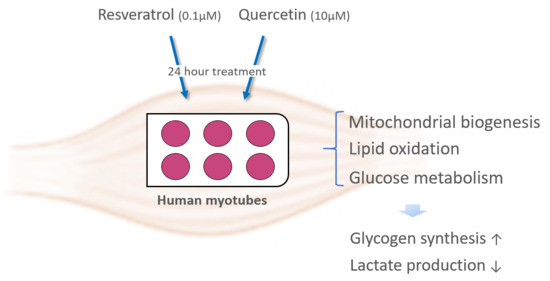
Effects of Physiological Doses of Resveratrol and Quercetin on Glucose Metabolism in Primary Myotubes
Abstract
:1. Introduction
2. Results
2.1. Results in Cytotoxicity Assay of RSV and Q
2.2. Effects of RSV and Q in the Expression of Mitochondrial and Cytosolic Genes
2.3. Effects of RSV and Q on Glucose Uptake and Oxidation and Palmitate Oxidation
2.4. Effects of RSV and Q in Glucose Homeostasis
2.5. The Effect of RSV and Q in Glycogen Synthesis and Lactate Production
3. Discussion
4. Materials and Methods
4.1. Experimental Design
4.2. Cell Treatment
4.3. Cytotoxicity Assay
4.4. Glycogen Synthesis Assay
4.5. Glucose Oxidation Assay
4.6. Palmitate Oxidation Assay
4.7. Measurement of Lactate Content in the Media
4.8. RNA Preparation and Quantitative Real Time PCR
4.9. Western Blot Analysis
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AK | Adenylate kinase |
| AKT | Protein kinase B (PKB) |
| AMPK | AMP-activated protein kinase |
| AS160 | AKT Substrate of 160 kDa |
| ATP5a1 | ATP synthase, H+ transporting, mitochondrial F1 complex, alpha subunit 1 |
| COMT | Catechol-O-methyltransferases |
| COX7C | Cytochrome c oxidase subunit7C |
| CYCS | Cytochrome C |
| GLUT4 | Glucose transporter 4 |
| GSK3β | Glycogen synthase kinase 3 |
| HK2 | Hexokinase 2 |
| IRS-1 | Insulin receptor substrate 1 |
| NDUFB8 | NADH dehydrogenase (ubiquinone) 1 beta subcomplex 8 |
| NRF1 | Nuclear respiratory factor 1 |
| PGC-1α | Peroxisome proliferator-activated receptor gamma coactivator 1-alpha |
| PI3K | Phosphoinositide 3-kinase |
| PKM | Pyruvate kinase M1/2 |
| RSV | Resveratrol |
| SDHA | Succinate dehydrogenase complex, subunit alpha |
| SULT | Sulfotransferases |
| TFAM | Mitochondrial transcription factor A |
| UGT | Uridine-5´-diphosphate glucuronosyltransferases |
| Q | Quercetin |
References
- Diabetes. World Health Organization: 2018; Volume 2019. Available online: https://www.who.int/health-topics/diabetes#tab=tab_1 (accessed on 12 May 2020).
