Paternal Inheritance of Bisphenol A Cardiotoxic Effects: The Implications of Sperm Epigenome
Abstract
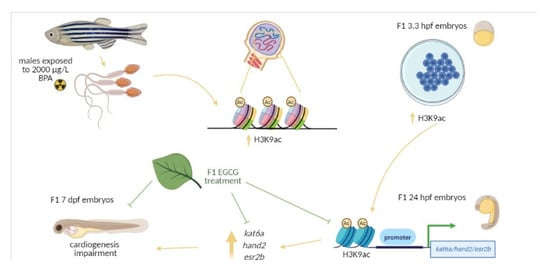
:1. Introduction
2. Results
2.1. Transcriptomic and Epigenetic Changes Triggered by Paternal BPA Exposure
2.2. Inhibition of BPA-Induced Histone Hyperacetylation with EGCG
2.3. Mitigation of Cardiac Toxicity Induced by Paternal BPA Exposure
3. Discussion
4. Materials and Methods
4.1. Ethics Statement
4.2. Zebrafish Maintenance and BPA Paternal Exposure
4.3. Embryo Collection and Experimental Design
4.4. Gene Expression
4.5. Whole Mount Immunostaining
4.6. Histone Acetylation in Gene Promoters
4.7. DNA Methylation in Gene Promoters
4.8. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Snijder, C.A.; Vlot, I.J.; Burdorf, A.; Obermann-Borst, S.A.; Helbing, W.A.; Wildhagen, M.F.; Steegers, E.A.P.; Steegers-Theunissen, R.P.M. Congenital heart defects and parental occupational exposure to chemicals. Hum. Reprod. 2012, 27, 1510–1517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernier, P.L.; Stefanescu, A.; Samoukovic, G.; Tchervenkov, C.I. The challenge of congenital heart disease worldwide: Epidemiologic and demographic facts. Semin. Thorac. Cardiovasc. Surg. Pediatr. Card. Surg. Annu. 2010, 13, 26–34. [Google Scholar] [CrossRef]
- Hoffman, J.I.E.; Kaplan, S. The incidence of congenital heart disease. J. Am. Coll. Cardiol. 2002, 39, 1890–1900. [Google Scholar] [CrossRef] [Green Version]
- Jenkins, D.J.A.; Kendall, C.W.C.; Nguyen, T.H.; Teitel, J.; Marchie, A.; Chiu, M.; Taha, A.Y.; Faulkner, D.A.; Kemp, T.; Wong, J.M.W.; et al. Effect on hematologic risk factors for coronary heart disease of a cholesterol reducing diet. Eur. J. Clin. Nutr. 2007, 61, 483–492. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.-P.; Bruneau, B.G. Epigenetics and Cardiovascular Development. Annu. Rev. Physiol. 2012, 74, 41–68. [Google Scholar] [CrossRef]
- Bogers, H.; Bos, D.; Schoenmakers, S.; Duvekot, J.J. Postpandemic influenza A/H1N1pdm09 is still causing severe perinatal complications. Mediterr. J. Hematol. Infect. Dis. 2015, 7, e2015007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colborn, T. Building Scientific Consensus on Endocrine Disruptors. Environ. Toxicol. Chem. 1998, 17, 1–2. [Google Scholar] [CrossRef]
- Bonde, J.; Giwercman, A. Environmental xenobiotics and male reproductive health. Asian J. Androl. 2014, 16, 3. [Google Scholar] [CrossRef]
- Thulstrup, A.M.; Bonde, J.P. Maternal occupational exposure and risk of specific birth defects. Occup. Med. 2006, 56, 532–543. [Google Scholar] [CrossRef] [Green Version]
- Chia, S.E.; Shi, L.M. Review of recent epidemiological studies on paternal occupations and birth defects. Occup. Environ. Med. 2002, 59, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Skinner, M.K. Endocrine disruptor induction of epigenetic transgenerational inheritance of disease. Mol. Cell. Endocrinol. 2014, 398, 4–12. [Google Scholar] [CrossRef] [Green Version]
