Cerebral Expression of Metabotropic Glutamate Receptor Subtype 5 in Idiopathic Autism Spectrum Disorder and Fragile X Syndrome: A Pilot Study
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Participants
4.1.1. Recruiting Sites
4.1.2. Inclusion Criteria
4.1.3. Exclusion Criteria
4.2. Procedures
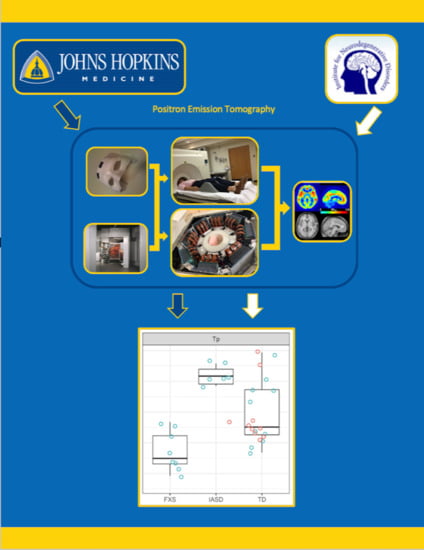
4.2.1. Positron Emission Tomography (PET)
4.2.2. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| [18F]FPEB | 3-[18F]fluoro-5-(2-pyridinylethynyl)benzonitrile |
| ADI-R | Autism Diagnostic Interview-Revised [50] |
| ADOS | Autism Diagnostic Observation Schedule [51] |
| ANOVA | analysis of variance |
| ASD | autism spectrum disorder |
| BMI | basal metabolic index |
| BPND | non-displaceable binding potential |
| CEA | Commissariat à l’Énergie Atomique et aux Énergies Alternatives |
| CDC | Centers for Disease Control and Prevention |
| CN | caudate nucleus |
| CNAMI | CNS Neuropsychopharmacology and Multimodal Imaging |
| CNMT | Certified Nuclear Medicine Technologist |
| CNRS | Centre National de la Recherche Scientifique |
| CNS | central nervous system |
| DSM-5 | Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition [1] |
| DTI | diffusion tensor imaging |
| EEG | electroencephalography |
| ERK | extracellular signal-regulated kinase |
| ERP | event-related brain potential |
| FORWARD | Fragile X Online Registry With Accessible Research Database of the National Fragile X Foundation (NFXF) |
| FMR1 | fragile X mental retardation 1 gene |
| fmr1 | fmr1 gene in knockout mouse model of fragile X syndrome |
| FMRP | Fragile X Mental Retardation Protein |
| FXS | fragile X syndrome |
| FXS-M | fragile X syndrome allele size mosaicism |
| GABA | gamma amino butyric acid |
| HRRT | high resolution research tomograph [53] |
| HSD | Honest Standard Differences [31] |
| IASD | idiopathic autism spectrum disorder |
| ID | intellectual disability |
| IND | Institute for Neurodegenerative Disorders |
| IJMS | International Journal of Molecular Sciences |
| JHU | Johns Hopkins University |
| KO | knockout |
| LTD | long-term depression |
| MDPI | Multidisciplinary Digital Publishing Institute |
| MPAK | mitogen-activated protein kinase (MAPK) |
| MBq | megabecquerel |
| mCi | millicurie |
| mGluR1/5 | metabotropic glutamate receptors subtypes 1 and 5 |
| mGluR5 | metabotropic glutamate receptor subtype 5 |
| mGluR-LTD | metabotropic glutamate receptor dependent longterm depression |
| MIRCen | Molecular Imaging Research Center |
| MR | magnetic resonance |
| MRS | magnetic resonance spectroscopy |
| mTOR | mammalian target of rapamycin |
| mTp | medial temporal cortex |
| NAM | negative allosteric modulator |
| NFXF | National Fragile X Foundation |
| NMTCB | Nuclear Medicine Technology Certification Board |
| Oc | occipital cortex |
| Pa | parietal cortex |
| pCg | posterior cingulate cortex |
| PCR | polymerase chain reaction |
| PET | positron emission tomography |
| PET/MRI | positron emission tomography/magnetic resonance imaging |
| Pu | putamen |
| ROI | region of interest |
| rs-fMRI | resting state functional magnetic resonance imaging |
| RTGA | reference tissue graphical analysis |
| SPM | Statistical Parametric Mapping |
| TD | typical development |
| Th | thalamus |
| Tp | temporal cortex |
| (period) | missing data |
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, (DSM-5), 5th ed.; American Psychiatric Association: Arlington, VA, USA, 2013. [Google Scholar]
- Brasic, J.R.; Farhadi, F.; Elshourbagy, T. Autism Spectrum Disorder. Medscape Drugs Dis. Updated on 18 March 2020. 2020. Available online: http://emedicine.medscape.com/article/912781-overview (accessed on 6 March 2021).
