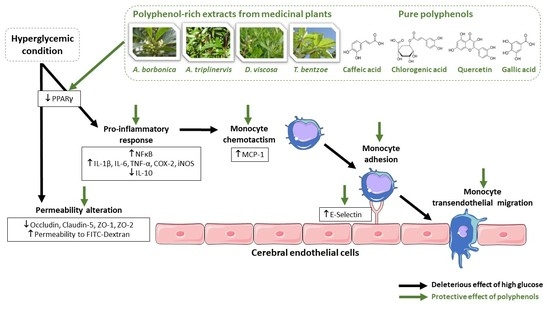
Hyperglycemic Condition Causes Pro-Inflammatory and Permeability Alterations Associated with Monocyte Recruitment and Deregulated NFκB/PPARγ Pathways on Cerebral Endothelial Cells: Evidence for Polyphenols Uptake and Protective Effect
Abstract
:1. Introduction
2. Results
2.1. Effect of Hyperglycemic Condition and Polyphenols on the Production of Inflammatory Markers on Cerebral Endothelial Cells
2.2. Effect of Hyperglycemic Condition and Polyphenols on the Monocyte Adhesion and Transendothelial Migration
2.3. Effect of Hyperglycemic Condition and Polyphenols on the Production of Permeability Markers on Cerebral Endothelial Cells
2.4. Effect of PPARγ agonist and Antagonist on the Inflammatory and Permeability Alterations Caused by Hyperglycemic Condition on Cerebral Endothelial Cells
2.5. Detection of Polyphenols at the Intracellular Level or Membrane-Bound to Cerebral Endothelial Cells in Normoglycemic or Hyperglycemic Condition
3. Discussion
4. Materials and Methods
4.1. Preparation and Characterization of Polyphenol-Rich Medicinal Plant Extracts
4.2. Cell Culture
4.3. Evaluation of NFκB Activation
4.4. Evaluation of Relative Gene Expression
4.5. Quantification of Cytokine Secretion
4.6. Evaluation of Endothelial Permeability
4.7. Evaluation of Monocyte Adhesion on Cerebral Endothelial Cells
4.8. Evaluation of Monocyte Transendothelial Migration
4.9. Evaluation of Polyphenols Uptake by Cerebral Endothelial Cells
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| A. borbonica | Antirhea borbonica |
| A. triplinervis | Ayapana triplinervis |
| BBB | blood-brain barrier |
| BCA | bicinchoninic acid |
| BCECF-AM | 2′,7′-bis-(2-carboxyethyl)-5-(and-6)-carboxyfluorescein acetoxymethyl ester |
| BCRP | breast cancer resistance protein |
| bEnd3 | brain endothelial cells |
| COX | cyclooxygenase |
| DMEM | Dulbecco’s Modified Eagle Medium |
| D. viscosa | Dodonaea viscosa |
| E-selectin | endothelial-leukocyte adhesion molecule |
| FITC | fluorescein isothiocyanate |
| GLUT-1 | glucose transporter type 1 |
| HBSS | Hanks’ balanced salt solution |
| HG | hyperglycemic condition |
| IκB | inhibitor protein of NFκB |
| IL | interleukin |
| iNOS | inducible nitric oxide synthase |
| MCP-1 | monocyte chemoattractant protein 1 |
| MMP | matrix metalloproteinase |
| NFκB | nuclear factor kappa B |
| NG | normoglycemic condition |
| Nrf2 | nuclear factor erythroid 2-related factor 2 |
| PBS | phosphate buffer saline |
| PPARγ | peroxisome proliferator-activated receptor gamma |
| Pgp | P-glycoprotein |
| RPMI | Roswell park memorial institute medium |
| SEAP | secreted alkaline phosphatase |
| T. bentzoe | Terminalia bentzoe |
| TNF-α | tumor necrosis factor alpha |
| UPLC-ESI-MS-MS | ultra-performance liquid chromatography coupled to electrospray ionization-tandem mass spectrometry |
| ZO | zona occludens |
References