- Combarnous, Y.; Nguyen, T.M.D. Comparative overview of the mechanisms of action of hormones and endocrine disruptor compounds. Toxics 2019, 7, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.; Choi, K.; Park, J.; Moon, H.B.; Choi, G.; Lee, J.J.; Suh, E.; Kim, H.J.; Eun, S.H.; Kim, G.H.; et al. Bisphenol A distribution in serum, urine, placenta, breast milk, and umbilical cord serum in a birth panel of mother–neonate pairs. Sci. Total Environ. 2018, 626, 1494–1501. [Google Scholar] [CrossRef] [PubMed]
- Wetherill, Y.B.; Akingbemi, B.T.; Kanno, J.; McLachlan, J.A.; Nadal, A.; Sonnenschein, C.; Watson, C.S.; Zoeller, R.T.; Belcher, S.M. In vitro molecular mechanisms of bisphenol A action. Reprod. Toxicol. 2007, 24, 178–198. [Google Scholar] [CrossRef] [PubMed]
- Gore, A.C.; Chappell, V.A.; Fenton, S.E.; Flaws, J.A.; Nadal, A.; Prins, G.S.; Toppari, J.; Zoeller, R.T. Executive Summary to EDC-2: The Endocrine Society’s second Scientific Statement on endocrine-disrupting chemicals. Endocr. Rev. 2015, 36, 593–602. [Google Scholar] [CrossRef] [Green Version]
- Moreman, J.; Lee, O.; Trznadel, M.; David, A.; Kudoh, T.; Tyler, C.R. Acute Toxicity, Teratogenic, and Estrogenic Effects of Bisphenol A and Its Alternative Replacements Bisphenol S, Bisphenol F, and Bisphenol AF in Zebrafish Embryo-Larvae. Environ. Sci. Technol. 2017, 51, 12796–12805. [Google Scholar] [CrossRef] [PubMed]
- Ross Brown, A.; Green, J.M.; Moreman, J.; Gunnarsson, L.M.; Mourabit, S.; Ball, J.; Winter, M.J.; Trznadel, M.; Correia, A.; Hacker, C.; et al. Cardiovascular Effects and Molecular Mechanisms of Bisphenol A and Its Metabolite MBP in Zebrafish. Environ. Sci. Technol. 2019, 53, 463–474. [Google Scholar] [CrossRef] [Green Version]
- Lombó, M.; González-Rojo, S.; Fernández-Díez, C.; Herráez, M.P. Cardiogenesis impairment promoted by bisphenol A exposure is successfully counteracted by epigallocatechin gallate. Environ. Pollut. 2019, 246, 1008–1019. [Google Scholar] [CrossRef]
- Chapalamadugu, K.C.; VandeVoort, C.A.; Settles, M.L.; Robison, B.D.; Murdoch, G.K. Maternal bisphenol a exposure impacts the fetal heart transcriptome. PLoS ONE 2014, 9, e89096. [Google Scholar] [CrossRef] [Green Version]
- Lombó, M.; Fernández-Díez, C.; González-Rojo, S.; Navarro, C.; Robles, V.; Herráez, M.P. Transgenerational inheritance of heart disorders caused by paternal bisphenol A exposure. Environ. Pollut. 2015, 206, 667–678. [Google Scholar] [CrossRef]
- González-Rojo, S.; Lombó, M.; Fernández-Díez, C.; Herráez, M.P. Male exposure to bisphenol a impairs spermatogenesis and triggers histone hyperacetylation in zebrafish testes. Environ. Pollut. 2019, 248, 368–379. [Google Scholar] [CrossRef]
- Lombó, M.; Fernández-díez, C.; González-rojo, S.; Herráez, M.P. Genetic and epigenetic alterations induced by bisphenol A exposure during different periods of spermatogenesis: From spermatozoa to the progeny. Sci. Rep. 2019, 9, 18029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toyoshima, Y.; Monson, C.; Duan, C.; Wu, Y.; Gao, C.; Yakar, S.; Sadler, K.C.; LeRoith, D. The role of insulin receptor signaling in zebrafish embryogenesis. Endocrinology 2008, 149, 5996–6005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carrell, D.T.; Hammoud, S.S. The human sperm epigenome and its potential role in embryonic development. Mol. Hum. Reprod. 2010, 16, 37–47. [Google Scholar] [CrossRef] [Green Version]