- Carnagarin, R.; Dharmarajan, A.M.; Dass, C.R. Molecular aspects of glucose homeostasis in skeletal muscle—A focus on the molecular mechanisms of insulin resistance. Mol. Cell. Endocrinol. 2015, 417, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Lauritzen, H.P.; Schertzer, J.D. Measuring GLUT4 translocation in mature muscle fibers. Am. J. Physiol. Metab. 2010, 299, E169–E179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saltiel, A.R.; Kahn, C.R. Insulin signalling and the regulation of glucose and lipid metabolism. Nature 2001, 414, 799–806. [Google Scholar] [CrossRef]
- Villarroel-Espíndola, F.; Maldonado, R.; Mancilla, H.; Stelt, K.V.; Acuña, A.I.; Covarrubias, A.; López, C.; Angulo, C.; Castro, M.A.; Slebe, J.C.; et al. Muscle glycogen synthase isoform is responsible for testicular glycogen synthesis: Glycogen overproduction induces apoptosis in male germ cells. J. Cell. Biochem. 2013, 114, 1653–1664. [Google Scholar] [CrossRef] [PubMed]
- Day, E.A.; Ford, R.J.; Steinberg, G.R. AMPK as a Therapeutic Target for Treating Metabolic Diseases. Trends Endocrinol. Metab. 2017, 28, 545–560. [Google Scholar] [CrossRef]
- Osler, M.E.; Zierath, J.R. Minireview: Adenosine 5′-Monophosphate-Activated Protein Kinase Regulation of Fatty Acid Oxidation in Skeletal Muscle. Endocrinology 2007, 149, 935–941. [Google Scholar] [CrossRef] [Green Version]
- Jiang, H.; Yamashita, Y.; Nakamura, A.; Croft, K.D.; Ashida, H. Quercetin and its metabolite isorhamnetin promote glucose uptake through different signalling pathways in myotubes. Sci. Rep. 2019, 9, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Klip, A.; Schertzer, J.D.; Bilan, P.J.; Thong, F.; Antonescu, C.N. Regulation of glucose transporter 4 traffic by energy deprivation from mitochondrial compromise. Acta Physiol. 2009, 196, 27–35. [Google Scholar] [CrossRef]
- Alvim, R.O.; Cheuhen, M.R.; Machado, S.R.; Sousa, A.G.P.; Santos, P.C. General aspects of muscle glucose uptake. An. Acad. Bras. Ciências 2015, 87, 351–368. [Google Scholar] [CrossRef] [Green Version]
- Szkudelski, T.; Szkudelski, T. Resveratrol, obesity and diabetes. Eur. J. Pharmacol. 2010, 635, 1–8. [Google Scholar] [CrossRef]
- Portillo, M.P.; Aguirre, L.; Arias, N.; Macarulla, M.T.; Gracia, A. Beneficial Effects of Quercetin on Obesity and Diabetes. Open Nutraceuticals J. 2011, 4, 189–198. [Google Scholar] [CrossRef] [Green Version]
- Carpene, C.; Gomez-Zorita, S.; Deleruyelle, S.; Carpene, M. Novel Strategies for Preventing Diabetes and Obesity Complications with Natural Polyphenols. Curr. Med. Chem. 2014, 22, 150–164. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Quintela, A.; Milton-Laskibar, I.; González, M.; Portillo, M.P. Antiobesity effects of resveratrol: Which tissues are involved? Ann. N. Y. Acad. Sci. 2017, 1403, 118–131. [Google Scholar] [CrossRef] [PubMed]
- Haddad, P.S.; Eid, H.M. The Antidiabetic Potential of Quercetin: Underlying Mechanisms. Curr. Med. Chem. 2017, 24, 355–364. [Google Scholar] [CrossRef]
- Patel, K.R.; Scott, E.; Brown, V.A.; Gescher, A.J.; Steward, W.P.; Brown, K. Clinical trials of resveratrol. Ann. N. Y. Acad. Sci. 2011, 1215, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, D.M.; Yan, J.; Soleas, G.J. Absorption of three wine-related polyphenols in three different matrices by healthy sub-jects. Clin. Biochem. 2003, 36, 79–87. [Google Scholar] [CrossRef]
- Timmers, S.; Konings, E.; Bilet, L.; Houtkooper, R.H.; Van De Weijer, T.; Goossens, G.H.; Hoeks, J.; Van Der Krieken, S.; Ryu, D.; Kersten, S.; et al. Calorie Restriction-like Effects of 30 Days of Resveratrol Supplementation on Energy Metabolism and Metabolic Profile in Obese Humans. Cell Metab. 2011, 14, 612–622. [Google Scholar] [CrossRef] [Green Version]