- Genovese, A.; Butler, M.G. Clinical assessment, genetics, and treatment approaches in autism spectrum disorder (ASD). Int. J. Mol. Sci. 2020, 21, 4726. [Google Scholar] [CrossRef]
- Budimirovic, D.; Haas-Givler, B.; Blitz, R.; Esler, A.; Kaufmann, W.; Sudhalter, V.; Stackhouse, T.M.; Scharfenaker, S.K.; Berry-Kravis, E. Consensus of the Fragile X Clinical & Research Consortium on Clinical Practices: Autism Spectrum Disorder in Fragile X Syndrome. The Fragile X Clinical & Research Consortium. Available online: https://fragilex.org/wp-content/uploads/2012/08/Autism-Spectrum-Disorder-in-Fragile-X-Syndrome-2014-Nov.pdf (accessed on 6 March 2021).
- Budimirovic, D.B.; Kaufmann, W.E. What can we learn about autism from studying fragile X syndrome? Dev. Neurosci. 2011, 33, 379–394. [Google Scholar] [CrossRef] [Green Version]
- Haas-Givler, B.; Taylor, C.M.; Riley, K.; Braden, M.; Budimirovic, D.; Frazier, J.; Kinney, M.; Stackhouse, T.; Scharfenaker, S.; Kaufmann, W. Consensus of the Fragile X Clinical & Research Consortium on Clinical Practices: Behavioral challenges in fragile X syndrome. The Fragile X Clinical & Research Consortium. 2018. Available online: https://fragilex.org/wp-content/uploads/2018/12/Behavior-Challenges-in-Fragile-X-Treatment-Guidelines.pdf (accessed on 6 March 2021).
- Jęśko, H.; Cieślik, M.; Gromadzka, G.; Adamczyk, A. Dysfunctional proteins in neuropsychiatric disorders: From neurodegeneration to autism spectrum disorders. Neurochem. Int. 2020, 141, 104853. [Google Scholar] [CrossRef] [PubMed]
- Zantomio, D.; Chana, G.; Laskaris, L.; Testa, R.; Everall, I.; Pantelis, C.; Skafidas, E. Convergent evidence for mGluR5 in synaptic and neuroinflammatory pathways implicated in ASD. Neurosci. Biobehav. Rev. 2015, 52, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Budimirovic, D.B.; Berry-Kravis, E.; Erickson, C.A.; Hall, S.S.; Hessl, D.; Reiss, A.L.; King, M.K.; Abbeduto, L.; Kaufmann, W.E. Updated report on tools to measure outcomes of clinical trials in fragile X syndrome. J. Neurodev. Disord. 2017, 9, 14. [Google Scholar] [CrossRef]
- Budimirovic, D.B.; Schlageter, A.; Filopovic-Sadic, S.; Protic, D.D.; Bram, E.; Mahone, E.M.; Nicholson, K.; Culp, K.; Javanmardi, K.; Kemppainnen, J.; et al. A genotype-phenotype study of high-resolution FMR1 nucleic acid and protein analyses in fragile X patients with neurobehavioral assessments. Brain Sci. 2020, 10, 694. [Google Scholar] [CrossRef]
- Budimirovic, D.B.; Subramanian, M. Neurobiology of Autism and Intellectual Disability: Fragile X Syndrome. In Neurobiology of Disease, 2nd ed.; Johnston, M.V., Ed.; Oxford University Press: New York, NY, USA, 2016; pp. 375–384. [Google Scholar]
- Duy, P.Q.; Budimirovic, D.B. Fragile X syndrome: Lessons learned from the most translated neurodevelopmental disorder in clinical trials. Transl. Neurosci. 2017, 8, 7–8. [Google Scholar] [CrossRef] [Green Version]