- Brykczynska, U.; Hisano, M.; Erkek, S.; Ramos, L.; Oakeley, E.J.; Roloff, T.C.; Beisel, C.; Schübeler, D.; Stadler, M.B.; Peters, A.H.F.M. Repressive and active histone methylation mark distinct promoters in human and mouse spermatozoa. Nat. Struct. Mol. Biol. 2010, 17, 679–687. [Google Scholar] [CrossRef] [PubMed]
- Anway, M.D.; Cupp, A.S.; Uzumcu, M.; Skinner, M.K. Epigenetic transgenerational actions of endocrine disruptors and male fertility. Science 2005, 308, 1466–1469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doyle, T.J.; Bowman, J.L.; Windell, V.L.; McLean, D.J.; Kim, K.H. Transgenerational effects of di-(2-ethylhexyl) phthalate on testicular germ cell associations and spermatogonial stem cells in mice. Biol. Reprod. 2013, 88, 112. [Google Scholar] [CrossRef]
- Anway, M.D.; Skinner, M.K. Symposium: Nuclear reprogramming and the control of differentiation in mammalian embryos: Epigenetic programming of the germ line: Effects of endocrine disruptors on the development of transgenerational disease. Reprod. Biomed. Online 2008, 16, 23–25. [Google Scholar] [CrossRef]
- Jiang, L.; Zhang, J.; Wang, J.J.; Wang, L.; Zhang, L.; Li, G.; Yang, X.; Ma, X.; Sun, X.; Cai, J.; et al. Sperm, but not oocyte, DNA methylome is inherited by zebrafish early embryos. Cell 2013, 153, 773–784. [Google Scholar] [CrossRef] [Green Version]
- Potok, M.E.; Nix, D.A.; Parnell, T.J.; Cairns, B.R. Reprogramming the maternal zebrafish genome after fertilization to match the paternal methylation pattern. Cell 2013, 153, 759–772. [Google Scholar] [CrossRef] [Green Version]
- Baccarelli, A.; Rienstra, M.; Benjamin, E.J. Cardiovascular epigenetics: Basic concepts and results from animal and human studies. Circ. Cardiovasc. Genet. 2010. [Google Scholar] [CrossRef] [Green Version]
- Yerra, V.G.; Advani, A. Histones and heart failure in diabetes. Cell. Mol. Life Sci. 2018, 75, 3193–3213. [Google Scholar] [CrossRef] [Green Version]
- Gaikwad, A.B.; Sayyed, S.G.; Lichtnekert, J.; Tikoo, K.; Anders, H.J. Renal failure increases cardiac histone H3 acetylation, dimethylation, and phosphorylation and the induction of cardiomyopathy-related genes in type 2 diabetes. Am. J. Pathol. 2010, 176, 1079–1083. [Google Scholar] [CrossRef] [Green Version]
- Gilsbach, R.; Preissl, S.; Grüning, B.A.; Schnick, T.; Burger, L.; Benes, V.; Würch, A.; Bönisch, U.; Günther, S.; Backofen, R.; et al. Dynamic DNA methylation orchestrates cardiomyocyte development, maturation and disease. Nat. Commun. 2014, 5. [Google Scholar] [CrossRef] [Green Version]