- Abbott, N.J.; Patabendige, A.A.; Dolman, D.E.; Yusof, S.R.; Begley, D.J. Structure and function of the blood-brain barrier. Neurobiol. Dis. 2010, 37, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Daneman, R.; Prat, A. The blood-brain barrier. Cold Spring Harb. Perspect. Biol. 2015, 7, a020412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagy, Z.; Peters, H.; Hüttner, I. Fracture faces of cell junctions in cerebral endothelium during normal and hyperosmotic conditions. Lab. Investig. J. Tech. Methods Pathol. 1984, 50, 313–322. [Google Scholar]
- Brooks, T.A.; Hawkins, B.T.; Huber, J.D.; Egleton, R.D.; Davis, T.P. Chronic inflammatory pain leads to increased blood-brain barrier permeability and tight junction protein alterations. Am. J. Physiol. Heart Circ. Physiol. 2005, 289, H738–H743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, R.C.; Morris, A.P.; O’Neil, R.G. Tight junction protein expression and barrier properties of immortalized mouse brain microvessel endothelial cells. Brain Res. 2007, 1130, 17–30. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.; Lin, L.; Liu, N.; Wang, Q.; Yuan, J.; Li, Y.; Chung, K.K.; Guo, S. FGF21 Protects against Aggravated Blood-Brain Barrier Disruption after Ischemic Focal Stroke in Diabetic db/db Male Mice via Cerebrovascular PPARγ Activation. Int. J. Mol. Sci. 2020, 21, 824. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Z.; Zhang, X.; Dai, Y.; Pan, K.; Deng, Y.; Meng, Y.; Xu, T. PPAR-γ promotes p38 MAP kinase-mediated endothelial cell permeability through activating Sirt3. BMC Neurol. 2019, 19, 289. [Google Scholar] [CrossRef]
- Yang, S.; Jin, H.; Zhao, Z. Paracellular tightness and the functional expression of efflux transporters P-gp and BCRP in bEnd3 cells. Neurol. Res. 2018, 40, 644–649. [Google Scholar] [CrossRef]
- Rempe, R.G.; Hartz, A.M.S.; Bauer, B. Matrix metalloproteinases in the brain and blood-brain barrier: Versatile breakers and makers. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 2016, 36, 1481–1507. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Jin, X.; Liu, K.J.; Liu, W. Matrix metalloproteinase-2-mediated occludin degradation and caveolin-1-mediated claudin-5 redistribution contribute to blood-brain barrier damage in early ischemic stroke stage. J. Neurosci. Off. J. Soc. Neurosci. 2012, 32, 3044–3057. [Google Scholar] [CrossRef]
- Somade, O.T.; Ajayi, B.O.; Tajudeen, N.O.; Atunlute, E.M.; James, A.S.; Kehinde, S.A. Camphor elicits up-regulation of hepatic and pulmonary pro-inflammatory cytokines and chemokines via activation of NF-kB in rats. Pathophysiol. Off. J. Int. Soc. Pathophysiol. 2019, 26, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Mohammadian Haftcheshmeh, S.; Karimzadeh, M.R.; Azhdari, S.; Vahedi, P.; Abdollahi, E.; Momtazi-Borojeni, A.A. Modulatory effects of curcumin on the atherogenic activities of inflammatory monocytes: Evidence from in vitro and animal models of human atherosclerosis. Biofactors 2019, 46, 341–355. [Google Scholar] [CrossRef] [PubMed]
- Hijmans, J.G.; Bammert, T.D.; Stockelman, K.A.; Reiakvam, W.R.; Greiner, J.J.; DeSouza, C.A. High glucose-induced endothelial microparticles increase adhesion molecule expression on endothelial cells. Diabetol. Int. 2019, 10, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Birk, P.; Jürgen, D.; Stephan, K.; Jörg, H.; Heike, G. Regulation of BCRP (ABCG2) and P-Glycoprotein (ABCB1) by Cytokines in a Model of the Human Blood–Brain Barrier. Cell. Mol. Neurobiol. 2010, 30, 63–70. [Google Scholar]
