Endocrine-Disrupting Chemicals and Infectious Diseases: From Endocrine Disruption to Immunosuppression
Abstract
:1. Introduction
2. Sources of EDCs Exposure
3. EDC-Related Diseases
3.1. Metabolic Disorders and Obesity
3.2. Diabetes
3.3. Hypertension and Cardiovascular Diseases
3.4. Kidney Diseases
3.5. Cancer
3.6. Lung Diseases
3.7. Neurodegenerative Diseases
3.8. Immune Function
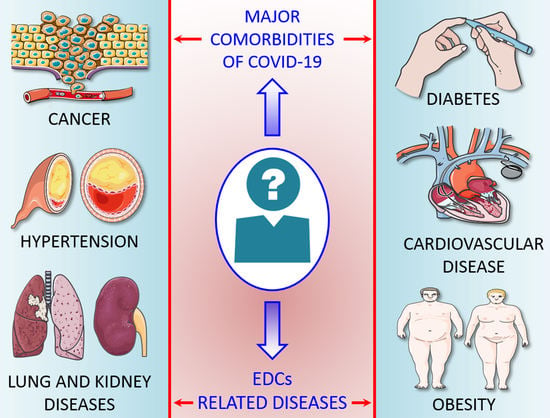
4. Underlying Comorbidities of COVID-19
5. Lesson from the Previous Pandemic
6. Current Knowledge on EDCs and COVID-19 Risks
7. Recommended Methodologies for Assessing the Role of EDCs in COVID-19 Severity
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Matuszczak, E.; Komarowska, M.D.; Debek, W.; Hermanowicz, A. The impact of bisphenol A on fertility, reproductive system, and development: A review of the literature. Int. J. Endocrinol. 2019, 2019, 4068717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- EFSA CEF Panel (EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids). Scientific opinion on the risks to public health related to the presence of bisphenol A (BPA) in foodstuffs: Executive summary. EFSA J. 2015, 13, 3978. [Google Scholar] [CrossRef]
- Kozul, C.D.; Ely, K.H.; Enelow, R.I.; Hamilton, J.W. Low-dose arsenic compromises the immune response to influenza A infection in vivo. Environ. Health Perspect. 2009, 117, 144–1447. [Google Scholar] [CrossRef] [Green Version]
- Warren, T.K.; Mitchell, K.A.; Lawrence, B.P. Exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) suppresses the humoral and cell-mediated immune responses to influenza A virus without affecting cytolytic activity in the lung. Toxicol. Sci. 2000, 56, 114–123. [Google Scholar] [CrossRef] [Green Version]
- Wehbe, Z.; Nasser, S.A.; El-Yazbi, A.; Nasreddine, S.; Eid, A.H. Estrogen and Bisphenol A in Hypertension. Curr. Hypertens. Rep. 2020, 22, 23. [Google Scholar] [CrossRef] [PubMed]
- De Coster, S.; van Larebeke, N. Endocrine-disrupting chemicals: Associated disorders and mechanisms of action. J. Environ. Public Health 2012, 2012, 713696. [Google Scholar] [CrossRef]
- Rochester, J.R. Bisphenol A and human health: A review of the literature. Reprod. Toxicol. 2013, 42, 132–155. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Similarities and Differences between Flu and COVID-19. Available online: https://www.cdc.gov/flu/symptoms/flu-vs-covid19.htm (accessed on 27 May 2020).
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical Characteristics of 138 Hospitalized Patients with 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA 2020, 323, 106–1069. [Google Scholar] [CrossRef] [PubMed]
- WHO Coronavirus Disease (COVID-19) Dashboard. Available online: https://covid19.who.int (accessed on 22 May 2020).