- Olthof, M.R.; Hollman, P.C.H.; Vree, T.B.; Katan, M.B. Bioavailabilities of Quercetin-3-Glucoside and Quercetin-4′-Glucoside Do Not Differ in Humans. J. Nutr. 2000, 130, 1200–1203. [Google Scholar] [CrossRef]
- Wang, S.; Moustaid-Moussa, N.; Chen, L.; Mo, H.; Shastri, A.; Su, R.; Bapat, P.; Kwun, I.; Shen, C.-L. Novel insights of dietary polyphenols and obesity. J. Nutr. Biochem. 2014, 25, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Rasouli, H.; Farzaei, M.H.; Khodarahmi, R. Polyphenols and their benefits: A review. Int. J. Food Prop. 2017, 20, 1–42. [Google Scholar] [CrossRef] [Green Version]
- Öztürk, E.; Arslan, A.K.K.; Yerer, M.B.; Bishayee, A. Resveratrol and diabetes: A critical review of clinical studies. Biomed. Pharmacother. 2017, 95, 230–234. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.-D.; Shi, G.; Jiang, Y.; Yao, C.; Zhu, C. A review on the potential of Resveratrol in prevention and therapy of diabetes and diabetic complications. Biomed. Pharmacother. 2020, 125, 109767. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Liao, D.; Dong, Y.; Pu, R. Effect of quercetin supplementation on plasma lipid profiles, blood pressure, and glucose levels: A systematic review and meta-analysis. Nutr. Rev. 2020, 78, 615–626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zamora-Ros, R.; Knaze, V.; Rothwell, J.A.; Hémon, B.; Moskal, A.; Overvad, K.; Tjønneland, A.; Kyrø, C.; Fagherazzi, G.; Boutron-Ruault, M.-C.; et al. Dietary polyphenol intake in Europe: The European Prospective Investigation into Cancer and Nutrition (EPIC) study. Eur. J. Nutr. 2016, 55, 1359–1375. [Google Scholar] [CrossRef] [PubMed]
- Harwood, M.; Danielewska-Nikiel, B.; Borzelleca, J.; Flamm, G.; Williams, G.; Lines, T. A critical review of the data related to the safety of quercetin and lack of evidence of in vivo toxicity, including lack of genotoxic/carcinogenic properties. Food Chem. Toxicol. 2007, 45, 2179–2205. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.P.; Singh, R.; Verma, S.S.; Rai, V.; Kaschula, C.H.; Maiti, P.; Gupta, S.C. Health benefits of resveratrol: Evidence from clinical studies. Med. Res. Rev. 2019, 39, 1851–1891. [Google Scholar] [CrossRef]
- Andres, S.; Pevny, S.; Ziegenhagen, R.; Bakhiya, N.; Schäfer, B.; Hirsch-Ernst, K.I.; Lampen, A. Safety Aspects of the Use of Quercetin as a Dietary Supplement. Mol. Nutr. Food Res. 2018, 62. [Google Scholar] [CrossRef]
- Shi, G.-J.; Li, Y.; Cao, Q.-H.; Wu, H.-X.; Tang, X.-Y.; Gao, X.-H.; Yu, J.-Q.; Chen, Z.; Yang, Y. In vitro and in vivo evidence that quercetin protects against diabetes and its complications: A systematic review of the literature. Biomed. Pharmacother. 2019, 109, 1085–1099. [Google Scholar] [CrossRef]
- Szkudelski, T.; Szkudelska, K. Resveratrol and diabetes: From animal to human studies. Biochim. Biophys. Acta 2015, 1852, 1145–1154. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Jiang, H.; Wu, X.; Fang, J. Therapeutic Effects of Quercetin on Inflammation, Obesity, and Type 2 Diabetes. Mediat. Inflamm. 2016, 2016, 9340637. [Google Scholar] [CrossRef]
- Boocock, D.J.; Faust, G.E.; Patel, K.R.; Schinas, A.M.; Brown, V.A.; Ducharme, M.P.; Booth, T.D.; Crowell, J.A.; Perloff, M.; Gescher, A.J.; et al. Phase I Dose Escalation Pharmacokinetic Study in Healthy Volunteers of Resveratrol, a Potential Cancer Chemopreventive Agent. Cancer Epidemiol. Biomark. Prev. 2007, 16, 1246–1252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boocock, D.J.; Patel, K.R.; Faust, G.E.; Normolle, D.P.; Marczylo, T.H.; Crowell, J.A.; Brenner, D.E.; Booth, T.D.; Gescher, A.; Steward, W.P. Quantitation of trans-resveratrol and detection of its metabolites in human plasma and urine by high performance liquid chromatography. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2007, 848, 182–187. [Google Scholar] [CrossRef] [Green Version]