- Scalbert, A.; Williamson, G. Dietary intake and bioavailability of polyphenols. J. Nutr. 2000, 130 (Suppl. 8S), 2073S–2085S. [Google Scholar] [CrossRef]
- Monfoulet, L.E.; Mercier, S.; Bayle, D.; Tamaian, R.; Barber-Chamoux, N.; Morand, C.; Milenkovic, D. Curcumin modulates endothelial permeability and monocyte transendothelial migration by affecting endothelial cell dynamics. Free Radic. Biol. Med. 2017, 112, 109–120. [Google Scholar] [CrossRef]
- Jiang, R.; Hodgson, J.M.; Mas, E.; Croft, K.D.; Ward, N.C. Chlorogenic acid improves ex vivo vessel function and protects endothelial cells against HOCl-induced oxidative damage, via increased production of nitric oxide and induction of Hmox-1. J. Nutr. Biochem. 2016, 27, 53–60. [Google Scholar] [CrossRef] [Green Version]
- Tang, J.S.; Bozonet, S.M.; McKenzie, J.L.; Anderson, R.F.; Melton, L.D.; Vissers, M.C.M. Physiological Concentrations of Blueberry-Derived Phenolic Acids Reduce Monocyte Adhesion to Human Endothelial Cells. Mol. Nutr. Food Res. 2019, 63, e1900478. [Google Scholar] [CrossRef]
- Ariff, A.M.; Bakar, N.A.A.; Muid, S.A.; Omar, E.; Ismail, N.H.; Ali, A.M.; Kasim, N.A.M.; Nawawi, H.M. Ficus deltoidea suppresses endothelial activation, inflammation, monocytes adhesion and oxidative stress via NF-κB and eNOS pathways in stimulated human coronary artery endothelial cells. BMC Complement. Med. Ther. 2020, 20, 56. [Google Scholar]
- Arcambal, A.; Taïlé, J.; Couret, D.; Planesse, C.; Veeren, B.; Diotel, N.; Gauvin-Bialecki, A.; Meilhac, O.; Gonthier, M.P. Protective Effects of Antioxidant Polyphenols Against Hyperglycemia-Mediated Alterations in Cerebral Endothelial Cells and a Mouse Stroke Model. Mol. Nutr. Food Res. 2020, 64, e1900779. [Google Scholar] [CrossRef]
- Taïlé, J.; Arcambal, A.; Clerc, P.; Gauvin-Bialecki, A.; Gonthier, M.P. Medicinal Plant Polyphenols Attenuate Oxidative Stress and Improve Inflammatory and Vasoactive Markers in Cerebral Endothelial Cells during Hyperglycemic Condition. Antioxidants 2020, 9, 573. [Google Scholar] [CrossRef] [PubMed]
- Desilles, J.P.; Syvannarath, V.; Ollivier, V.; Journé, C.; Delbosc, S.; Ducroux, C.; Boisseau, W.; Louedec, L.; Di Meglio, L.; Loyau, S.; et al. Exacerbation of Thromboinflammation by Hyperglycemia Precipitates Cerebral Infarct Growth and Hemorrhagic Transformation. Stroke 2017, 48, 1932–1940. [Google Scholar] [CrossRef] [PubMed]
- Zonneveld, T.P.; Nederkoorn, P.J.; Westendorp, W.F.; Brouwer, M.C.; van de Beek, D.; Kruyt, N.D. Hyperglycemia predicts poststroke infections in acute ischemic stroke. Neurology 2017, 88, 1415–1421. [Google Scholar] [CrossRef] [PubMed]
- Omidi, Y.; Campbell, L.; Barar, J.; Connell, D.; Akhtar, S.; Gumbleton, M. Evaluation of the immortalised mouse brain capillary endothelial cell line, b.End3, as an in vitro blood-brain barrier model for drug uptake and transport studies. Brain Res. 2003, 990, 95–112. [Google Scholar] [CrossRef]
