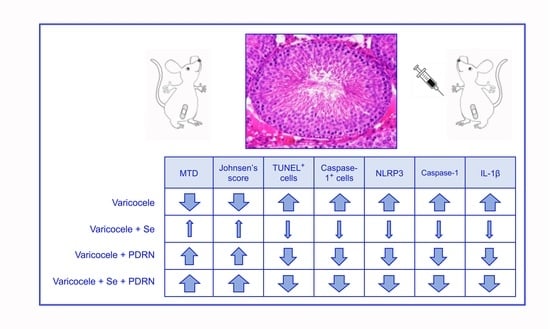
2.4. Effects of Se, PDRN and Their Association on NLRP3, IL-1β and Caspase-1 Expression
2.5. Administration of Se, PDRN and Their Association Counteracts Testes Changes
2.6. Administration of Se, PDRN and Their Association Modulates Sperm Cells Apoptosis
2.7. Administration of Se, PDRN and Their Association Modulates Caspase-1 Activity
2.8. Administration of Se, PDRN and Their Association Counteracts Ultrastructural Testes Changes
3. Discussion
4. Material and Methods
4.1. Experimental Protocol
4.2. Determination of Testosterone
4.3. Analysis of Cytokine Expressions through Real-Time PCR
4.4. Evaluation of NLRP3 and IL-1β Levels in Testis
4.5. Determination of Glutathion (GSH) and Glutathion Peroxidase (GPx)
4.6. Histological Evaluation
4.7. Evaluation of Apoptosis with Terminal Deoxynucleotidyl Transferase dUTP Nick End Labeling (TUNEL) Assay
4.8. Immunohistochemistry for Caspase-1
4.9. Morphometric Evaluation
4.10. Scanning Electron Microscopy
4.11. Drugs
4.12. Statistical Analysis
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cho, C.L.; Esteves, S.C.; Agarwal, A. Novel insights into the pathophysiology of varicocele and its association with reactive oxygen species and sperm DNA fragmentation. Asian J. Androl. 2016, 18, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Alsaikhan, B.; Alrabeeah, K.; Delouya, G.; Zini, A. Epidemiology of varicocele. Asian J. Androl. 2016, 18, 179–181. [Google Scholar] [CrossRef] [PubMed]
- Hassanin, A.M.; Ahmed, H.H.; Kaddah, A.N. A global view of the pathophysiology of varicocele. Andrology 2018, 6, 654–661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pastuszak, A.W.; Wang, R. Varicocele and testicular function. Asian J. Androl. 2015, 17, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Barati, E.; Nikzad, H.; Karimian, M. Oxidative stress and male infertility: Current knowledge of pathophysiology and role of antioxidant therapy in disease management. Cell. Mol. Life. Sci. 2020, 77, 93–113. [Google Scholar] [CrossRef]
- Aitken, R.J.; De Iuliis, G.N.; Finnie, J.M.; Hedges, A.; McLachlan, R. Analysis of the relationships between oxidative stress, DNA damage and sperm vitality in a patient population: Development of diagnostic criteria. Hum. Reprod. 2010, 25, 2415–2426. [Google Scholar] [CrossRef] [Green Version]
- Walczak-Jedrzejowska, R.; Wolski, J.K.; Slowikowska-Hilczer, J. The role of oxidative stress and antioxidants in male fertility. Cent. Eur. J. Urol. 2013, 66, 60–67. [Google Scholar] [CrossRef] [Green Version]
- Schroder, K.; Tschopp, J. The inflammasomes. Cell 2010, 140, 821–832. [Google Scholar] [CrossRef] [Green Version]
- Martinon, F.; Burns, K.; Tschopp, J. The inflammasome: A molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol. Cell 2002, 10, 417–426. [Google Scholar] [CrossRef]
- Kayagaki, N.; Warming, S.; Lamkanfi, M.; Vande Walle, L.; Louie, S.; Dong, J.; Newton, K.; Qu, Y.; Liu, J.; Heldens, S.; et al. Non-canonical inflammasome activation targets caspase-11. Nature 2011, 479, 117–121. [Google Scholar] [CrossRef]