- Gambini, J.; Inglés, M.; Olaso, G.; Lopezgrueso, R.; Bonetcosta, V.; Gimenomallench, L.; Mas-Bargues, C.; Abdelaziz, K.M.; Gomezcabrera, M.C.; Vina, J.; et al. Properties of Resveratrol:In VitroandIn VivoStudies about Metabolism, Bioavailability, and Biological Effects in Animal Models and Humans. Oxidative Med. Cell. Longev. 2015, 2015, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Rémésy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230S–242S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conquer, J.A.; Maiani, G.; Azzini, E.; Raguzzini, A.; Holub, B.J. Supplementation with Quercetin Markedly Increases Plasma Quercetin Concentration without Effect on Selected Risk Factors for Heart Disease in Healthy Subjects. J. Nutr. 1998, 128, 593–597. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.; Spagnuolo, C.; Tedesco, I.; Bilotto, S.; Russo, G.L. The flavonoid quercetin in disease prevention and therapy: Facts and fancies. Biochem. Pharmacol. 2012, 83, 6–15. [Google Scholar] [CrossRef]
- Brown, V.A.; Patel, K.R.; Viskaduraki, M.; Crowell, J.A.; Perloff, M.; Booth, T.D.; Vasilinin, G.; Sen, A.; Schinas, A.M.; Piccirilli, G.; et al. Repeat Dose Study of the Cancer Chemopreventive Agent Resveratrol in Healthy Volunteers: Safety, Pharmacokinetics, and Effect on the Insulin-like Growth Factor Axis. Cancer Res. 2010, 70, 9003–9011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dolinsky, V.W.; Dyck, J.R.B. Experimental Studies of the Molecular Pathways Regulated by Exercise and Resveratrol in Heart, Skeletal Muscle and the Vasculature. Molecules 2014, 19, 14919–14947. [Google Scholar] [CrossRef] [Green Version]
- Malaguti, M.; Angeloni, C.; Hrelia, S. Polyphenols in Exercise Performance and Prevention of Exercise-Induced Muscle Damage. Oxidative Med. Cell. Longev. 2013, 2013, 825928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lagouge, M.; Argmann, C.; Gerhart-Hines, Z.; Meziane, H.; Lerin, C.; Daussin, F.; Messadeq, N.; Milne, J.; Lambert, P.; Elliott, P.; et al. Resveratrol Improves Mitochondrial Function and Protects against Metabolic Disease by Activating SIRT1 and PGC-1α. Cell 2006, 127, 1109–1122. [Google Scholar] [CrossRef]
- Krishnamoorthy, R.M.; Venkatraman, A.C. Polyphenols activate energy sensing network in insulin resistant models. Chem. Interact. 2017, 275, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Montesano, A.; Luzi, L.; Senesi, P.; Mazzocchi, N.; Terruzzi, I. Resveratrol promotes myogenesis and hypertrophy in murine myoblasts. J. Transl. Med. 2013, 11, 310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haddad, P.S.; Eid, H.M.; Nachar, A.; Thong, F.; Sweeney, G. The molecular basis of the antidiabetic action of quercetin in cultured skeletal muscle cells and hepatocytes. Pharmacogn. Mag. 2015, 11, 74–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skrobuk, P.; Von Kraemer, S.; Semenova, M.M.; Zitting, A.; Koistinen, H.A. Acute exposure to resveratrol inhibits AMPK activity in human skeletal muscle cells. Diabetologia 2012, 55, 3051–3060. [Google Scholar] [CrossRef] [PubMed]