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Xu, S.; Yu, M.; Wang, K.; Tao, Y.; Zhou, Y.; Shi, J.; Zhou, M.; Wu, B.; Yang, Z.; et al. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J. Allergy Clin. Immunol. 2020, 146, 110–118. [Google Scholar] [CrossRef]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.; Jia, X.; Li, J.; Hu, K.; Chen, G.; Wei, J.; Gong, Z.; Zhou, C.; Yu, H.; et al. Risk factors for disease severity, unimprovement, and mortality in COVID-19 patients in Wuhan, China. Clin. Microbiol. Infect. 2020, 26, 767–772. [Google Scholar] [CrossRef]
- Li, G.; Hu, R.; Gu, X. A close-up on COVID-19 and cardiovascular diseases. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 1057–1060. [Google Scholar] [CrossRef] [PubMed]
- Bornstein, S.R.; Dalan, R.; Hopkins, D.; Mingrone, G.; Boehm, B.O. Endocrine and metabolic link to coronavirus infection. Nat. Rev. Endocrinol. 2020, 16, 297–298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsatsakis, A.; Petrakis, D.; Nikolouzakis, T.K.; Docea, A.O.; Calina, D.; Vinceti, M.; Goumenou, M.; Kostoff, R.N.; Mamoulakis, C.; Aschner, M.; et al. COVID-19, an opportunity to reevaluate the correlation between long-term effects of anthropogenic pollutants on viral epidemic/pandemic events and prevalence. Food Chem. Toxicol. 2020, 141, 111418. [Google Scholar] [CrossRef] [PubMed]
- Zahra, A.; Sisu, C.; Silva, E.; De Aguiar Greca, S.C.; Randeva, H.S.; Chatha, K.; Kyrou, I.; Karteris, E. Is There a Link between Bisphenol A (BPA), a Key Endocrine Disruptor, and the Risk for SARS-CoV-2 Infection and Severe COVID-19? J. Clin. Med. 2020, 9, 3296. [Google Scholar] [CrossRef]
- Kostoff, R.N.; Briggs, M.B.; Porter, A.L.; Hernández, A.F.; Abdollahi, M.; Aschner, M.; Tsatsakis, A. The under-reported role of toxic substance exposures in the COVID-19 pandemic. Food Chem. Toxicol. 2020, 145, 111687. [Google Scholar] [CrossRef]
- Wu, Q.; Coumoul, X.; Grandjean, P.; Barouki, R.; Audouze, K. Endocrine disrupting chemicals and COVID-19 relationships: A computational systems biology approach. Environ. Int. 2020. [Google Scholar] [CrossRef]
- Ben Maamar, M.; Lesné, L.; Desdoits-Lethimonier, C.; Coiffec, I.; Lassurguère, J.; Lavoué, V.; Deceuninck, Y.; Antignac, J.P.; Le Bizec, B.; Perdu, E.; et al. An investigation of the endocrine-disruptive effects of bisphenol a in human and rat fetal testes. PLoS ONE 2015, 10, e0117226. [Google Scholar]
- Desdoits-Lethimonier, C.; Lesné, L.; Gaudriault, P.; Zalko, D.; Antignac, J.P.; Deceuninck, Y.; Platel, C.; Dejucq-Rainsford, N.; Mazaud-Guittot, S.; Jégou, B. Parallel assessment of the effects of bisphenol A and several of its analogs on the adult human testis. Hum. Reprod. 2017, 32, 1465–1473. [Google Scholar] [CrossRef]
- Henley, D.V.; Korach, K.S. Endocrine-disrupting chemicals use distinct mechanisms of action to modulate endocrine system function. Endocrinology 2006, 147, S25–S32. [Google Scholar] [CrossRef] [Green Version]
- Genoa, R.W.; Jodi, A.F. Bisphenol A and Phthalates: How environmental chemicals are reshaping toxicology. Toxicol. Sci. 2018, 166, 246–249. [Google Scholar]
- Cao, X.L.; Zhao, W.; Churchill, R.; Hilts, C. Occurrence of di-(2-ethylhexyl) adipate and phthalate plasticizers in samples of meat, fish, and cheese and their packaging films. J. Food Prot. 2014, 77, 610–620. [Google Scholar] [CrossRef]
- CDC. Fourth National Report on Human Exposure to Environmental Chemicals. Updated Tables, January 2019, Volume One. Available online: https://dtsc.ca.gov/wp-content/uploads/sites/31/2019/07/FourthReport_UpdatedTables_Volume1.pdf (accessed on 22 May 2020).
- Kitamura, S.; Suzuki, T.; Sanoh, S.; Kohta, R.; Jinno, N.; Sugihara, K.; Yoshihara, S.; Fujimoto, N.; Watanabe, H.; Ohta, S. Comparative study of the endocrine-disrupting activity of bisphenol A and 19 related compounds. Toxicol. Sci. 2005, 84, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Clark, E. Sulfolane and sulfones. In Kirk-Othmer Encyclopedia of Chemical Technology; John Wiley & Sons: New York, NY, USA, 2006. [Google Scholar]
- Office of Environmental Health Hazard Assessment. Potential Designated Chemicals: P, p’-Bisphenols and Diglycidyl Ethers of p, p-Bisphenols. 2012. Available online: https://biomonitoring.ca.gov/sites/default/files/downloads/110812Bisphenols.pdf (accessed on 25 July 2020).
- Pacyga, D.C.; Sathyanarayana, S.; Strakovsky, R. Dietary Predictors of Phthalate and Bisphenol Exposures in Pregnant Women. Adv. Nutr. 2019, 10, 803–815. [Google Scholar] [CrossRef] [PubMed]
- National Institute of Environmental Health Science. Endocrine Disruptors. Available online: https://www.niehs.nih.gov/health/topics/agents/endocrine/index.cfm (accessed on 19 May 2020).