- Lu, Y.; Xu, S.; Chen, H.; He, M.; Deng, Y.; Cao, Z.; Pi, H.; Chen, C.; Li, M.; Ma, Q.; et al. CdSe/ZnS quantum dots induce hepatocyte pyroptosis and liver inflammation via NLRP3 inflammasome activation. Biomaterials 2016, 90, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Hajipour, E.; Mashayekhi, F.J.; Mosayebi, G.; Baazm, M.; Zendedel, A. Resveratrol decreases apoptosis and NLRP3 complex expressions in experimental varicocele rat model. Iran J. Basic Med. Sci. 2018, 21, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Baazm, M.; Ghafarizadeh, A.A.; Noshad Kamran, A.R.; Beyer, C.; Zendedel, A. Presence of The NLRP3 Inflammasome Components in Semen of Varicocele Patients. Int. J. Fertil. Steril. 2020, 14, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Cho, C.L.; Esteves, S.C.; Agarwal, A. Indications and outcomes of varicocele repair. Panminerva Med. 2019, 61, 152–163. [Google Scholar] [CrossRef] [PubMed]
- Grasso, M.; Lania, C.; Castelli, M.; Galli, L.; Franzoso, F.; Rigatti, P. Low-grade left varicocele in patients over 30 years old: The effect of spermatic vein ligation on fertility. B.J.U. Int. 2000, 85, 305–307. [Google Scholar] [CrossRef]
- Jensen, C.F.S.; Østergren, P.; Dupree, J.M.; Ohl, D.A.; Sønksen, J.; Fode, M. Varicocele and male infertility. Nat. Rev. Urol. 2017, 14, 523–533. [Google Scholar] [CrossRef]
- Minutoli, L.; Arena, S.; Bonvissuto, G.; Bitto, A.; Polito, F.; Irrera, N.; Arena, F.; Fragalà, E.; Romeo, C.; Nicotina, P.A.; et al. Activation of adenosine A2A receptors by polydeoxyribonucleotide increases vascular endothelial growth factor and protects against testicular damage induced by experimental varicocele in rats. Fertil. Steril. 2011, 95, 1510–1513. [Google Scholar] [CrossRef]
- Arena, S.; Minutoli, L.; Arena, F.; Nicotina, P.A.; Romeo, C.; Squadrito, F.; Altavilla, D.; Morgia, G.; Magno, C. Polydeoxyribonucleotide administration improves the intra-testicular vascularization in rat experimental varicocele. Fertil. Steril. 2012, 97, 165–168. [Google Scholar] [CrossRef]
- Chen, Y.W.; Niu, Y.H.; Wang, D.Q.; Li, H.; Pokhrel, G.; Xu, H.; Wang, T.; Wang, S.G.; Liu, J.H. Effect of adjuvant drug therapy after varicocelectomy on fertility outcome in males with varicocele-associated infertility: Systematic review and meta-analysis. Andrologia 2018, 50, e13070. [Google Scholar] [CrossRef]
- Busetto, G.M.; Agarwal, A.; Virmani, A.; Antonini, G.; Ragonesi, G.; Del Giudice, F.; Micic, S.; Gentile, V.; De Berardinis, E. Effect of metabolic and antioxidant supplementation on sperm parameters in oligo-astheno-teratozoospermia, with and without varicocele: A double-blind placebo-controlled study. Andrologia 2018, 50. [Google Scholar] [CrossRef] [Green Version]