- Yang, S.; Mei, S.; Jin, H.; Zhu, B.; Tian, Y.; Huo, J.; Cui, X.; Guo, A.; Zhao, Z. Identification of two immortalized cell lines, ECV304 and bEnd3, for in vitro permeability studies of blood-brain barrier. PLoS ONE 2017, 12, e0187017. [Google Scholar] [CrossRef]
- Arcambal, A.; Taïlé, J.; Rondeau, P.; Viranaïcken, W.; Meilhac, O.; Gonthier, M.P. Hyperglycemia modulates redox, inflammatory and vasoactive markers through specific signaling pathways in cerebral endothelial cells: Insights on insulin protective action. Free Radic. Biol. Med. 2019, 130, 59–70. [Google Scholar] [CrossRef]
- Samoisy, A.K.; Mahomoodally, M.F. Ethnopharmacological analysis of medicinal plants used against non-communicable diseases in Rodrigues Island, Indian Ocean. J. Ethnopharmacol. 2015, 173, 20–38. [Google Scholar] [CrossRef]
- Ruskovska, T.; Massaro, M.; Carluccio, M.A.; Arola-Arnal, A. Systematic bioinformatic analysis of nutrigenomic data of flavanols in cell models of cardiometabolic disease. Food Funct. 2020, 11, 5040–5064. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Remesy, C.; Jimenez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [Green Version]
- Williams, C.R.; Lu, X.; Sutliff, R.L.; Hart, C.M. Rosiglitazone attenuates NF-κB-mediated Nox4 upregulation in hyperglycemia-activated endothelial cells. Am. J. Physiol. Cell Physiol. 2012, 303, C213–C223. [Google Scholar] [CrossRef] [Green Version]
- Yuan, T.; Yang, T.; Chen, H.; Fu, D.; Hu, Y.; Wang, J.; Yuan, Q.; Yu, H.; Xu, W.; Xie, X. New insights into oxidative stress and inflammation during diabetes mellitus-accelerated atherosclerosis. Redox Biol. 2019, 20, 247–260. [Google Scholar] [CrossRef] [PubMed]
- Markovics, A.; Biró, A. Effect of Anthocyanin-Rich Extract of Sour Cherry for Hyperglycemia-Induced Inflammatory Response and Impaired Endothelium-Dependent Vasodilation. Nutrients 2020, 12, 3373. [Google Scholar] [CrossRef] [PubMed]
- Sheu, M.L.; Ho, F.M.; Yang, R.S.; Chao, K.F.; Lin, W.W.; Lin-Shiau, S.Y.; Liu, S.H. High glucose induces human endothelial cell apoptosis through a phosphoinositide 3-kinase-regulated cyclooxygenase-2 pathway. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 539–545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajesh, M.; Mukhopadhyay, P.; Bátkai, S.; Haskó, G.; Liaudet, L.; Drel, V.R.; Obrosova, I.G.; Pacher, P. Cannabidiol attenuates high glucose-induced endothelial cell inflammatory response and barrier disruption. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H610–H619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aljofan, M.; Ding, H. High glucose increases expression of cyclooxygenase-2, increases oxidative stress and decreases the generation of nitric oxide in mouse microvessel endothelial cells. J. Cell. Physiol. 2010, 222, 669–675. [Google Scholar] [CrossRef] [PubMed]
- Xing, C.; Xiang, D.; Caiying, L. Effects of troxerutin on vascular inflammatory mediators and expression of microRNA-146a/NF-κB signaling pathway in aorta of healthy and diabetic rats. Korean J. Physiol. Pharmacol. Off. J. Korean Physiol. Soc. Korean Soc. Pharmacol. 2020, 24, 395–402. [Google Scholar] [CrossRef]