- Chana, G.; Laskaris, L.; Pantelis, C.; Gillett, P.; Testa, R.; Zantomio, D.; Burrows, E.L.; Hannan, A.J.; Everall, I.P.; Skafidas, E. Decreased expression of mGluR5 within the dorsolateral prefrontal cortex in autism and increased microglial number in mGluR5 knockout mice: Pathophysiological and neurobehavioral implications. Brain Behav. Immun. 2015, 49, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Fatemi, S.H.; Wong, D.F.; Brašić, J.R.; Kuwabara, H.; Mathur, A.; Folsom, T.D.; Jacob, S.; Realmuto, G.M.; Pardo, J.V.; Lee, S. Metabotropic glutamate receptor 5 tracer [18F]-FPEB displays increased binding potential in postcentral gyrus and cerebellum of male individuals with autism: A pilot PET study. Cerebellum Ataxias 2018, 5, 3. Available online: http://rdcu.be/GQb3 (accessed on 6 March 2021). [CrossRef]
- van Gelder, C.A.G.H.; Penning, R.; Veth, T.S.; Catsburg, L.A.D.; Hoogenraad, C.C.; MacGillavry, H.D.; Altelaar, M. Temporal quantitative proteomics of mGluR-induced protein translation and phosphorylation in neurons. Mol. Cell. Proteomics 2020, 19, 1952–1967. [Google Scholar] [CrossRef]
- Bear, M.F.; Huber, K.M.; Warren, S.T. The mGluR theory of fragile X mental retardation. Trends Neurosci. 2004, 27, 370–377. [Google Scholar] [CrossRef]
- Brašić, J.R.; Nandi, A.; Russell, D.S.; Jennings, D.; Barret, O.; Mathur, A.; Slifer, K.; Sedlak, S.; Martin, S.D.; Brinson, Z.; et al. Reduced cerebral expression of metabotropic glutamate receptor subtype 5 in men with fragile X syndrome. Brain Sci. 2020, 10, 899. [Google Scholar] [CrossRef] [PubMed]
- Eltokhi, A.; Santuy, A.; Merchan-Perez, A.; Sprengel, R. Glutamatergic dysfunction and synaptic ultrastructural alterations in schizophrenia and autism spectrum disorder: Evidence from human and rodent studies. Int. J. Mol. Sci. 2021, 22, 59. [Google Scholar] [CrossRef] [PubMed]
- Basilico, B.; Morandell, J.; Novarino, G. Molecular mechanisms for targeted ASD treatments. Curr. Opin. Genet. Dev. 2020, 65, 126–137. [Google Scholar] [CrossRef] [PubMed]
- Burket, J.A.; Herndon, A.L.; Winebarger, E.E.; Jacome, L.F.; Deutsch, S.I. Complex effects of mGluR5 antagonism on sociability and stereotypic behaviors in mice: Possible implications for the pharmacotherapy of autism spectrum disorders. Brain Res. Bull. 2011, 86, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Hooshmandi, M.; Wong, C.; Khoutorsky, A. Dysregulation of translational control signaling in autism spectrum disorders. Cell. Signal. 2020, 75, 109746. [Google Scholar] [CrossRef] [PubMed]
- Zoicas, I.; Kornhuber, J. The role of metabotropic glutamate receptors in social behavior in rodents. Int. J. Mol. Sci. 2019, 20, 1412. [Google Scholar] [CrossRef] [Green Version]
- Brasic, J.; Mishra, C.; Mathur, A.; Sweeney, K.; Folsom, T.; Kitzmiller, K.; Mellinger-Pilgrim, R.; Wong, D.; Fatemi, S. Microdose PET for the metabotropic glutamate receptor type 5 (mGluR5). J. Nucl. Med. 2018, 59 (Suppl. 1), 1774. Available online: http://jnm.snmjournals.org/content/59/supplement_1/1774.abstract (accessed on 6 March 2021).