- Kefer, J.C.; Agarwal, A.; Sabanegh, E. Role of antioxidants in the treatment of male infertility. Int. J. Urol. 2009, 16, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Razi, M.; Tavalaee, M.; Sarrafzadeh-Rezaei, F.; Moazamian, A.; Gharagozloo, P.; Drevet, J.R.; Nasr-Eshafani, M.H. Varicocele and Oxidative Stress: New Perspectives from Animal and Human Studies. Andrology 2020. [Google Scholar] [CrossRef] [PubMed]
- Antonuccio, P.; Micali, A.; Puzzolo, D.; Romeo, C.; Vermiglio, G.; Squadrito, V.; Freni, J.; Pallio, G.; Trichilo, V.; Righi, M.; et al. Nutraceutical Effects of Lycopene in Experimental Varicocele: An “In Vivo” Model to Study Male Infertility. Nutrients 2020, 12, 1536. [Google Scholar] [CrossRef] [PubMed]
- Minutoli, L.; Marini, H. Selenium and prostate health: A new possible nutraceutical challenge. Selenium sources, functions and health effects. In Public Health in the 21st Century, Nutrition and Diet Research Progressed; Aomori, C., Hokkaido, M., Eds.; Nova Science Publishers Inc.: Hauppauge, NY, USA, 2012; pp. 209–218. ISBN 978-1-61942-061-8. [Google Scholar]
- Minutoli, L.; Squadrito, F.; Altavilla, D.; Marini, H. Therapy with Selenium Cocktails and Co-use of Lycopene and Selenium. In Selenium: Chemistry, Analysis, Function and Effects (Food and Nutritional Components in Focus); Preedy, V.R., Ed.; Royal Society of Chemistry: London, UK, 2015; Volume 9, pp. 363–376. [Google Scholar] [CrossRef]
- Huang, H.; Jiao, X.Y.; Xu, Y.M.; Han, Q.; Jiao, W.Y.; Liu, Y.Y.; Li, S.; Teng, X. Dietary selenium supplementation alleviates immune toxicity in the hearts of chickens with lead-added drinking water. Avian Pathol. 2019, 48, 230e7. [Google Scholar] [CrossRef] [PubMed]
- Benvenga, S.; Micali, A.; Pallio, G.; Vita, R.; Malta, C.; Puzzolo, D.; Irrera, N.; Squadrito, F.; Altavilla, D.; Minutoli, L. Effects of Myo-inositol Alone and in Combination with Seleno-L-methionine on Cadmium-Induced Testicular Damage in Mice. Curr. Mol. Pharmacol. 2019, 12, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Li, X.; Wang, Z.; Lin, X.; Tian, Y.; Zhao, Q.; Zheng, P. Anti-inflammatory effect of selenium on lead-induced testicular inflammation by inhibiting NLRP3 inflammasome activation in chickens. Theriogenology 2020, 155, 139–149. [Google Scholar] [CrossRef]
- Taghizadeh, L.; Eidi, A.; Mortazavi, P.; Rohani, A.H. Effect of selenium on testicular damage induced by varicocele in adult male Wistar rats. J. Trace Elem. Med. Biol. 2017, 44, 177–185. [Google Scholar] [CrossRef]
- Ardestani Zadeh, A.; Arab, D.; Kia, N.S.; Heshmati, S.; Amirkhalili, S.N. The role of Vitamin E—Selenium—Folic Acid Supplementation in Improving Sperm Parameters After Varicocelectomy: A Randomized Clinical Trial. Urol. J. 2019, 16, 495–500. [Google Scholar] [CrossRef]
- Altavilla, D.; Bitto, A.; Polito, F.; Marini, H.; Minutoli, L.; Di Stefano, V.; Irrera, N.; Cattarini, G.; Squadrito, F. Polydeoxyribonucleotide (PDRN): A safe approach to induce therapeutic angiogenesis in peripheral artery occlusive disease and in diabetic foot ulcers. Cardiovasc. Hematol. Agents Med. Chem. 2009, 7, 313–321. [Google Scholar] [CrossRef]