- Breen, D.M.; Sanli, T.; Giacca, A.; Tsiani, E. Stimulation of muscle cell glucose uptake by resveratrol through sirtuins and AMPK. Biochem. Biophys. Res. Commun. 2008, 374, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Minakawa, M.; Kawano, A.; Miura, Y.; Yagasaki, K. Hypoglycemic effect of resveratrol in type 2 diabetic model db/db mice and its actions in cultured L6 myotubes and RIN-5F pancreatic β-cells. J. Clin. Biochem. Nutr. 2011, 48, 237–244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fröjdö, S.; Cozzone, D.; Vidal, H.; Pirola, L. Resveratrol is a class IA phosphoinositide 3-kinase inhibitor. Biochem. J. 2007, 406, 511–518. [Google Scholar] [CrossRef]
- Kaminski, J.; Lançon, A.; Tili, E.; Aires, V.; Demarquoy, J.; Lizard, G.; Michaille, J.; Latruffe, N. Dietary Resveratrol Modu-lates Metabolic Functions in Skeletal Muscle Cells. J. Food Drug Anal. 2012, 20, 398–401. [Google Scholar]
- Barger, J.L.; Kayo, T.; Vann, J.M.; Arias, E.B.; Wang, J.; Hacker, T.A.; Wang, Y.; Raederstorff, D.; Morrow, J.D.; Leeuwenburgh, C.; et al. A Low Dose of Dietary Resveratrol Partially Mimics Caloric Restriction and Retards Aging Parameters in Mice. PLoS ONE 2008, 3, e2264. [Google Scholar] [CrossRef]
- Gulati, N.; Laudet, B.; Zohrabian, V.M.; Murali, R.; Jhanwar-Uniyal, M. The antiproliferative effect of Quercetin in cancer cells is mediated via inhibition of the PI3K-Akt/PKB pathway. Anticancer. Res. 2006, 26, 1177–1181. [Google Scholar]
- Yoneyama, Y.; Inamitsu, T.; Chida, K.; Iemura, S.-I.; Natsume, T.; Maeda, T.; Hakuno, F.; Takahashi, S.-I. Serine Phosphorylation by mTORC1 Promotes IRS-1 Degradation through SCFβ-TRCP E3 Ubiquitin Ligase. iScience 2018, 5, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, F.; Liao, W.; Yu, L.; Hu, Z.; Li, M.; Xia, H. Curcumin suppresses glioblastoma cell proliferation by p-AKT/mTOR pathway and increases the PTEN expression. Arch. Biochem. Biophys. 2020, 689, 108412. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.-L.; Li, W.-Q.; Tong, X.-L.; Liu, X.-Y.; Zhou, W. Xanthohumol attenuates isoprenaline-induced cardiac hypertrophy and fibrosis through regulating PTEN/AKT/mTOR pathway. Eur. J. Pharmacol. 2020, 891, 173690. [Google Scholar] [CrossRef]
- Milton-Laskíbar, I.; Aguirre, L.; Macarulla, M.; Etxeberria, U.; Milagro, F.I.; Martínez, J.; Contreras, J.; Portillo, M.P. Comparative effects of energy restriction and resveratrol intake on glycemic control improvement. BioFactors 2017, 43, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Eid, H.M.; Martineau, L.C.; Saleem, A.; Muhammad, A.; Vallerand, D.; Benhaddou-Andaloussi, A.; Nistor, L.; Afshar, A.; Arnason, J.T.; Haddad, P.S. Stimulation of AMP-activated protein kinase and enhancement of basal glucose uptake in muscle cells by quercetin and quercetin glycosides, active principles of the antidiabetic medicinal plant Vaccinium vitis-idaea. Mol. Nutr. Food Res. 2010, 54, 991–1003. [Google Scholar] [CrossRef]
- Dai, X.; Ding, Y.; Zhang, Z.; Cai, X.; Bao, L.; Li, Y. Quercetin but not quercitrin ameliorates tumor necrosis fac-tor-alpha-induced insulin resistance in C2C12 skeletal muscle cells. Biol. Pharm. Bull. 2013, 36, 788–795. [Google Scholar] [CrossRef] [Green Version]
- Kleinert, M.; Sylow, L.; Richter, E.A. Regulation of glycogen synthase in muscle and its role in Type 2 diabetes. Diabetes Manag. 2013, 3, 81–90. [Google Scholar] [CrossRef]
- Christ-Roberts, C.Y.; Pratipanawatr, T.; Pratipanawatr, W.; Berria, R.; Belfort, R.; Kashyap, S.; Mandarino, L.J. Exercise training increases glycogen synthase activity and GLUT4 expression but not insulin signaling in overweight nondiabetic and type 2 diabetic subjects. Metabolism 2004, 53, 1233–1242. [Google Scholar] [CrossRef]
- Zachwieja, J.; Pirner, M.; Smith, J. Methods of Reducing Blood Lactate Content. 2017. Available online: https://patents.google.com/patent/EP2615931A1/en (accessed on 19 June 2020).