- Eladak, S.; Grisin, T.; Moison, D.; Guerquin, M.; N’Tumba-Byn, T.; Pozzi-Gaudin, S.; Benachi, A.; Livera, G.; Rouiller-Fabre, V.; Habert, R. A new chapter in the bisphenol a story: Bisphenol S and bisphenol F are not safe alternatives to this compound. Fertil. Steril. 2015, 103, 11–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zalko, D.; Jacques, C.; Duplan, H.; Bruel, S.; Perdu, E. Viable skin efficiently absorbs and metabolizes bisphenol A. Chemosphere 2011, 82, 424–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schug, T.T.; Janesick, A.; Blumberg, B.; Heindel, J.J. Endocrine disrupting chemicals and disease susceptibility. J. Steroid Biochem. Mol. Biol. 2011, 127, 204–215. [Google Scholar] [CrossRef] [Green Version]
- Nowak, K.; Jabłońska, E.; Ratajczak-Wrona, W. Immunomodulatory effects of synthetic endocrine disrupting chemicals on the development and functions of human immune cells. Environ. Int. 2019, 125, 350–364. [Google Scholar] [CrossRef]
- Rahman, M.S.; Kwon, W.S.; Karmakar, P.C.; Yoon, S.J.; Ryu, B.Y.; Pang, M.G. Gestational Exposure to Bisphenol-A Affects the Function and Proteome Profile of F1 Spermatozoa in Adult Mice. Environ. Health Perspect. 2017, 125, 238–245. [Google Scholar] [CrossRef]
- Cohn, B.A.; Wolff, M.S.; Cirillo, P.M. DDT and breast cancer in young women: New data on the significance of age at exposure. Environ. Health Perspect. 2007, 115, 1406–1414. [Google Scholar] [CrossRef]
- Vandenberg, L.N.; Maffini, M.V.; Schaeberle, C.M.; Ucci, A.A.; Sonnenschein, C.; Rubin, B.S.; Soto, A.M. Perinatal exposure to the xenoestrogen bisphenol-A induces mammary intraductal hyperplasias in adult CD-1 mice. Reprod. Toxicol. 2008, 26, 210–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diamanti-Kandarakis, E.; Bourguignon, J.P.; Giudice, L.C.; Hauser, R.; Prins, G.S.; Soto, A.M. Endocrine-disrupting chemicals: An endocrine society scientific statement. Endocr. Rev. 2009, 30, 293–342. [Google Scholar] [CrossRef] [PubMed]
- Janesick, A.S.; Blumberg, B. Endocrine disrupting chemicals and the developmental programming of adipogenesis and obesity. Birth Defects Res. C Embryo Today 2011, 93, 34–50. [Google Scholar] [CrossRef] [Green Version]
- Darbre, P.D. Endocrine disruptors and obesity. Curr. Obes. Rep. 2017, 6, 18–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grun, F.; Blumberg, B. Endocrine disrupters as obesogens. Mol. Cell. Endocrinol. 2009, 304, 19–29. [Google Scholar] [CrossRef] [Green Version]
- Meldrum, D.R.; Morris, M.A.; Gambone, J.C. Obesity pandemic: Causes, consequences, and solutions-but do we have the will? Fertil. Steril. 2017, 107, 833–839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelly, T.; Yang, W.; Chen, C.S.; Reynolds, K.; He, J. Global burden of obesity in 2005 and projections to 2030. Int. J. Obes. 2008, 32, 1431–1437. [Google Scholar] [CrossRef] [Green Version]
- Salmon, A.B. Beyond diabetes: Does obesity-induced oxidative stress drive the aging process? Antioxidants 2006, 5, 24. [Google Scholar] [CrossRef] [Green Version]
- Esposito, K.; Nappo, F.; Marfella, R.; Giugliano, G.; Giugliano, F.; Ciotola, M.; Quagliaro, L.; Ceriello, A.; Giugliano, D. Inflammatory cytokine concentrations are acutely increased by hyperglycemia in humans: Role of oxidative stress. Circulation 2002, 106, 2067–2072. [Google Scholar] [CrossRef] [Green Version]