- Squadrito, F.; Micali, A.; Rinaldi, M.; Irrera, N.; Marini, H.; Puzzolo, D.; Pisani, A.; Lorenzini, C.; Valenti, A.; Laurà, R.; et al. Polydeoxyribonucleotide, an Adenosine-A2A Receptor Agonist, Preserves Blood Testis Barrier from Cadmium-Induced Injury. Front. Pharmacol. 2017, 7, 537. [Google Scholar] [CrossRef] [Green Version]
- Minutoli, L.; Arena, S.; Antonuccio, P.; Romeo, C.; Bitto, A.; Magno, C.; Rinaldi, M.; Micali, A.; Irrera, N.; Pizzino, G.; et al. Role of Inhibitors of Apoptosis Proteins in Testicular Function and Male Fertility: Effects of Polydeoxyribonucleotide Administration in Experimental Varicocele. Biomed. Res. Int. 2015, 2015, 248976. [Google Scholar] [CrossRef] [PubMed]
- Boivin, J.; Bunting, L.; Collins, J.A.; Nygren, K.G. International estimates of infertility prevalence and treatment-seeking: Potential need and demand for infertility medical care. Hum. Reprod. 2007, 22, 1506–1512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leaver, R.B. Male infertility: An overview of causes and treatment options. Br. J. Nurs. 2016, 25, S35–S40. [Google Scholar] [CrossRef] [PubMed]
- Krausz, C.; Riera-Escamilla, A. Genetics of male infertility. Nat. Rev. Urol. 2018, 15, 369–384. [Google Scholar] [CrossRef]
- Fainberg, J.; Kashanian, J.A. Recent advances in understanding and managing male infertility. F1000Res. 2019, 8. [Google Scholar] [CrossRef]
- Al-Said, S.; Al-Naimi, A.; Al-Ansari, A.; Younis, N.; Shamsodini, A.; A-sadiq, K.; Shokeir, A.A. Varicocelectomy for male infertility: A comparative study of open, laparoscopic and microsurgical approaches. J. Urol. 2008, 180, 266–270. [Google Scholar] [CrossRef]
- Mohamed, E.E.; Gawish, M.; Mohamed, A. Semen parameters and pregnancy rates after microsurgical varicocelectomy in primary versus secondary infertile men. Hum Fertil. 2017, 20, 293–296. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, X.; Wang, F.; Lin, Q.; Wang, W. Effects of Morinda officinalis Polysaccharide on Experimental Varicocele Rats. Evid. Based Complement Alternat. Med. 2016, 2016, 5365291. [Google Scholar] [CrossRef] [Green Version]
- Mendes, T.B.; Paccola, C.C.; De Oliveira Neves, F.M.; Simas, J.N.; Da Costa Vaz, A.; Cabral, R.E.; Vendramini, V.; Miraglia, S.M. Resveratrol improves reproductive parameters of adult rats varicocelized in peripuberty. Reproduction 2016, 152, 23–35. [Google Scholar] [CrossRef] [Green Version]
- Missassi, G.; Dos Santos Borges, C.; De Lima Rosa, J.; Villela, E.; Silva, P.; Da Cunha Martins, A., Jr.; Barbosa, F., Jr.; De Grava Kempinas, W. Chrysin Administration Protects against Oxidative Damage in Varicocele-Induced Adult Rats. Oxid. Med. Cell. Longev. 2017, 2017, 2172981. [Google Scholar] [CrossRef]
- Asadi, N.; Kheradmand, A.; Gholami, M.; Moradi, F.H. Effect of ghrelin on the biochemical and histopathology parameters and spermatogenesis cycle following experimental varicocele in rat. Andrologia 2018, 50, e13106. [Google Scholar] [CrossRef] [PubMed]