- Tsai, S.J.; Chao, C.Y.; Yin, M.C. Preventive and therapeutic effects of caffeic acid against inflammatory injury in striatum of MPTP-treated mice. Eur. J. Pharm. 2011, 670, 441–447. [Google Scholar] [CrossRef]
- Rozentsvit, A.; Vinokur, K.; Samuel, S.; Li, Y.; Gerdes, A.M.; Carrillo-Sepulveda, M.A. Ellagic Acid Reduces High Glucose-Induced Vascular Oxidative Stress Through ERK1/2/NOX4 Signaling Pathway. Cell Physiol. Biochem. 2017, 44, 1174–1187. [Google Scholar] [CrossRef]
- Park, C.S.; Lee, J.Y.; Choi, H.Y.; Lee, K.; Heo, Y.; Ju, B.G.; Choo, H.P.; Yune, T.Y. Gallic acid attenuates blood-spinal cord barrier disruption by inhibiting Jmjd3 expression and activation after spinal cord injury. Neurobiol. Dis. 2020, 145, 105077. [Google Scholar] [CrossRef]
- Fratantonio, D.; Speciale, A. Low nanomolar caffeic acid attenuates high glucose-induced endothelial dysfunction in primary human umbilical-vein endothelial cells by affecting NF-κB and Nrf2 pathways. Biofactors 2017, 43, 54–62. [Google Scholar] [CrossRef]
- Ahmed, S.M.; Luo, L.; Namani, A.; Wang, X.J.; Tang, X. Nrf2 signaling pathway: Pivotal roles in inflammation. Biochim. Biophys. Acta. Mol. Basis Dis. 2017, 1863, 585–597. [Google Scholar] [CrossRef] [PubMed]
- Le Sage, F.; Meilhac, O.; Gonthier, M.P. Anti-inflammatory and antioxidant effects of polyphenols extracted from Antirhea borbonica medicinal plant on adipocytes exposed to Porphyromonas gingivalis and Escherichia coli lipopolysaccharides. Pharm. Res. 2017, 119, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J.; Karin, M. Nuclear factor-kappaB: A pivotal transcription factor in chronic inflammatory diseases. N. Engl. J. Med. 1997, 336, 1066–1071. [Google Scholar] [CrossRef] [PubMed]
- Blankenberg, S.; Barbaux, S.; Tiret, L. Adhesion molecules and atherosclerosis. Atherosclerosis 2003, 170, 191–203. [Google Scholar] [CrossRef]
- Jin, Z.; Ke, J.; Guo, P.; Wang, Y.; Wu, H. Quercetin improves blood-brain barrier dysfunction in rats with cerebral ischemia reperfusion via Wnt signaling pathway. Am. J. Transl. Res. 2019, 11, 4683–4695. [Google Scholar]
- Lee, K.; Lee, J.S.; Jang, H.J.; Kim, S.M.; Chang, M.S.; Park, S.H.; Kim, K.S.; Bae, J.; Park, J.W.; Lee, B.; et al. Chlorogenic acid ameliorates brain damage and edema by inhibiting matrix metalloproteinase-2 and 9 in a rat model of focal cerebral ischemia. Eur. J. Pharm. 2012, 689, 89–95. [Google Scholar] [CrossRef]
- Chien, M.Y.; Chuang, C.H.; Chern, C.M.; Liou, K.T.; Liu, D.Z.; Hou, Y.C.; Shen, Y.C. Salvianolic acid A alleviates ischemic brain injury through the inhibition of inflammation and apoptosis and the promotion of neurogenesis in mice. Free Radic. Biol. Med. 2016, 99, 508–519. [Google Scholar] [CrossRef]
- Chen, Y.C.; Wu, J.S.; Tsai, H.D.; Huang, C.Y.; Chen, J.J.; Sun, G.Y.; Lin, T.N. Peroxisome proliferator-activated receptor gamma (PPAR-γ) and neurodegenerative disorders. Mol. Neurobiol. 2012, 46, 114–124. [Google Scholar] [CrossRef]