- Kandimalla, R.; Thirumala, V.; Reddy, P.H. Is Alzheimer’s disease a Type 3 Diabetes? A critical appraisal. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 1078–1089. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Magdalena, P.; Quesada, I.; Nadal, A. Endocrine disruptors in the etiology of type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2011, 7, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Magdalena, P.; Ropero, A.B.; Soriano, S.; Quesada, I.; Nadal, A. Bisphenol-A: A new diabetogenic factor? Hormones 2010, 9, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Hectors, T.L.; Vanparys, C.; van der Ven, K.; Martens, G.A.; Jorens, P.G.; Van Gaal, L.F.; Covaci, A.; De Coen, W.; Blust, R. Environmental pollutants and type 2 diabetes: A review of mechanisms that can disrupt beta cell function. Diabetologia 2011, 54, 1273–1290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grun, F.; Blumberg, B. Minireview the case for obesogens. Mol. Endocrinol. 2009, 23, 1127–1134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, D.H.; Lee, I.K.; Song, K.; Steffes, M.; Toscano, W.; Baker, B.A.; Jacobs, D.R. A strong dose-response relation between serum concentrations of persistent organic pollutants and diabetes: Results from the National Health and Examination Survey 1999–2002. Diabetes Care 2006, 29, 1638–1644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruiz, D.; Becerra, M.; Jagai, J.S.; Ard, K.; Sargis, R.M. Disparities in environmental exposures to endocrine-disrupting chemicals and diabetes risk in vulnerable populations. Diabetes Care 2018, 41, 193–205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gore, A.C.; Chappell, V.A.; Fenton, S.E.; Flaws, J.A.; Nadal, A.; Prins, G.S.; Toppari, J.; Zoeller, R.T. Executive Summary to EDC-2: The Endocrine Society’s Second Scientific Statement on Endocrine-Disrupting Chemicals. Endocr. Rev. 2015, 36, 593–602. [Google Scholar] [CrossRef] [Green Version]
- Marroqui, L.; Tuduri, E.; Alonso-Magdalena, P.; Quesada, I.; Nadal, A.; Dos Santos, R.S. Mitochondria as target of endocrine-disrupting chemicals: Implications for type 2 diabetes. J. Endocrinol. 2018, 239, R27–R45. [Google Scholar] [CrossRef] [PubMed]
- Cardenas, A.; Gold, D.R.; Hauser, R.; Kleinman, K.P.; Hivert, M.F.; Calafat, A.M.; Ye, X.; Webster, T.F.; Horton, E.S.; Oken, E. Plasma Concentrations of Per- and Polyfluoroalkyl Substances at Baseline and Associations with Glycemic Indicators and Diabetes Incidence among High-Risk Adults in the Diabetes Prevention Program Trial. Environ. Health Perspect. 2017, 125, 107001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uslu, U.; Sandal, S.; Cumbul, A.; Yildiz, S.; Aydin, M.; Yilmaz, B. Evaluation of estrogenic effects of polychlorinated biphenyls and organochlorinated pesticides using immature rat utero trophic assay. Hum. Exp. Toxicol. 2013, 32, 476–482. [Google Scholar] [CrossRef]
- Garcia-Arevalo, M.; Alonso-Magdalena, P.; Rebelo Dos Santos, J.; Quesada, I.; Carneiro, E.M. Exposure to bisphenol-a during pregnancy partially mimics the effects of a high-fat diet altering glucose homeostasis and gene expression in adult male mice. PLoS ONE 2014, 9, e100214. [Google Scholar] [CrossRef] [Green Version]
- Yan, S.; Chen, Y.; Dong, M.; Song, W.; Belcher, S.M.; Wang, H.S. Bisphenol A and 17beta-estradiol promote arrhythmia in the female heart via alteration of calcium handling. PLoS ONE 2011, 6, e25455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aekplakorn, W.; Chailurkit, L.O.; Ongphiphadhanakul, B. Association of serum bisphenol a with hypertension in thai population. Int. J. Hypertens. 2015, 2015, 594189. [Google Scholar] [CrossRef]
- Bae, S.; Kim, J.H.; Lim, Y.H.; Park, H.Y.; Hong, Y.C. Associations of bisphenol A exposure with heart rate variability and blood pressure. Hypertension 2012, 60, 786. [Google Scholar] [CrossRef]
- Khalil, N.; Ebert, J.R.; Wang, L.; Belcher, S.; Lee, M.; Czerwinski, S.A.; Kannan, K. Bisphenol A and cardiometabolic risk factors in obese children. Sci. Total Environ. 2014, 470–471, 726–732. [Google Scholar] [CrossRef] [PubMed]
- Shankar, A.; Teppala, S. Urinary bisphenol A and hypertension in a multiethnic sample of US adults. J. Environ. Public Health 2012, 2012, 481641. [Google Scholar] [CrossRef]