- Mazhari, S.; Razi, M.; Sadrkhanlou, R. Silymarin and celecoxib ameliorate experimental varicocele-induced pathogenesis: Evidences for oxidative stress and inflammation inhibition. Int. Urol. Nephrol. 2018, 50, 1039–1052. [Google Scholar] [CrossRef] [PubMed]
- Hassani-Bafrani, H.; Najaran, H.; Razi, M.; Rashtbari, H. Berberine ameliorates experimental varicocele-induced damages at testis and sperm levels; evidences for oxidative stress and inflammation. Andrologia 2019, 51, e13179. [Google Scholar] [CrossRef] [PubMed]
- Karna, K.K.; Choi, B.R.; Kim, M.J.; Kim, H.K.; Park, J.K. The Effect of Schisandra chinensis Baillon on Cross-Talk between Oxidative Stress, Endoplasmic Reticulum Stress, and Mitochondrial Signaling Pathway in Testes of Varicocele-Induced SD Rat. Int. J. Mol. Sci. 2019, 20, 5785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abderrazak, A.; Syrovets, T.; Couchie, D.; El Hadri, K.; Friguet, B.; Simmet, T.; Rouis, M. NLRP3 inflammasome: From a danger signal sensor to a regulatory node of oxidative stress and inflammatory diseases. Redox Biol. 2015, 4, 296–307. [Google Scholar] [CrossRef]
- Tschopp, J.; Schroder, K. NLRP3 inflammasome activation: The convergence of multiple signalling pathways on ROS production? Nat. Rev. Immunol. 2010, 10, 210–215. [Google Scholar] [CrossRef]
- Minutoli, L.; Antonuccio, P.; Irrera, N.; Rinaldi, M.; Bitto, A.; Marini, H.; Pizzino, G.; Romeo, C.; Pisani, A.; Santoro, G.; et al. NLRP3 Inflammasome Involvement in the Organ Damage and Impaired Spermatogenesis Induced by Testicular Ischemia and Reperfusion in Mice. J. Pharmacol. Exp. Ther. 2015, 355, 370–380. [Google Scholar] [CrossRef] [Green Version]
- Bazrafkan, M.; Nikmehr, B.; Shahverdi, A.; Hosseini, S.R.; Hassani, F.; Poorhassan, M.; Mokhtari, T.; Abolhassani, F.; Choobineh, H.; Beyer, C.; et al. Lipid Peroxidation and Its Role in the Expression of NLRP1a and NLRP3 Genes in Testicular Tissue of Male Rats: A Model of Spinal Cord Injury. Iran Biomed. J. 2018, 22, 151–159. [Google Scholar] [CrossRef]
- Khodamoradi, K.; Amini-Khoei, H.; Khosravizadeh, Z.; Hosseini, S.R.; Dehpour, A.R.; Hassanzadeh, G. Oxidative stress, inflammatory reactions and apoptosis mediated the negative effect of chronic stress induced by maternal separation on the reproductive system in male mice. Reprod. Biol. 2019, 19, 340–348. [Google Scholar] [CrossRef]
- Huang, H.; Chen, J.; Sun, Q.; Liu, Y.; Tang, Y.; Teng, X. NLRP3 inflammasome is involved in the mechanism of mitigative effect of selenium on lead-induced inflammatory damage in chicken kidneys. Environ. Sci. Pollut. Res. Int. 2020. [Google Scholar] [CrossRef]