- Brasic, J.R.; Mathur, A.K.; Budimirovic, D.B. Clinical trials of pharmacological agents for developmental disabilities: Potential tools to demonstrate target engagement in children and adolescents. Md. Reg. Counc. Child Adolesc. Psychiatry (MRCCAP) 2020, 1, 2. [Google Scholar]
- Wong, D.F.; Waterhouse, R.; Kuwabara, H.; Kim, J.; Brašić, J.R.; Chamroonrat, W.; Stabins, M.; Holt, D.P.; Dannals, R.F.; Hamill, T.G.; et al. 18F-FPEB, a PET radiopharmaceutical for quantifying metabotropic glutamate 5 receptors: A first-in-human study of radiochemical safety, biokinetics, and radiation dosimetry. J. Nucl. Med. 2013, 54, 388–396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brašić, J.R.; Nandi, A.; Russell, D.S.; Jennings, D.; Barret, O.; Mathur, A.; Slifer, K.; Sedlak, S.; Martin, S.D.; Brinson, Z.; et al. Reduced expression of cerebral metabotropic glutamate receptor subtype 5 in men with fragile X syndrome. Zenodo 2020, v1. [Google Scholar] [CrossRef]
- Innis, R.B.; Cunningham, V.J.; Delforge, J.; Fujita, M.; Gjedde, A.; Gunn, R.N.; Holden, J.; Houle, S.; Huang, S.C.; Ichise, M.; et al. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J. Cereb. Blood Flow Metab. 2007, 27, 1533–1539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brasic, J.R.; Syed, A.B.; Farhadi, F.; Wong, D.F. PET Scanning in Autism Spectrum Disorder. Medscape Drugs Dis. Updated on 16 April 2020. 2020. Available online: http://emedicine.medscape.com/article/1155568-overview (accessed on 6 March 2021).
- Weissgerber, T.L.; Savic, M.; Winham, S.J.; Stanisavljevic, D.; Garovic, V.D.; Milic, N.M. Data visualization, bar naked: A free tool for creating interactive graphics. J. Biol. Chem. 2017, 292, 20592–20598. [Google Scholar] [CrossRef] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing; R Core Team: Vienna, Austria, 2020; Available online: https://www.R-project-org (accessed on 6 March 2021).
- Bretz, F.; Hothorn, T.; Westfall, P. Multiple Comparisons Using R; Chapman & Hall/CRC, Taylor and Francis Group, LLC: Boca Raton, FL, USA, 2011. [Google Scholar]
- Wong, D.F.; Wagner, H.N.; Dannals, R.F.; Links, J.M.; Frost, J.J.; Ravert, H.T.; Wilson, A.A.; Rosenbaum, A.E.; Gjedde, A.; Douglass, K.; et al. Effects of age on dopamine and serotonin receptors measured by positron tomography in the living human brain. Science 1984, 226, 1393–1396. [Google Scholar]
- Chugani, H.T. Positron emission tomography in pediatric neurodegenerative disorders. Pediatr. Neurol. 2019, 100, 12–25. [Google Scholar] [CrossRef] [PubMed]
- Brašić, J.R.; Bibat, G.; Kumar, A.; Zhou, Y.; Hilton, J.; Yablonski, M.E.; Dogan, A.S.; Guevara, M.R.; Stephane, M.; Johnston, M.; et al. Correlation of the vesicular acetylcholine transporter densities in the striata to the clinical abilities of women with Rett syndrome. Synapse 2012, 66, 471–482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, D.F.; Blue, M.E.; Brašić, J.R.; Nandi, A.; Valentine, H.; Stansfield, K.H.; Rousset, O.; Bibat, G.; Yablonski, M.E.; Johnston, M.V.; et al. Are dopamine receptor and transporter changes in Rett syndrome reflected in Mecp2-deficient mice? Exp. Neurol. 2018, 307, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Carson, R.E. Tracer Kinetic Modeling in PET. In Positron Emission Tomography: Basic Science and Clinical Practice; Valk, P.E., Bailey, D.L., Townsend, D.W., Maisey, M.N., Eds.; Springer-Verlag: London, UK, 2003; pp. 147–179. [Google Scholar]