- Lang, I.A.; Galloway, T.S.; Scarlett, A.; Henley, W.E.; Depledge, M.; Wallace, R.B.; Melzer, D. Association of urinary bisphenol A concentration with medical disorders and laboratory abnormalities in adults. JAMA 2008, 300, 1303–1310. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Hong, Y. Bisphenol A, Hypertension, and Cardiovascular Diseases: Epidemiological, Laboratory, and Clinical Trial Evidence. Curr. Hypertens. Rep. 2016, 18, 11. [Google Scholar] [CrossRef] [PubMed]
- Melzer, D.; Osborne, N.J.; Henley, W.E.; Cipelli, R.; Young, A.; Money, C.; McCormack, P.; Luben, R.; Khaw, K.; Wareham, N.J.; et al. Urinary bisphenol A concentration and risk of future coronary artery disease in apparently healthy men and women. Circulation 2012, 125, 1482–1490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kjeldsen, S.E. Hypertension and cardiovascular risk: General aspects. Pharmacol. Res. 2018, 129, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Mariana, M.; Feiteiro, J.; Verde, I.; Cairrao, E. The effects of phthalates in the cardiovascular and reproductive systems: A review. Environ. Int. 2016, 94, 758–776. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Bi, Y.; Qi, L.; Wang, T.; Xu, M.; Huang, Y.; Xu, Y.; Chen, Y.; Lu, J.; Wang, W.; et al. Exposure to bisphenol A is associated with low-grade albuminuria in Chinese adults. Kidney Int. 2012, 81, 1131–1139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trasande, L.; Attina, T.M.; Trachtman, H. Bisphenol A exposure is associated with low-grade urinary albumin excretion in children of the United States. Kidney Int. 2013, 83, 741–748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsai, H.J.; Chen, B.; Wu, C.F.; Wang, S.; Huang, P.; Tsai, Y.; Chen, M.; Ho, C.; Chao, A.; Hsiung, C.A.; et al. Intake of phthalate-tainted foods and microalbuminuria in children: The 2011 Taiwan food scandal. Environ. Int. 2016, 89–90, 129–137. [Google Scholar] [CrossRef]
- David, R.M.; Moore, M.R.; Finney, D.C.; Guest, D. Chronic toxicity of di(2-ethylhexyl) phthalate in mice. Toxicol. Sci. 2000, 58, 377–385. [Google Scholar] [CrossRef] [Green Version]
- Wood, C.E.; Jokinen, M.P.; Johnson, C.L.; Olson, G.R.; Hester, S.; George, M.; Chorley, B.N.; Carswell, G.; Carter, J.H.; Wood, C.R.; et al. Comparative time course profiles of phthalate stereoisomers in mice. Toxicol. Sci. 2014, 139, 21–34. [Google Scholar] [CrossRef] [Green Version]
- Kobroob, A.; Peerapanyasut, W.; Chattipakorn, N.; Wongmekiat, O. Damaging Effects of Bisphenol A on the Kidney and the Protection by Melatonin: Emerging Evidences from In Vivo and In Vitro Studies. Oxid. Med. Cell. Longev. 2018, 2018, 3082438. [Google Scholar] [CrossRef] [PubMed]
- Gowder, S.J.T. Nephrotoxicity of Bisphenol A (BPA)—An Updated Review. Curr. Mol. Pharmacol. 2013, 6, 163–172. [Google Scholar] [CrossRef]
- Koch, C.A.; Diamanti-Kandarakis, E. Introduction to endocrine disrupting chemicals—Is it time to act? Rev. Endocr. Metab. Disord. 2015, 6, 269–270. [Google Scholar] [CrossRef] [Green Version]
- Lerma, E.V.; Koch, C.A. Nephroendocrinology: When endocrinology meets nephrology. Rev. Endocr. Metab. Disord. 2017, 18, 1–3. [Google Scholar] [CrossRef] [Green Version]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Del Pup, L.; Mantovani, A.; Cavaliere, C.; Facchini, G.; Luce, A.; Sperlongano, P.; Caraglia, M.; Berretta, M. Carcinogenetic mechanisms of endocrine disruptors in female cancers (Review). Oncol. Rep. 2016, 36, 603–612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soto, A.M.; Sonnenschein, C. Environmental causes of cancer: Endocrine disruptors as carcinogens. Nat. Rev. Endocrinol. 2010, 6, 363–370. [Google Scholar] [CrossRef] [Green Version]
- Wogan, G.N.; Hecht, S.S.; Felton, J.S.; Conney, A.H.; Loeb, L.A. Environmental and chemical carcinogenesis. Semin. Cancer Biol. 2004, 14, 473–486. [Google Scholar] [CrossRef] [PubMed]
- Volinia, S.; Calin, G.A.; Liu, C.G.; Ambs, S.; Cimmino, A.; Petrocca, F.; Visone, R.; Iorio, M.; Roldo, C.; Ferracin, M.; et al. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc. Natl. Acad. Sci. USA 2006, 103, 2257–2261. [Google Scholar] [CrossRef] [Green Version]