- Rho, J.H.; Ko, I.G.; Jin, J.J.; Hwang, L.; Kim, S.H.; Chung, J.Y.; Hwang, T.J.; Han, J.H. Polydeoxyribonucleotide Ameliorates Inflammation and Apoptosis in Achilles Tendon-Injury Rats. Int. Neurourol. J. 2020, 24 (Suppl. 2), 79–87. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.E.; Ko, I.G.; Jin, J.J.; Hwang, L.; Kim, C.J.; Kim, S.H.; Han, J.H.; Jeon, J.W. Polydeoxyribonucleotide Exerts Therapeutic Effect by Increasing VEGF and Inhibiting Inflammatory Cytokines in Ischemic Colitis Rats. Biomed. Res. Int. 2020, 2020, 2169083. [Google Scholar] [CrossRef] [PubMed]
- Irrera, N.; Bitto, A.; Vaccaro, M.; Mannino, F.; Squadrito, V.; Pallio, G.; Arcoraci, V.; Minutoli, L.; Ieni, A.; Lentini, M.; et al. PDRN, a Bioactive Natural Compound, Ameliorates Imiquimod-Induced Psoriasis through NF-κB Pathway Inhibition and Wnt/β-Catenin Signaling Modulation. Int. J. Mol. Sci. 2020, 21, 1215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marini, H.R.; Puzzolo, D.; Micali, A.; Adamo, E.B.; Irrera, N.; Pisani, A.; Pallio, G.; Trichilo, V.; Malta, C.; Bitto, A.; et al. Neuroprotective Effects of Polydeoxyribonucleotide in a Murine Model of Cadmium Toxicity. Oxid. Med. Cell. Longev. 2018, 2018, 4285694. [Google Scholar] [CrossRef] [PubMed]
- Pizzino, G.; Irrera, N.; Galfo, F.; Oteri, G.; Atteritano, M.; Pallio, G.; Mannino, F.; D’Amore, A.; Pellegrino, E.; Aliquò, F.; et al. Adenosine Receptor Stimulation Improves Glucocorticoid-Induced Osteoporosis in a Rat Model. Front. Pharmacol. 2017, 8, 558. [Google Scholar] [CrossRef] [Green Version]
- Pallio, G.; Bitto, A.; Pizzino, G.; Galfo, F.; Irrera, N.; Squadrito, F.; Squadrito, G.; Pallio, S.; Anastasi, G.P.; Cutroneo, G.; et al. Adenosine Receptor Stimulation by Polydeoxyribonucleotide Improves Tissue Repair and Symptomology in Experimental Colitis. Front. Pharmacol. 2016, 7, 273. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.J.; Kim, M.J.; Kweon, D.K.; Lim, S.T.; Lee, S.J. Polydeoxyribonucleotide Activates Mitochondrial Biogenesis but Reduces MMP-1 Activity and Melanin Biosynthesis in Cultured Skin Cells. Appl. Biochem. Biotechnol. 2020, 191, 540–554. [Google Scholar] [CrossRef]
- Squadrito, F.; Bitto, A.; Altavilla, D.; Arcoraci, V.; De Caridi, G.; De Feo, M.E.; Corrao, S.; Pallio, G.; Sterrantino, C.; Minutoli, L.; et al. The effect of PDRN, an adenosine receptor A2A agonist, on the healing of chronic diabetic foot ulcers: Results of a clinical trial. J. Clin. Endocrinol. Metab. 2014, 99, E746–E753, Erratum in 2015, 100, 763. [Google Scholar]
- Kim, M.S.; Cho, R.K.; In, Y. The efficacy and safety of polydeoxyribonucleotide for the treatment of knee osteoarthritis: Systematic review and meta-analysis of randomized controlled trials. Medicine 2019, 98, e17386. [Google Scholar] [CrossRef]
- Gugliandolo, E.; D’Amico, R.; Cordaro, M.; Fusco, R.; Siracusa, R.; Crupi, R.; Impellizzeri, D.; Cuzzocrea, S.; Di Paola, R. Neuroprotective Effect of Artesunate in Experimental Model of Traumatic Brain Injury. Front. Neurol. 2018, 9, 590. [Google Scholar] [CrossRef] [Green Version]