- Zhao, X.; Strong, R.; Zhang, J.; Sun, G.; Tsien, J.Z.; Cui, Z.; Grotta, J.C.; Aronowski, J. Neuronal PPARgamma deficiency increases susceptibility to brain damage after cerebral ischemia. J. Neurosci. Off. J. Soc. Neurosci. 2009, 29, 6186–6195. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.S.; Tsai, H.D.; Cheung, W.M.; Hsu, C.Y.; Lin, T.N. PPAR-γ Ameliorates Neuronal Apoptosis and Ischemic Brain Injury via Suppressing NF-κB-Driven p22phox Transcription. Mol. Neurobiol. 2016, 53, 3626–3645. [Google Scholar] [CrossRef]
- Collino, M.; Patel, N.S.; Thiemermann, C. PPARs as new therapeutic targets for the treatment of cerebral ischemia/reperfusion injury. Ther. Adv. Cardiovasc. Dis. 2008, 2, 179–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Szwajgier, D.; Borowiec, K.; Pustelniak, K. The Neuroprotective Effects of Phenolic Acids: Molecular Mechanism of Action. Nutrients 2017, 9, 477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishisaka, A.; Mukai, R.; Terao, J.; Shibata, N.; Kawai, Y. Specific localization of quercetin-3-O-glucuronide in human brain. Arch. Biochem. Biophys. 2014, 557, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Spencer, J.P.; Kuhnle, G.G.; Williams, R.J.; Rice-Evans, C. Intracellular metabolism and bioactivity of quercetin and its in vivo metabolites. Biochem. J. 2003, 372 Pt 1, 173–181. [Google Scholar] [CrossRef] [Green Version]
- Mateos, R.; Goya, L.; Bravo, L. Uptake and metabolism of hydroxycinnamic acids (chlorogenic, caffeic, and ferulic acids) by HepG2 cells as a model of the human liver. J. Agric. Food Chem. 2006, 54, 8724–8732. [Google Scholar] [CrossRef] [Green Version]
- Mohos, V.; Fliszár-Nyúl, E.; Ungvári, O.; Kuffa, K.; Needs, P.W.; Kroon, P.A.; Telbisz, Á.; Özvegy-Laczka, C.; Poór, M. Inhibitory Effects of Quercetin and Its Main Methyl, Sulfate, and Glucuronic Acid Conjugates on Cytochrome P450 Enzymes, and on OATP, BCRP and MRP2 Transporters. Nutrients 2020, 12, 2306. [Google Scholar] [CrossRef]
- Kaur, M.; Badhan, R.K. Phytochemical mediated-modulation of the expression and transporter function of breast cancer resistance protein at the blood-brain barrier: An in-vitro study. Brain Res. 2017, 1654 Pt A, 9–23. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Chen, Z.; Yan, M.; He, P.; Chen, Z.; Dai, H. The protective role of isorhamnetin on human brain microvascular endothelial cells from cytotoxicity induced by methylglyoxal and oxygen-glucose deprivation. J. Neurochem. 2016, 136, 651–659. [Google Scholar] [CrossRef]
- Yokozawa, T.; Kim, H.Y.; Cho, E.J.; Choi, J.S.; Chung, H.Y. Antioxidant effects of isorhamnetin 3,7-di-O-beta-D-glucopyranoside isolated from mustard leaf (Brassica juncea) in rats with streptozotocin-induced diabetes. J. Agric. Food Chem. 2002, 50, 5490–5495. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Bainor, A.; Chang, L.; McQuade, T.J.; Webb, B.; Gestwicki, J.E. Bicinchoninic acid (BCA) assay in low volume. Anal. Biochem. 2011, 410, 310–312. [Google Scholar] [CrossRef] [PubMed]
- Iwashima, T.; Kudome, Y.; Kishimoto, Y.; Saita, E.; Tanaka, M.; Taguchi, C.; Hirakawa, S.; Mitani, N.; Kondo, K.; Iida, K. Aronia berry extract inhibits TNF-α-induced vascular endothelial inflammation through the regulation of STAT3. Food Nutr. Res. 2019, 63. [Google Scholar] [CrossRef] [PubMed]