- Tilghman, S.L.; Bratton, M.R.; Segar, H.C.; Martin, E.C.; Rhodes, L.V.; Li, M.; McLachlan, J.A.; Wiese, T.E.; Nephew, K.P.; Burow, M.E. Endocrine disruptor regulation of microRNA expression in breast carcinoma cells. PLoS ONE 2012, 7, e32754. [Google Scholar] [CrossRef]
- Park, M.A.; Hwang, K.A.; Choi, K.C. Diverse animal models to examine potential role(s) and mechanism of endocrine disrupting chemicals on the tumor progression and prevention: Do they have tumorigenic or anti-tumorigenic property. Lab. Anim. Res. 2011, 27, 265–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, F.; Lerro, C.; Lavoué, J.; Huang, H.; Siemiatycki, J.; Zhao, N.; Ma, S.; Deziel, N.C.; Friesen, M.C.; Udelsman, R.; et al. Occupational exposure to pesticides and other biocides and risk of thyroid cancer. Occup. Environ. Med. 2017, 74, 502–510. [Google Scholar] [CrossRef] [Green Version]
- Sprague, B.L.; Trentham-Dietz, A.; Hedman, C.J.; Wang, J.; Hemming, J.D.; Hampton, J.M.; Buist, D.S.; Aiello Bowles, E.J.; Sisney, G.S.; Burnside, E.S. Circulating serum xenoestrogens and mammographic breast density. Breast Cancer Res. 2013, 15, R45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kabir, E.R.; Rahman, M.S.; Rahman, I. A review on endocrine disruptors and their possible impacts on human health. Environ. Toxicol. Pharmacol. 2015, 40, 241–258. [Google Scholar] [CrossRef]
- Polyzos, S.A.; Kountouras, J.; Deretzi, G.; Zavos, C.; Mantzoros, C.S. The emerging role of endocrine disruptors in pathogenesis of insulin resistance: A concept implicating nonalcoholic fatty liver disease. Curr. Mol. Med. 2012, 12, 68–82. [Google Scholar] [CrossRef]
- Darbre, P.D. Endocrine Disruption and Human Health; Academic Press: Cambridge, MA, USA, 2015; pp. 27–45. [Google Scholar]
- Rudel, R.A.; Perovich, L.J. Endocrine disrupting chemicals in indoor and outdoor air. Atmos. Environ. 2009, 43, 170–181. [Google Scholar] [CrossRef] [Green Version]
- Butte, W.; Heinzow, B. Pollutants in house dust as indicators of indoor contamination. Rev. Environ. Contam. Toxicol. 2002, 175, 1–46. [Google Scholar]
- Paciência, I.; Cavaleiro, R.J.; Silva, D.; Carla Martins, C.; Francisca Mendes, F.; Farraia, M.; Delgado, L.; Fernandes, E.O.; Padrão, P.; Moreira, P.; et al. Exposure to indoor endocrine-disrupting chemicals and childhood asthma and obesity. Allergy 2019, 74, 1277–1291. [Google Scholar] [CrossRef]
- Nazaroff, W.W.; Weschler, C.J. Cleaning products and air fresheners: Exposure to primary and secondary air pollutants. Atmos. Environ. 2004, 38, 2841–2865. [Google Scholar] [CrossRef]
- Lynch, R.M. Modeling of exposure to carpet-cleaning chemicals preceding irritant-induced asthma in one patient. Environ. Health Perspect. 2000, 108, 911–913. [Google Scholar] [CrossRef] [PubMed]
- Purohit, A.; Kopferschmitt-Kubler, M.C.; Moreau, C.; Popin, E.; Blaumeiser, M.; Pauli, G. Quaternary ammonium compounds and occupational asthma. Int. Arch. Occup. Environ. Health 2000, 73, 423–427. [Google Scholar] [CrossRef]
- Karjalainen, A.; Martikainen, R.; Karjalainen, J.; Klaukka, T.; Kurppa, K. Excess incidence of asthma among Finnish cleaners employed in different industries. Eur. Respir. J. 2002, 19, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Zock, J.P.; Kogevinas, M.; Sunyer, J.; Almar, E.; Muniozguren, N.; Payo, F.; Sánchez, J.L.; Antó, J.M. Asthma risk, cleaning activities and use of specific cleaning products among Spanish indoor cleaners. Scand. J. Work. Environ. Health 2001, 27, 76–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, K.S.; Chen, H.Q.; Chen, Y.S.; Qiu, K.F.; Zheng, X.B.; Li, G.C.; Yang, H.D.; Wen, C.J. Bisphenol A stimulates human lung cancer cell migration via upregulation of matrix metalloproteinases by GPER/EGFR/ERK1/2 signal pathway. Biomed. Pharmacother. 2014, 68, 1037–1043. [Google Scholar] [CrossRef]