- Irrera, N.; D’Ascola, A.; Pallio, G.; Bitto, A.; Mannino, F.; Arcoraci, V.; Rottura, M.; Ieni, A.; Minutoli, L.; Metro, D.; et al. β-Caryophyllene Inhibits Cell Proliferation through a Direct Modulation of CB2 Receptors in Glioblastoma Cells. Cancers 2020, 12, 1038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Irrera, N.; Arcoraci, V.; Mannino, F.; Vermiglio, G.; Pallio, G.; Minutoli, L.; Bagnato, G.; Anastasi, G.P.; Mazzon, E.; Bramanti, P.; et al. Activation of A2A Receptor by PDRN Reduces Neuronal Damage and Stimulates WNT/β-CATENIN Driven Neurogenesis in Spinal Cord Injury. Front. Pharmacol. 2018, 9, 506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Irrera, N.; D’Ascola, A.; Pallio, G.; Bitto, A.; Mazzon, E.; Mannino, F.; Squadrito, V.; Arcoraci, V.; Minutoli, L.; Campo, G.M.; et al. β-Caryophyllene Mitigates Collagen Antibody Induced Arthritis (CAIA) in Mice Through a Cross-Talk between CB2 and PPAR-γ Receptors. Biomolecules 2019, 9, 326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pallio, G.; Micali, A.; Benvenga, S.; Antonelli, A.; Marini, H.R.; Puzzolo, D.; Macaione, V.; Trichilo, V.; Santoro, G.; Irrera, N.; et al. Myo-inositol in the protection from cadmium-induced toxicity in mice kidney: An emerging nutraceutical challenge. Food Chem. Toxicol. 2019, 132, 110675. [Google Scholar] [CrossRef]
- Pallio, G.; Bitto, A.; Ieni, A.; Irrera, N.; Mannino, F.; Pallio, S.; Altavilla, D.; Squadrito, F.; Scarpignato, C.; Minutoli, L. Combined Treatment with Polynucleotides and Hyaluronic Acid Improves Tissue Repair in Experimental Colitis. Biomedicines 2020, 8, 438. [Google Scholar] [CrossRef]
- Johnsen, S.G. Testicular biopsy score count—A method for registration of spermatogenesis in human testes: Normal values and results in 335 hypogonadal males. Hormones 1970, 1, 2–25. [Google Scholar] [CrossRef]
- Erdemir, F.; Atilgan, D.; Markoc, F.; Boztepe, O.; Suha-Parlaktas, B.; Sahin, S. The effect of diet induced obesity on testicular tissue and serum oxidative stress parameters. Actas Urol. Esp. 2012, 36, 153–159. [Google Scholar] [CrossRef]
- Tsounapi, P.; Saito, M.; Dimitriadis, F.; Kitatani, K.; Kinoshita, Y.; Shomori, K.; Takenaka, A.; Satoh, K. The role of K ATP channels on ischemia-reperfusion injury in the rat testis. Life Sci. 2012, 90, 649–656. [Google Scholar] [CrossRef]






| Groups | Testis Weight (g) | Testosterone (ng/ml) | MTD (μm) | JS |
|---|---|---|---|---|
| Sham | 1.65 ± 0.17 | 6.1 ± 0.9 | 249 ± 21 | 9.7 ± 0.2 |
| Varicocele | 0.83 ± 0.11 a | 2.6 ± 0.5 a | 127 ± 19 a | 2.6 ± 0.5 a |
| Varicocele CL | 1.12 ± 0.36 a,b | 168 ± 15 a,b | 7.3 ± 0.8 a,b | |
| Varicocele + Se | 1.18 ± 0.25 a,b | 4.2 ± 0.8 a,b | 173 ± 12 a,b | 6.7 ± 0.7 a,b |
| Varicocele + Se CL | 1.39 ± 0.33 a,b | 203 ± 16 a,b | 8.8 ± 0.6 b | |
| Varicocele + PDRN | 1.28 ± 0.10 a,b | 5.1 ± 0.5 b | 210 ± 15 a,b | 8.4 ± 1.3 b |
| Varicocele + PDRN CL | 1.55 ± 0.51 b | 221 ± 13 b | 9.1 ± 0.7 b | |
| Varicocele + PDRN + Se | 1.58 ± 0.35 b | 5.9 ± 0.7 b | 242 ± 17 b | 9.0 ± 0.5 b |
| Varicocele + PDRN + Se CL | 1.62 ± 0.39 b | 251 ± 14 b | 9.3 ± 0.5 b |