- Carey, C.; Dunn, J.; Mendez, M.A.; Velthuis, H.; Pereira, A.C.; Pretzsch, C.; Horder, J.; Veronese, M.; Lythgoe, D.; Murphy, D.; et al. mGluR5 receptor density using positron emission tomography in autism spectrum disorder versus healthy controls: Comparison with magnetic resonance spectroscopy. Eur. Neuropsychopharmacol. 2019, 29 (Suppl. 6), S540–S541. [Google Scholar]
- Catana, C. Principles of simultaneous PET/MR imaging. Magn. Reson. Imaging Clin. N. Am. 2017, 25, 231–243. [Google Scholar] [CrossRef] [Green Version]
- Elshourbagy, T.; Mousa, A.; Mohamed, M.A.; Brasic, J.R. Differentiation of zaghrouta, ululation to express joy in the Middle East, from movement disorders and other conditions. Int. J. Health Life Sci. 2021, 7, e106655. [Google Scholar] [CrossRef]
- Razak, K.A.; Dominick, K.C.; Erickson, C.A. Developmental studies in fragile X syndrome. J. Neurodev. Disord. 2020, 12, 13. [Google Scholar] [CrossRef]
- Li, W.; Kutas, M.; Gray, J.A.; Hagerman, R.H.; Olichney, J.M. The role of glutamate in language and language disorders—Evidence from ERP and pharmacological studies. Neurosci. Biobehav. Rev. 2020, 119, 217–241. [Google Scholar] [CrossRef]
- McKay, G.N.; Harrigan, T.P.; Brasic, J.R. A low-cost quantitative continuous measurement of movements in the extremities of people with Parkinson’s disease. MethodsX 2019, 6, 169–189. [Google Scholar] [CrossRef]
- Martin, S.D.; Berry-Kravis, E.M.; Russell, D.R.; Jennings, D.; Barret, O.; Nandi, A.; Seibyl, J.P.; Slifer, K.; Wong, D.F.; Budimirovic, D.B.; et al. Fragile X Mental Retardation Protein and metabotropic glutamate receptor subtype 5 in fragile X syndrome. [poster]. Society for Neuroscience Global Connectome; Virtual. 12 January 2021. [Google Scholar]
- Johnson, M.H.; Charman, T.; Pickles, A.; Jones, E.J.H. Annual Research Review: Anterior Modifiers in the Emergence of Neurodevelopmental Disorders (AMEND)—A systems neuroscience approach to common developmental disorders. J. Child Psychol. Psychiatry 2021. [Google Scholar] [CrossRef]
- Darnell, R.B. The genetic control of stoichiometry underlying autism. Annu. Rev. Neurosci. 2020, 43, 509–533. [Google Scholar] [CrossRef]
- Fatemi, S.H.; Folsom, T.D. GABA receptor subunit distribution and FMRP–mGluR5 signaling abnormalities in the cerebellum of subjects with schizo-phrenia, mood disorders, and autism. Schizophr. Res. 2015, 167, 42–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Russell, D. A PET Brain Imaging Study of mGluR5 in Subjects with Neuropsychiatric Conditions (FPEB). ClinicalTrials.gov Identifier: NCT00870974 2017. Available online: https://clinicaltrials.gov/ct2/show/NCT00870974 (accessed on 7 March 2021).
- International Committee of Medical Journal Editors (ICMJE). Recommendations for the Conduct, Reporting, Editing, and Publication of Scholarly Work in Medical Journals. 2019. Available online: http://www.icmje.org/icmje-recommendations.pdf (accessed on 7 March 2021).