- León, L.E.; Benavides, F.; Espinoza, K.; Vial, C.; Alvarez, P.; Palomares, M.; Lay-Son, G.; Miranda, M.; Repetto, G.M. Partial microduplication in the histone acetyltransferase complex member KANSL1 is associated with congenital heart defects in 22q11.2 microdeletion syndrome patients. Sci. Rep. 2017, 7, 1795. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.-Y.; Zhao, C.-N.; Gan, R.-Y.; Xu, X.-Y.; Wei, X.-L.; Corke, H.; Atanasov, A.G.; Li, H.-B. Effects and mechanisms of tea and its bioactive compounds for the prevention and treatment of cardiovascular diseases: An updated review. Antioxidants 2019, 8, 166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eng, Q.Y.; Thanikachalam, P.V.; Ramamurthy, S. Molecular understanding of Epigallocatechin gallate (EGCG) in cardiovascular and metabolic diseases. J. Ethnopharmacol. 2018, 210, 296–310. [Google Scholar] [CrossRef]
- Kuruto-Niwa, R.; Inoue, S.; Ogawa, S.; Muramatsu, M.; Nozawa, R. Effects of tea catechins on the ERE-regulated estrogenic activity. J. Agric. Food Chem. 2000, 48, 6355–6361. [Google Scholar] [CrossRef]
- Farabegoli, F.; Barbi, C.; Lambertini, E.; Piva, R. (-)-Epigallocatechin-3-gallate downregulates estrogen receptor alpha function in MCF-7 breast carcinoma cells. Cancer Detect. Prev. 2007, 31, 499–504. [Google Scholar] [CrossRef]
- Choi, K.C.; Myung, G.J.; Lee, Y.H.; Joo, C.Y.; Seung, H.K.; Kang, H.B.; Kim, M.J.; Cha, J.H.; Young, J.K.; Woo, J.J.; et al. Epigallocatechin-3-gallate, a histone acetyltransferase inhibitor, inhibits EBV-induced B lymphocyte transformation via suppression of RelA acetylation. Cancer Res. 2009, 69, 583–592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, N.; Lu, R.; Lin, J.; Zhi, S.; Tian, J.; Zhu, J. Islet-1 promotes the cardiac-specific differentiation of mesenchymal stem cells through the regulation of histone acetylation. Int. J. Mol. Med. 2014, 33, 1075–1082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lam, S.H.; Hlaing, M.M.; Zhang, X.; Yan, C.; Duan, Z.; Zhu, L.; Ung, C.Y.; Mathavan, S.; Ong, C.N.; Gong, Z. Toxicogenomic and phenotypic analyses of bisphenol-a early-life exposure toxicity in zebrafish. PLoS ONE 2011, 6, e28273. [Google Scholar] [CrossRef] [Green Version]
- Makarova, K.; Siudem, P.; Zawada, K.; Kurkowiak, J. Screening of toxic effects of Bisphenol A and products of its degradation: Zebrafish (Danio rerio) embryo test and molecular docking. Zebrafish 2016, 13, 466–474. [Google Scholar] [CrossRef] [PubMed]
- Moreman, J.; Takesono, A.; Trznadel, M.; Winter, M.J.; Perry, A.; Wood, M.E.; Rogers, N.J.; Kudoh, T.; Tyler, C.R. Estrogenic mechanisms and cardiac responses following early life exposure to Bisphenol A (BPA) and its metabolite 4-methyl-2,4-bis(p -hydroxyphenyl)pent-1-ene (MBP) in zebrafish. Environ. Sci. Technol. 2018, 52, 6656–6665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roth, S.Y.; Denu, J.M.; Allis, C.D. Histone acetyltransferases. Annu. Rev. Biochem. 2001, 70, 81–120. [Google Scholar] [CrossRef]
- Abi Khalil, C. The emerging role of epigenetics in cardiovascular disease. Ther. Adv. Chronic Dis. 2014, 5, 178–187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Butyrate, B.; Davie, J.R. Nutritional Proteomics in Cancer Prevention Inhibition of Histone Deacetylase Activity. J. Nutr. 2003, 133, 2485–2493. [Google Scholar]