- Milenkovic, D.; Berghe, W.V.; Morand, C.; Claude, S.; van de Sandt, A.; Gorressen, S.; Monfoulet, L.E. A systems biology network analysis of nutri(epi)genomic changes in endothelial cells exposed to epicatechin metabolites. Sci. Rep. 2018, 8, 15487. [Google Scholar] [CrossRef] [PubMed]







| Concentration (nM) | NG | NG + Pgpi | NG + BCRPi | HG | HG + Pgpi | HG + BCRPi |
|---|---|---|---|---|---|---|
| Intracellular | ||||||
| Caffeic acid | 2.11 ± 0.02 | 1.91 ± 0.14 | 1.91 ± 0.24 | 1.96 ± 0.19 | 1.65 ± 0.36 | 1.60 ± 0.15 |
| Chlorogenic acid | nd | nd | nd | nd | nd | nd |
| Gallic acid | nd | nd | nd | nd | nd | nd |
| Quercetin | 15.70 ± 1.14 | 18.68 ± 1.51 | 24.36 ± 1.70 ** | 17.36 ± 0.80 | 21.77 ± 0.08 | 39.00 ± 9.27 # |
| Isorhamnetin | 58.59 ± 7.92 | 57.22 ± 8.13 | 124.64 ± 13.09 ** | 65.22 ± 2.49 | 76.24 ± 9.55 | 158.49 ± 22.51 ## |
| Membrane-bound | ||||||
| Caffeic acid | 26.18 ± 2.74 | 21.06 ± 4.22 | 18.00 ± 2.67 | 14.25 ± 2.80 * | 14.26 ± 1.96 * | 17.74 ± 3.52 * |
| Chlorogenic acid | 16.25 ± 0.94 | 12.38 ± 3.28 | 11.33 ± 1.86 | 7.70 ± 0.90 * | 5.73 ± 0.26 * | 9.06 ± 3.00 * |
| Gallic acid | 30.03 ± 3.75 | 26.43 ± 8.81 | 28.57 ± 8.31 | 12.35 ± 2.55 * | 13.11 ± 2.27 * | 10.21 ± 2.17 * |
| Quercetin | 11.13 ± 2.23 | 9.14 ± 3.16 | 7.00 ± 2.94 | 8.81 ± 1.81 | 8.08 ± 3.97 | 9.85 ± 4.50 |
| Isorhamnetin | 69.14 ± 18.60 | 57.74 ± 17.78 | 86.24 ± 9.08 | 46.17 ± 8.69 | 50.12 ± 17.01 | 76.52 ± 14.28 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taïlé, J.; Patché, J.; Veeren, B.; Gonthier, M.-P. Hyperglycemic Condition Causes Pro-Inflammatory and Permeability Alterations Associated with Monocyte Recruitment and Deregulated NFκB/PPARγ Pathways on Cerebral Endothelial Cells: Evidence for Polyphenols Uptake and Protective Effect. Int. J. Mol. Sci. 2021, 22, 1385. https://doi.org/10.3390/ijms22031385
Taïlé J, Patché J, Veeren B, Gonthier M-P. Hyperglycemic Condition Causes Pro-Inflammatory and Permeability Alterations Associated with Monocyte Recruitment and Deregulated NFκB/PPARγ Pathways on Cerebral Endothelial Cells: Evidence for Polyphenols Uptake and Protective Effect. International Journal of Molecular Sciences. 2021; 22(3):1385. https://doi.org/10.3390/ijms22031385
Chicago/Turabian StyleTaïlé, Janice, Jessica Patché, Bryan Veeren, and Marie-Paule Gonthier. 2021. "Hyperglycemic Condition Causes Pro-Inflammatory and Permeability Alterations Associated with Monocyte Recruitment and Deregulated NFκB/PPARγ Pathways on Cerebral Endothelial Cells: Evidence for Polyphenols Uptake and Protective Effect" International Journal of Molecular Sciences 22, no. 3: 1385. https://doi.org/10.3390/ijms22031385
APA StyleTaïlé, J., Patché, J., Veeren, B., & Gonthier, M. -P. (2021). Hyperglycemic Condition Causes Pro-Inflammatory and Permeability Alterations Associated with Monocyte Recruitment and Deregulated NFκB/PPARγ Pathways on Cerebral Endothelial Cells: Evidence for Polyphenols Uptake and Protective Effect. International Journal of Molecular Sciences, 22(3), 1385. https://doi.org/10.3390/ijms22031385