- World Medical Association. Declaration of Helsinki: Medical Research Involving Human Subjects. 2013. Available online: https://www.wma.net/what-we-do/medical-ethics/declaration-of-helsinki/ (accessed on 7 March 2021).
- Lord, C.; Rutter, M.; Le Couteur, A. Autism Diagnostic Interview-Revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev. Disord. 1994, 24, 659–685. [Google Scholar] [CrossRef]
- Lord, C.; Rutter, M.; Goode, S.; Heemsbergen, J.; Jordan, H.; Mawhood, L.; Schopler, E. Autism Diagnostic Observation Schedule: A standardized observation of communicative and social behavior. J. Autism Dev. Disord. 1989, 19, 185–212. [Google Scholar] [CrossRef]
- Brix, G.; Zaers, J.; Adam, L.-E.; Bellemann, M.E.; Ostertag, H.; Trojan, H.; Haberkorn, U.; Doll, J.; Oberdorfer, F.; Lorenz, W.J. Performance evaluation of a whole-body PET scanner using the NEMA protocol. J. Nucl. Med. 1997, 38, 1614–1623. [Google Scholar]
- de Jong, H.W.A.M.; van Velden, F.H.P.; Kloet, R.W.; Buijs, F.L.; Boellaard, R.; Lammertsma, A.A. Performance evaluation of the ECAT HRRT: An LSO-LYSO double layer high resolution, high sensitivity scanner. Phys. Med. Biol. 2005, 52, 1505–1526. [Google Scholar]
- Sullivan, J.M.; Lim, K.; Labaree, D.; Lin, S.-F.; McCarthy, T.J.; Seibyl, J.P.; Tamagnan, G.; Huang, Y.; Carson, R.E.; Ding, Y.-S.; et al. Kinetic analysis of the metabotropic glutamate subtype 5 tracer [18F]FPEB in bolus and bolus plus-constant-infusion studies in humans. J. Cereb. Blood Flow Metab. 2013, 33, 532–541. [Google Scholar] [CrossRef] [Green Version]
- Logan, J.; Alexoff, D.; Fowler, J.S. The use of alternative forms of graphical analysis to balance bias and precision in PET images. J. Cereb. Blood Flow Metab. 2011, 31, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Budimirovic, D.B.; Bukelis, I.; Cox, C.; Gray, R.M.; Tierney, E.; Kaufman, W.E. Autism spectrum disorder in fragile X syndrome: Differential contribution of adaptive socialization and social withdrawal. Am. J. Med. Genet. Part A 2006, 140A, 1814–1826. [Google Scholar]



| Participant | Group | Institution | Sex | Age in Years | BMI |
|---|---|---|---|---|---|
| INDTD01 | TD | IND | Male | 44 | . |
| INDTD02 | TD | IND | Male | 57 | . |
| INDTD07 | TD | IND | Female | 62 | . |
| INDTD08 | TD | IND | Female | 62 | . |
| INDTD14 | TD | IND | Male | 28 | 25.8 |
| INDTD16 | TD | IND | Male | 31 | 29.8 |
| INDTD17 | TD | IND | Male | 38 | 25.0 |
| INDTD30 | TD | IND | Female | 28 | . |
| INDTD35 | TD | IND | Female | 56 | 42.3 |
| INDTD47 | TD | IND | Female | 22 | . |
| INDTD48 | TD | IND | Female | 29 | . |
| INDTD49 | TD | IND | Female | 20 | . |
| JHUTD4 | TD | JHU | Female | 19 | 28.4 |
| JHUTD6 | TD | JHU | Female | 19 | . |
| JHUTD14 | TD | JHU | Male | 24 | 21.7 |
| JHUTD105 | TD | JHU | Male | 26 | . |
| JHUTD1001 | TD | JHU | Male | 32 | 27.1 |
| JHUTD1002 | TD | JHU | Male | 27 | 28.6 |
| JHUTD1005 | TD | JHU | Male | 39 | 29.3 |
| JHUASD3 | IASD | JHU | Male | 18 | 28.8 |
| JHUASD4 | IASD | JHU | Male | 18 | . |
| JHUASD5 | IASD | JHU | Male | 19 | 22.2 |
| JHUASD7 | IASD | JHU | Female | 18 | 22.3 |
| JHUASD8 | IASD | JHU | Male | 23 | 28.5 |