- Jaakkola, J.J.; Knight, T.L. The role of exposure to phthalates from polyvinyl chloride products in the development of asthma and allergies: A systematic review and meta-analysis. Environ. Health Perspect. 2008, 116, 845–853. [Google Scholar] [CrossRef]
- Hsu, N.Y.; Lee, C.C.; Wang, J.Y.; Li, Y.C.; Chang, H.W.; Chen, C.Y.; Bornehag, C.G.; Wu, P.C.; Sundell, J.; Su, H.J. Predicted risk of childhood allergy, asthma, and reported symptoms using measured phthalate exposure in dust and urine. Indoor Air 2012, 22, 186–199. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.M.; Kim, J.; Cheong, H.K. Exposure to phthalates aggravates pulmonary function and airway inflammation in asthmatic children. PLoS ONE 2018, 13, e0208553. [Google Scholar] [CrossRef]
- Kim, J.H.; Bae, S.L.; Kiyoung, S.J.; Hong, Y. Exposure to Bisphenol A and Phthalates Affects Lung Function and Oxidative Stress in the Elderly. Epidemiology 2009, 20, S154. [Google Scholar] [CrossRef]
- Brookmeyer, R.; Johnson, E.; Ziegler-Graham, K.; Arrighi, H.M. Forecasting the global burden of Alzheimer’s disease. Alzheimer’s Dement. 2007, 3, 186–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yegambaram, M.; Manivannan, B.; Beach, T.G.; Halden, R.U. Role of environmental contaminants in the etiology of Alzheimer’s disease: A review. Curr. Alzheimer. Res. 2015, 12, 116–146. [Google Scholar] [CrossRef] [PubMed]
- Kodavanti, P.R. Cell signaling and neurotoxicity: Protein kinase C in vitro and in vivo. Methods Mol. Biol. 2011, 758, 307–319. [Google Scholar]
- Zaganas, I.; Kapetanaki, S.; Mastorodemos, V.; Kanavouras, K.; Colosio, C.; Wilks, M.F.; Tsatsakis, A.M. Linking pesticide exposure and dementia What is the evidence? Toxicology 2013, 307, 3–11. [Google Scholar] [CrossRef]
- Steenland, K.; Hein, M.J.; Cassinelli, R.T.; Prince, M.M.; Nilsen, N.B.; Whelan, E.A.; Waters, M.A.; Ruder, A.M.; Schnorr, T.M. Polychlorinated Biphenyls and Neurodegenerative Disease Mortality in an Occupational Cohort. Epidemiology 2006, 17, 8–13. [Google Scholar] [CrossRef]
- Freire, C.; Koifman, S. Pesticide exposure and Parkinson’s disease: Epidemiological evidence of association. Neurotoxicology 2012, 33, 947–971. [Google Scholar] [CrossRef]
- Parrón, T.; Requena, M.; Hernández, A.F.; Alarcón, R. Association between environmental exposure to pesticides and neurodegenerative diseases. Toxicol. Appl. Pharmacol. 2011, 256, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Hayden, K.M.; Norton, M.C.; Darcey, D.; Ostbye, T.; Zandi, P.P.; Breitner, J.C.; Welsh-Bohmer, K.A. Occupational exposure to pesticides increases the risk of incident AD: The Cache County study. Neurology 2010, 74, 1524–1530. [Google Scholar] [CrossRef] [Green Version]
- Chalubinski, M.; Kowalski, M.L. Endocrine disrupters—Potential modulators of the immune system and allergic response. Allergy 2006, 61, 1326–1335. [Google Scholar] [CrossRef] [PubMed]
- Robinson, L.; Miller, R. The impact of bisphenol A and phthalates on allergy, asthma, and immune function: A review of latest findings. Curr. Environ. Health Rep. 2015, 2, 379–387. [Google Scholar] [CrossRef] [Green Version]
- Dewailly, E.; Ayotte, P.; Bruneau, S.; Gingras, S.; Belles-Isles, M.; Roy, R. Susceptibility to infections and immune status in Inuit infants exposed to organochlorines. Environ. Health Perspect. 2000, 108, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Heilmann, C.; Grandjean, P.; Weihe, P.; Nielsen, F.; Budtz-Jørgensen, E. Reduced antibody responses to vaccinations in children exposed to polychlorinated biphenyls. PLoS Med. 2006, 3, e311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clayton, E.M.; Todd, M.; Dowd, J.B.; Aiello, A.E. The impact of bisphenol A and triclosan on immune parameters in the U.S. population, NHANES 2003–2006. Environ. Health Perspect. 2011, 119, 390–396. [Google Scholar] [CrossRef]