- Goodin, M.G.; Fertuck, K.C.; Zacharewski, T.R.; Rosengren, R.J. Estrogen receptor-mediated actions of polyphenolic catechins in vivo and in vitro. Toxicol. Sci. 2002, 69, 354–361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Amicis, F.; Russo, A.; Avena, P.; Santoro, M.; Vivacqua, A.; Bonofiglio, D.; Mauro, L.; Aquila, S.; Tramontano, D.; Fuqua, S.A.; et al. In vitro mechanism for downregulation of ER-alpha expression by epigallocatechin gallate in ER+/PR+ human breast cancer cells. Mol. Nutr. Food Res. 2013, 57, 840–853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leal, M.C.; Cardoso, E.R.; Nóbrega, R.H.; Batlouni, S.R.; Bogerd, J.; França, L.R.; Schulz, R.W. Histological and stereological evaluation of zebrafish (Danio rerio) Spermatogenesis with an emphasis on spermatogonial generations1. Biol. Reprod. 2009, 81, 177–187. [Google Scholar] [CrossRef] [Green Version]
- Willhite, C.C.; Ball, G.L.; McLellan, C.J. Derivation of a bisphenol a oral reference dose (RfD) and drinking-water equivalent concentration. J. Toxicol. Environ. Health Part B Crit. Rev. 2008, 11, 69–146. [Google Scholar] [CrossRef] [PubMed]
- Wilk, B.K.; Fudala-Ksiazek, S.; Szopińska, M.; Luczkiewicz, A. Landfill leachates and wastewater of maritime origin as possible sources of endocrine disruptors in municipal wastewater. Environ. Sci. Pollut. Res. 2019, 26, 25690–25701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marty, M.S.; Borgert, C.; Coady, K.; Green, R.; Levine, S.L.; Mihaich, E.; Ortego, L.; Wheeler, J.R.; Yi, K.D.; Zorrilla, L.M. Distinguishing between endocrine disruption and non-specific effects on endocrine systems. Regul. Toxicol. Pharmacol. 2018, 99, 142–158. [Google Scholar] [CrossRef]
- Tohmé, M.; Prud’homme, S.M.; Boulahtouf, A.; Samarut, E.; Brunet, F.; Bernard, L.; Bourguet, W.; Gibert, Y.; Balaguer, P.; Laudet, V. Estrogen-related receptor γ is an in vivo receptor of bisphenol A. FASEB J. 2014, 28, 3124–3133. [Google Scholar] [CrossRef]
- Bardet, P.L.; Horard, B.; Robinson-Rechavi, M.; Laudet, V.; Vanacker, J.M. Characterization of oestrogen receptors in zebrafish (Danio rerio). J. Mol. Endocrinol. 2002, 28, 153–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stainier, D.Y.R. Zebrafish genetics and vertebrate heart formation. Nat. Rev. Genet. 2001, 2, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Santos-Pereira, J.M.; Gallardo-Fuentes, L.; Neto, A.; Acemel, R.D.; Tena, J.J. Pioneer and repressive functions of p63 during zebrafish embryonic ectoderm specification. Nat. Commun. 2019, 10, 3049. [Google Scholar] [CrossRef] [PubMed]






| Normalized Published Data | Normalized Original Data | |||
|---|---|---|---|---|
| F1 Embryos from Males Exposed to 2000 μg/L BPA | Reference | F1 Embryos from Control Unexposed Males Males, Treated with 50 μM EGCG | F1 Embryos from Males Exposed to 2000 μg/L BPA, Treated with 50 μM EGCG | |