- Alway, S.E.; McCrory, J.L.; Kearcher, K.; Vickers, A.; Frear, B.; Gilleland, D.L.; Bonner, D.E.; Thomas, J.M.; Donley, D.A.; Lively, M.W.; et al. Resveratrol Enhances Exercise-Induced Cellular and Functional Adaptations of Skeletal Muscle in Older Men and Women. J. Gerontol. A Boil. Sci. Med. Sci. 2017, 72, 1595–1606. [Google Scholar] [CrossRef] [Green Version]
- Pollack, R.M.; Barzilai, N.; Anghel, V.; Kulkarni, A.S.; Golden, A.; O’Broin, P.; Sinclair, D.A.; Bonkowski, M.S.; Coleville, A.J.; Powell, D.; et al. Resveratrol Improves Vascular Function and Mitochondrial Number but Not Glucose Metabolism in Older Adults. J. Gerontol. A Boil. Sci. Med. Sci. 2017, 72, 1703–1709. [Google Scholar] [CrossRef]
- Scribbans, T.D.; Ma, J.K.; Edgett, B.A.; Vorobej, K.A.; Mitchell, A.S.; Zelt, J.G.; Simpson, C.A.; Quadrilatero, J.; Gurd, B.J. Resveratrol supplementation does not augment performance adaptations or fibre-type–specific responses to high-intensity interval training in humans. Appl. Physiol. Nutr. Metab. 2014, 39, 1305–1313. [Google Scholar] [CrossRef] [PubMed]
- Olesen, J.; Gliemann, L.; Biensø, R.; Schmidt, J.; Hellsten, Y.; Pilegaard, H. Exercise training, but not resveratrol, improves metabolic and inflammatory status in skeletal muscle of aged men. J. Physiol. 2014, 592, 1873–1886. [Google Scholar] [CrossRef] [PubMed]
- Kjær, T.N.; Ornstrup, M.J.; Poulsen, M.M.; Stødkilde-Jørgensen, H.; Jessen, N.; Jørgensen, J.O.L.; Richelsen, B.; Pedersen, S.B. No Beneficial Effects of Resveratrol on the Metabolic Syndrome: A Randomized Placebo-Controlled Clinical Trial. J. Clin. Endocrinol. Metab. 2017, 102, 1642–1651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nieman, D.; Henson, D.A.; Maxwell, K.R.; Williams, A.S.; McAnulty, S.R.; Jin, F.; Shanely, R.A.; Lines, T.C. Effects of Quercetin and EGCG on Mitochondrial Biogenesis and Immunity. Med. Sci. Sports Exerc. 2009, 41, 1467–1475. [Google Scholar] [CrossRef] [PubMed]
- Nieman, D.; Williams, A.S.; Shanely, R.A.; Jin, F.; McAnulty, S.R.; Triplett, N.T.; Austin, M.D.; Henson, D.A. Quercetin’s Influence on Exercise Performance and Muscle Mitochondrial Biogenesis. Med. Sci. Sports Exerc. 2010, 42, 338–345. [Google Scholar] [CrossRef] [Green Version]
- Marín, L.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Bioavailability of Dietary Polyphenols and Gut Microbiota Metabolism: Antimicrobial Properties. BioMed Res. Int. 2015, 2015, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Ishizawa, K.; Yoshizumi, M.; Kawai, Y.; Terao, J.; Kihira, Y.; Ikeda, Y.; Tomita, S.; Minakuchi, K.; Tsuchiya, K.; Tamaki, T. Pharmacology in Health Food: Metabolism of Quercetin In Vivo and Its Protective Effect Against Arteriosclerosis. J. Pharmacol. Sci. 2011, 115, 466–470. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.; Sang, S. Metabolism and pharmacokinetics of resveratrol and pterostilbene. BioFactors 2018, 44, 16–25. [Google Scholar] [CrossRef]
- Luca, S.V.; Macovei, I.; Bujor, A.; Miron, A.; Skalicka-Woźniak, K.; Aprotosoaie, A.C.; Trifan, A. Bioactivity of dietary polyphenols: The role of metabolites. Crit. Rev. Food Sci. Nutr. 2020, 60, 626–659. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [Green Version]
- Lasa, A.; Churruca, I.; Eseberri, I.; Andrés-Lacueva, C.; Portillo, M.P. Delipidating effect of resveratrol metabolites in 3T3-L1 adipocytes. Mol. Nutr. Food Res. 2012, 56, 1559–1568. [Google Scholar] [CrossRef] [PubMed]