| JHUASD9 | IASD | JHU | Male | 20 | 19.4 |
| JHUASD12 | IASD | JHU | Male | 22 | 20.7 |
| INDFXS34 | FXS | IND | Male | 23 | 36.6 |
| INDFXS38 | FXS | IND | Male | 24 | 30.9 |
| INDFXS40 | FXS | IND | Male | 22 | 33.2 |
| INDFXS41 | FXS | IND | Male | 27 | 25.8 |
| INDFXS42 | FXS | IND | Male | 34 | . |
| INDFXS44 | FXS | IND | Male | 26 | 24.1 |
| INDFXS45 | FXS | IND | Male | 33 | 22.0 |
| INDFXS-M50 | FXS | IND | Male | 57 | 34.1 |
| JHUFXS2 | FXS | JHU | Male | 24 | 34.9 |
| JHUFXS4 | FXS | JHU | Male | 27 | 28.3 |
| Participant | CN | mTp | Oc | Pa | pCg | Pu | Th | Tp |
|---|---|---|---|---|---|---|---|---|
| INDTD01 | 3.01 | 1.94 | 1.41 | 1.72 | 1.92 | 2.52 | 1.08 | 2.37 |
| INDTD02 | 3.03 | 2.05 | 1.63 | 1.76 | 1.04 | 2.35 | 1.30 | 2.35 |
| INDTD07 | 3.31 | 2.04 | 1.50 | 1.76 | 2.07 | 2.70 | 1.60 | 2.56 |
| INDTD08 | 3.01 | 2.02 | 1.57 | 1.83 | 1.97 | 2.64 | 1.32 | 2.50 |
| INDTD14 | 2.12 | 1.41 | 1.16 | 1.43 | 1.72 | 1.85 | 1.06 | 1.68 |
| INDTD16 | 2.68 | 1.61 | 1.20 | 1.26 | 1.42 | 2.03 | 2.24 | 1.84 |
| INDTD17 | 2.63 | 1.82 | 1.37 | 1.36 | 1.55 | 2.03 | 1.16 | 2.04 |
| INDTD30 | 2.42 | 1.73 | 1.26 | 1.14 | 1.26 | 1.87 | 1.08 | 2.17 |
| INDTD35 | 3.82 | 1.90 | 1.74 | 1.86 | 2.15 | 2.48 | 1.43 | 2.39 |
| INDTD47 | 2.09 | 1.29 | 2.17 | 1.41 | 1.86 | 1.95 | 1.02 | 2.74 |
| INDTD48 | 3.30 | 1.76 | 1.94 | 1.95 | 2.25 | 2.49 | 1.51 | 2.51 |
| INDTD49 | 2.75 | 1.57 | 1.32 | 1.56 | 1.46 | 2.19 | 1.07 | 2.10 |
| JHUTD4 | 2.80 | 3.19 | 3.48 | 4.31 | 3.39 | 3.08 | 1.43 | 4.54 |
| JHUTD6 | 3.38 | 3.57 | 3.69 | 4.48 | 3.65 | 3.16 | 1.32 | 4.94 |
| JHUTD14 | 2.47 | 3.24 | 3.15 | 3.73 | 3.44 | 2.94 | 1.41 | 4.09 |
| JHUTD105 | 3.73 | . | 2.86 | . | . | 3.30 | 2.11 | 3.73 |
| JHUTD1001 | 4.59 | . | 3.64 | . | . | 4.29 | 2.57 | 4.86 |
| JHUTD1002 | 3.83 | . | 2.77 | . | . | 3.66 | 2.21 | 3.73 |
| JHUTD1005 | 3.58 | . | 2.77 | . | . | 3.24 | 2.02 | 3.26 |
| JHUASD3 | 2.17 | 3.06 | 3.26 | 4.05 | 3.43 | 3.87 | 1.27 | 3.97 |
| JHUASD4 | 2.62 | 3.43 | 3.10 | 4.17 | 3.99 | 3.03 | 1.45 | 3.85 |
| JHUASD5 | 2.79 | 3.25 | 3.48 | 3.84 | 3.25 | 2.78 | 1.46 | 4.17 |
| JHUASD7 | 3.13 | . | 2.03 | 2.74 | . | 2.84 | 1.67 | 2.72 |
| JHUASD8 | 3.12 | 3.54 | 3.42 | 4.18 | 3.74 | 3.36 | 1.55 | 4.21 |
| JHUASD9 | 3.06 | 3.35 | 3.43 | 4.23 | 3.64 | 3.36 | 1.32 | 4.58 |
| JHUASD12 | 3.25 | 2.46 | 3.41 | 4.11 | 3.34 | 2.99 | 1.75 | 4.58 |
| INDFXS34 | 1.96 | 1.00 | 1.06 | 1.42 | 1.37 | 2.01 | 0.83 | 1.40 |
| INDFXS38 | 1.58 | 0.69 | 0.59 | 0.82 | 0.79 | 1.08 | 0.61 | 0.92 |
| INDFXS40 | 1.65 | 0.81 | 0.82 | 0.96 | 1.40 | 1.13 | 0.39 | 1.14 |
| INDFXS41 | 2.14 | 1.25 | 1.08 | 1.36 | 1.56 | 1.65 | 0.56 | 1.59 |
| INDFXS42 | 3.45 | 2.24 | 1.78 | 2.09 | 2.18 | 2.55 | 1.25 | 2.68 |
| INDFXS44 | 1.76 | 1.01 | 1.03 | 1.23 | 1.23 | 1.69 | 0.73 | 1.37 |
| INDFXS45 | 2.89 | 1.74 | 1.47 | 1.73 | 1.91 | 2.16 | 1.32 | 2.13 |
| INDFXS-M50 | 2.99 | 2.24 | 1.73 | 1.77 | 1.82 | 2.50 | 1.19 | 2.53 |
| JHUFXS2 | 2.05 | 2.71 | . | . | 3.01 | 2.01 | 0.97 | . |
| JHUFXS4 | 2.00 | 2.21 | . | 2.7 | 2.25 | 1.98 | 0.89 | . |
| Analysis of Variance by Group Status and Region | |||
|---|---|---|---|
| Region | Degrees of freedom | Test statistic | Probability |