| H3K9ac | 5.24 ± 1.71 | [22] | 0.88 ± 0.25 | 0.79 ± 0.31 |
| H3K27ac | 2.79 ± 1.04 | [22] | 0.92 ± 0.51 | 0.87 ± 0.16 |
| kat6a expression (2−ΔCt) | 4.29 ± 5.32 | [22] | 1.14 ± 0.24 | 0.64 ± 0.31 |
| Mortality at 24 hpf | 2.27% ± 0.25 | [22] | 1.11% ± 0.7 | 0.58% ± 0.3 |
| Mortality at 48 hpf | 2.18% ± 0.24 | [22] | 1.21% ± 0.54 | 0.57% ± 0.3 |
| Mortality at 72 hpf | 1.97% ± 0.21 | [22] | 1.19% ± 0.52 | 0.58% ± 0.28 |
| Mortality at 96 hpf | 1.56% ± 0.14 | [22] | 0.38% ± 0.54 | 0.006% ± 0.009 |
| Mortality at 120 hpf | 1.59% ± 0.11 | [22] | 1.11% ± 0.32 | 0.55% ± 0.37 |
| Malformations At 7 dpf | 5.61 ± 0.49 | [20] | 2.01 ± 0.72 | 1.35 ± 1.34 |
| F1 Embryos from Males Exposed to 2000 μg/L BPA | F1 Embryos from Control Unexposed Males Males, Treated with 50 μM EGCG | F1 Embryos from Males Exposed to 2000 μg/L BPA, Treated with 50 μM EGCG | |
|---|---|---|---|
| esr2b expression (2−ΔCt) | 2.71 ± 0.56 | 0.77 ± 0.27 | 1.18 ± 0.85 |
| hand2 expression (2−ΔCt) | 2.15 ± 1.38 | 1.14 ± 0.24 | 0.64 ± 0.31 |
| Primers Used for qPCR | |||||
| Gene | Primers | Product Size (bp) | Temperature (°C) | Accesion Number | Efficiency (%) |
| gata4 | F: CCGCTCGTGGAGCAATAATC R: CTGGATCATCGGAGTCACCC | 154 | 64 | DQ886664.1 | 92 |
| bmp4 | F: GCGCTGGACCCAAGAAAAAC R: TTGCCGTCATGTCCGAATGT | 177 | 64 | NM_131342.2 | 99 |
| Primers Used for Gene Promoter Methylation | |||||
| Gene | Forward Primer | Reverse Primer | Product Size | Use | |
| esr2b | TAGGTTAGGGTTTTTTTTGT | AAACTAAATTATTCTCACCTACTC | 439 | Total PCR | |
| hand2 | TATTTTTTGAGTTGTTTGGG | CCCTTCACCAAAAATTTTAA | 549 | Total PCR | |
| esr2b | GAGGTTTGTTAGGATTATTTTTT | ATATATCTTAACCTCCTCCC | 233 | Nested PCR | |
| hand2 | TTAAAAGTAGTTAATTTATTGGT | ACTAATCCTTATACTACATTC | 295 | Nested PCR | |
| Primers Used for Pyrosequencing | |||||
| Sequences for esr2b | Product Size | Sequences for hand2 | Product Size | ||
| GTTTGTTAGGATTATTTTTT | 28 | TTATTTATTTAAAAAAAAAA | 36 | ||
| TTTAATTACGGAGTTTTA | 18 | GGTTTTTTTTTTTTTTTAGTGTGTG | 26 | ||
| TTGTAGTGTTCGGTTTTT | 33 | TAAATTAGTTTAAGTATATT | 15 | ||
| GTCGTTTTATTTTTTGTAT | 34 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lombó, M.; Herráez, M.P. Paternal Inheritance of Bisphenol A Cardiotoxic Effects: The Implications of Sperm Epigenome. Int. J. Mol. Sci. 2021, 22, 2125. https://doi.org/10.3390/ijms22042125
Lombó M, Herráez MP. Paternal Inheritance of Bisphenol A Cardiotoxic Effects: The Implications of Sperm Epigenome. International Journal of Molecular Sciences. 2021; 22(4):2125. https://doi.org/10.3390/ijms22042125
Chicago/Turabian StyleLombó, Marta, and María Paz Herráez. 2021. "Paternal Inheritance of Bisphenol A Cardiotoxic Effects: The Implications of Sperm Epigenome" International Journal of Molecular Sciences 22, no. 4: 2125. https://doi.org/10.3390/ijms22042125
APA StyleLombó, M., & Herráez, M. P. (2021). Paternal Inheritance of Bisphenol A Cardiotoxic Effects: The Implications of Sperm Epigenome. International Journal of Molecular Sciences, 22(4), 2125. https://doi.org/10.3390/ijms22042125