- Eseberri, I.; Miranda, J.; Lasa, A.; Mosqueda-Solís, A.; González-Manzano, S.; Santos-Buelga, C.; Portillo, M.P. Effects of Quercetin Metabolites on Triglyceride Metabolism of 3T3-L1 Preadipocytes and Mature Adipocytes. Int. J. Mol. Sci. 2019, 20, 264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galindo, P.; Rodríguez-Gómez, I.; González-Manzano, S.; Dueñas, M.; Jiménez, R.; Menéndez, C.; Vargas, F.; Tamargo, J.; Santos-Buelga, C.; Perez-Vizcaino, F.; et al. Glucuronidated Quercetin Lowers Blood Pressure in Spontaneously Hypertensive Rats via Deconjugation. PLoS ONE 2012, 7, e32673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishisaka, A.; Kawabata, K.; Miki, S.; Shiba, Y.; Minekawa, S.; Nishikawa, T.; Mukai, R.; Terao, J.; Kawai, Y. Mitochondrial Dysfunction Leads to Deconjugation of Quercetin Glucuronides in Inflammatory Macrophages. PLoS ONE 2013, 8, e80843. [Google Scholar] [CrossRef] [Green Version]
- Patel, K.R.; Andreadi, C.; Britton, R.G.; Horner-Glister, E.; Karmokar, A.; Sale, S.; Brown, V.A.; Brenner, D.E.; Singh, R.; Steward, W.P.; et al. Sulfate Metabolites Provide an Intracellular Pool for Resveratrol Generation and Induce Autophagy with Senescence. Sci. Transl. Med. 2013, 5, 205ra133. [Google Scholar] [CrossRef] [Green Version]
- Laurens, C.; Badin, P.-M.; Louche, K.; Mairal, A.; Tavernier, G.; Marette, A.; Tremblay, A.; Weisnagel, S.J.; Joanisse, D.R.; Langin, D.; et al. G0/G1 Switch Gene 2 controls adipose triglyceride lipase activity and lipid metabolism in skeletal muscle. Mol. Metab. 2016, 5, 527–537. [Google Scholar] [CrossRef]
- Coué, M.; Badin, P.-M.; Vila, I.K.; Laurens, C.; Louche, K.; Marquès, M.-A.; Bourlier, V.; Mouisel, E.; Tavernier, G.; Rustan, A.C.; et al. Defective Natriuretic Peptide Receptor Signaling in Skeletal Muscle Links Obesity to Type 2 Diabetes. Diabetes 2015, 64, 4033–4045. [Google Scholar] [CrossRef] [Green Version]
- Laurens, C.; Bourlier, V.; Mairal, A.; Louche, K.; Badin, P.-M.; Mouisel, E.; Montagner, A.; Marette, A.; Tremblay, A.; Weisnagel, J.S.; et al. Perilipin 5 fine-tunes lipid oxidation to metabolic demand and protects against lipotoxicity in skeletal muscle. Sci. Rep. 2016, 6, 38310. [Google Scholar] [CrossRef] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eseberri, I.; Laurens, C.; Miranda, J.; Louche, K.; Lasa, A.; Moro, C.; Portillo, M.P. Effects of Physiological Doses of Resveratrol and Quercetin on Glucose Metabolism in Primary Myotubes. Int. J. Mol. Sci. 2021, 22, 1384. https://doi.org/10.3390/ijms22031384
Eseberri I, Laurens C, Miranda J, Louche K, Lasa A, Moro C, Portillo MP. Effects of Physiological Doses of Resveratrol and Quercetin on Glucose Metabolism in Primary Myotubes. International Journal of Molecular Sciences. 2021; 22(3):1384. https://doi.org/10.3390/ijms22031384
Chicago/Turabian StyleEseberri, Itziar, Claire Laurens, Jonatan Miranda, Katie Louche, Arrate Lasa, Cedric Moro, and Maria P. Portillo. 2021. "Effects of Physiological Doses of Resveratrol and Quercetin on Glucose Metabolism in Primary Myotubes" International Journal of Molecular Sciences 22, no. 3: 1384. https://doi.org/10.3390/ijms22031384
APA StyleEseberri, I., Laurens, C., Miranda, J., Louche, K., Lasa, A., Moro, C., & Portillo, M. P. (2021). Effects of Physiological Doses of Resveratrol and Quercetin on Glucose Metabolism in Primary Myotubes. International Journal of Molecular Sciences, 22(3), 1384. https://doi.org/10.3390/ijms22031384