| Groups | GSH (nmol/mg Tissue) | GPx (nmol/min/mg Tissue) |
|---|---|---|
| Sham | 46 ± 3 | 52 ± 4 |
| Varicocele | 13 ± 2 a | 26 ± 2 a |
| Varicocele + CL | 25 ± 2 a,b | 37 ± 6 a,b |
| Varicocele + Se | 24 ± 3 a,b | 34 ± 5 a,b |
| Varicocele + Se CL | 27 ± 2 a,b | 44 ± 4 a,b |
| Varicocele + PDRN | 33 ± 2 a,b | 43 ± 5 a,b |
| Varicocele + PDRN CL | 37 ± 3 b | 49 ± 6 b |
| Varicocele + PDRN + Se | 42 ± 3 b | 47 ± 7 b |
| Varicocele + PDRN + Se CL | 45 ± 3 b | 50 ± 4 b |
| Groups | % TWAC | Apoptotic Index | Caspase-1 Positive Cells/MF |
|---|---|---|---|
| Sham | 0.2 ± 0.1 | 0.1 ± 0.1 | 0.3 ± 0.1 |
| Varicocele | 35 ± 5 a | 10 ± 2.6 a | 15.2 ± 4.1 a |
| Varicocele + CL | 7.5 ± 2.6 a,b | 4.1 ± 1.4 a,b | 7.5 ± 2.1 a,b |
| Varicocele + Se | 13.2 ± 2.7 a,b | 7.1 ± 1.4 a,b | 6.3 ± 1.7 a,b |
| Varicocele + Se CL | 3.3 ± 1.3 a,b | 3.9 ± 1.2 a,b | 3.5 ± 1.3 a,b |
| Varicocele + PDRN | 7.2 ± 1.7 a,b | 5.3 ± 1.1 a,b | 5.1 ± 1.6 a,b |
| Varicocele + PDRN CL | 1.8 ± 0.4 b | 2.2 ± 0.5 b | 2.1 ± 0.7 b |
| Varicocele + PDRN + Se | 4.4 ± 0.8 b | 1.5 ± 0.6 b | 1.3 ± 0.3 b |
| Varicocele + PDRN + Se CL | 1.3 ± 0.2 b | 0.2 ± 0.1 b | 0.4 ± 0.1 b |
| Gene | Sequence |
|---|---|
| β-actin | Fw:5′AGCCATGTACGTAGCCATCC3′ |
| Rw:5′CTCTCAGCTGTGGTGGTGAA3′ | |
| NLRP3 | Fw:5′ACGGCAAGTTCGAAAAAGGC3′ |
| Rw:5′AGACCTCGGCAGAAGCTAGA3′ | |
| IL-1β | Fw:5′AGGCTTCCTTGTGCAAGTGT3′ |
| Rw:5′TGAGTGACACTGCCTTCCTG3′ | |
| Caspase-1 | Fw:5′GACAAGATCCTGAGGGCAAA3′ |
| Rw:5′ GGTCTCGTGCCTTTTCCATA3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antonuccio, P.; Micali, A.G.; Romeo, C.; Freni, J.; Vermiglio, G.; Puzzolo, D.; Squadrito, F.; Irrera, N.; Marini, H.R.; Rana, R.A.; et al. NLRP3 Inflammasome: A New Pharmacological Target for Reducing Testicular Damage Associated with Varicocele. Int. J. Mol. Sci. 2021, 22, 1319. https://doi.org/10.3390/ijms22031319
Antonuccio P, Micali AG, Romeo C, Freni J, Vermiglio G, Puzzolo D, Squadrito F, Irrera N, Marini HR, Rana RA, et al. NLRP3 Inflammasome: A New Pharmacological Target for Reducing Testicular Damage Associated with Varicocele. International Journal of Molecular Sciences. 2021; 22(3):1319. https://doi.org/10.3390/ijms22031319
Chicago/Turabian StyleAntonuccio, Pietro, Antonio Girolamo Micali, Carmelo Romeo, Jose Freni, Giovanna Vermiglio, Domenico Puzzolo, Francesco Squadrito, Natasha Irrera, Herbert R. Marini, Rosa Alba Rana, and et al. 2021. "NLRP3 Inflammasome: A New Pharmacological Target for Reducing Testicular Damage Associated with Varicocele" International Journal of Molecular Sciences 22, no. 3: 1319. https://doi.org/10.3390/ijms22031319
APA StyleAntonuccio, P., Micali, A. G., Romeo, C., Freni, J., Vermiglio, G., Puzzolo, D., Squadrito, F., Irrera, N., Marini, H. R., Rana, R. A., Pallio, G., & Minutoli, L. (2021). NLRP3 Inflammasome: A New Pharmacological Target for Reducing Testicular Damage Associated with Varicocele. International Journal of Molecular Sciences, 22(3), 1319. https://doi.org/10.3390/ijms22031319