| Caudate nucleus | 2 | 6.77 | 0.00364 |
| Occipital cortex | 2 | 12.8 | 0.00010 |
| Parietal cortex | 2 | 16.2 | 0.00003 |
| Posterior cingulate cortex | 2 | 14.6 | 0.00006 |
| Putamen | 2 | 10.1 | 0.00043 |
| Thalamus | 2 | 10.3 | 0.00038 |
| Temporal cortex | 2 | 12.3 | 0.00014 |
| Post hoc Pairwise Comparisons by Tukey’s Honest Standard Differences [30,31] | ||||
|---|---|---|---|---|
| Region | Pairwise Comparison | Adjusted Mean Difference | Standard Error | Probability |
| Caudate nucleus | FXS-IASD | −0.81837 | 0.301489 | 0.028089 |
| TD-IASD | −0.02072 | 0.298712 | 0.997341 | |
| TD-FXS | 0.797653 | 0.259542 | 0.011671 | |
| Occipital cortex | FXS-IASD | −1.88121 | 0.409271 | 0.000218 |
| TD-IASD | −0.74904 | 0.384949 | 0.143727 | |
| TD-FXS | 1.132169 | 0.353062 | 0.008855 | |
| Parietal cortex | FXS-IASD | −2.31154 | 0.476181 | 0.000173 |
| TD-IASD | −1.56255 | 0.493592 | 0.010802 | |
| TD-FXS | 0.748987 | 0.462568 | 0.25626 | |
| Posterior cingulate cortex | FXS-IASD | −1.6965 | 0.362152 | 0.000203 |
| TD-IASD | −1.5125 | 0.425317 | 0.00414 | |
| TD-FXS | 0.184006 | 0.356537 | 0.863175 | |
| Putamen | FXS-IASD | −1.23401 | 0.29236 | 0.000529 |
| TD-IASD | −0.31288 | 0.289668 | 0.532202 | |
| TD-FXS | 0.921134 | 0.251683 | 0.002488 | |
| Thalamus | FXS-IASD | −0.69753 | 0.197012 | 0.003572 |
| TD-IASD | 0.084401 | 0.195197 | 0.902066 | |
| TD-FXS | 0.781931 | 0.169601 | 0.000214 | |
| Temporal cortex | FXS-IASD | −2.18986 | 0.490827 | 0.000294 |
| TD-IASD | −0.75798 | 0.461658 | 0.243837 | |
| TD-FXS | 1.431881 | 0.423417 | 0.005678 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brašić, J.R.; Nandi, A.; Russell, D.S.; Jennings, D.; Barret, O.; Martin, S.D.; Slifer, K.; Sedlak, T.; Seibyl, J.P.; Wong, D.F.; et al. Cerebral Expression of Metabotropic Glutamate Receptor Subtype 5 in Idiopathic Autism Spectrum Disorder and Fragile X Syndrome: A Pilot Study. Int. J. Mol. Sci. 2021, 22, 2863. https://doi.org/10.3390/ijms22062863
Brašić JR, Nandi A, Russell DS, Jennings D, Barret O, Martin SD, Slifer K, Sedlak T, Seibyl JP, Wong DF, et al. Cerebral Expression of Metabotropic Glutamate Receptor Subtype 5 in Idiopathic Autism Spectrum Disorder and Fragile X Syndrome: A Pilot Study. International Journal of Molecular Sciences. 2021; 22(6):2863. https://doi.org/10.3390/ijms22062863
Chicago/Turabian StyleBrašić, James Robert, Ayon Nandi, David S. Russell, Danna Jennings, Olivier Barret, Samuel D. Martin, Keith Slifer, Thomas Sedlak, John P. Seibyl, Dean F. Wong, and et al. 2021. "Cerebral Expression of Metabotropic Glutamate Receptor Subtype 5 in Idiopathic Autism Spectrum Disorder and Fragile X Syndrome: A Pilot Study" International Journal of Molecular Sciences 22, no. 6: 2863. https://doi.org/10.3390/ijms22062863
APA StyleBrašić, J. R., Nandi, A., Russell, D. S., Jennings, D., Barret, O., Martin, S. D., Slifer, K., Sedlak, T., Seibyl, J. P., Wong, D. F., & Budimirovic, D. B. (2021). Cerebral Expression of Metabotropic Glutamate Receptor Subtype 5 in Idiopathic Autism Spectrum Disorder and Fragile X Syndrome: A Pilot Study. International Journal of Molecular Sciences, 22(6), 2863. https://doi.org/10.3390/ijms22062863