- Hertz-Picciotto, I.; Park, H.Y.; Dostal, M.; Kocan, A.; Trnovec, Y. Pre-natal exposure to persistent and non-persistent organic compounds and effects on immune system development. Basic Clin. Pharmacol. Toxicol. 2008, 102, 146–154. [Google Scholar] [CrossRef]
- Noakes, P.S.; Taylor, P.; Wilkinson, S.; Prescott, S.L. The relationship between persistent organic pollutants in maternal and neonatal tissues and immune responses to allergens: A novel expoloratory study. Chemosphere 2006, 63, 1304–1311. [Google Scholar] [CrossRef]
- Horváthová, M.; Jahnová, E.; Palkovičová, L.; Trnovec, T.; Hertz-Picciotto, I. Dynamics of lymphocyte subsets in children living in an area polluted by polychlorinated biphenyls. J. Immunotoxicol. 2011, 8, 333–345. [Google Scholar] [CrossRef]
- Yang, X.; Yu, Y.; Xu, J.; Shu, H.; Xia, J.; Liu, H.; Wu, Y.; Zhang, L.; Yu, Z.; Fang, M.; et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Respir. Med. 2020, 8, 475–481. [Google Scholar] [CrossRef] [Green Version]
- NCDHHS. Available online: https://files.nc.gov/ncdhhs/documents/files/covid-19/Risk-Factors-for-Severe-Illness-from-COVID-19.pdf (accessed on 25 May 2020).
- Gold, J.A.W.; Wong, K.K.; Szablewski, C.M.; Patel, P.R.; Rossow, J.; da Silva, J.; Natarajan, P.; Morris, S.B.; Fanfair, R.N.; Rogers-Brown, J.; et al. Characteristics and Clinical Outcomes of Adult Patients Hospitalized with COVID-19—Georgia, March 2020. MMWR Morb. Mortal. Wkly Rep. 2020, 69, 545–550. [Google Scholar] [CrossRef] [PubMed]
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.H.; Ou, C.Q.; He, J.X.; Liu, L.; Shan, H.; Lei, C.L.; Hui, D.; et al. China Medical Treatment Expert Group for Covid-19. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Lian, J.S.; Hu, J.H.; Gao, J.; Zheng, L.; Zhang, Y.M.; Hao, S.R.; Jia, H.Y.; Cai, H.; Zhang, X.L.; et al. Epidemiological, clinical and virological characteristics of 74 cases of coronavirus-infected disease 2019 (COVID-19) with gastrointestinal symptoms. Gut 2020, 69, 1002–1009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guan, W.J.; Liang, W.H.; Zhao, Y.; Liang, H.R.; Chen, Z.S.; Li, Y.M.; Liu, X.Q.; Chen, R.C.; Tang, C.L.; Wang, T.; et al. Comorbidity and its impact on 1590 patients with COVID-19 in China: A nationwide analysis. Eur. Respir. J. 2020, 55, 2000547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y.; et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef] [Green Version]
- Wu, Z.; McGoogan, J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a report of 72,314 cases from the Chinese center for disease control and prevention. JAMA 2020, 323, 1239–1242. [Google Scholar] [CrossRef]
- CDC Centre for Disease Control. People Who Are at Higher Risk for Severe Illness. Available online: https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-at-higher-risk.html (accessed on 27 May 2020).
- Huang, R.; Zhu, L.; Xue, L.; Liu, L.; Yan, X.; Wang, J.; Zhang, B.; Xu, T.; Ji, F.; Zhao, Y.; et al. Clinical findings of patients with coronavirus disease 2019 in Jiangsu province, China: A retrospective, multi-center study. PLoS Negl. Trop. Dis. 2020, 14, e0008280. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, Y.; Shao, C.; Huang, J.; Gan, J.; Huang, X.; Bucci, E.; Piacentini, M.; Ippolito, G.; Melino, G. COVID-19 infection: The perspectives on immune responses. Cell Death Differ. 2020, 27, 1451–1454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.G.; Lebrec, H.; Burleson, G.R. Effect of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) on pulmonary influenza virus titer and natural killer (NK) activity in rats. Fundam. Appl. Toxicol. 1994, 23, 125–131. [Google Scholar] [CrossRef]
- Lawrence, B.P.; Roberts, A.D.; Neumiller, J.J.; Cundiff, J.A.; Woodland, D.L. Aryl hydrocarbon receptor activation impairs the priming but not the recall of influenza virus specific CD8+ T cells in the lung. J. Immunol. 2006, 177, 5819–5828. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, K.A.; Lawrence, B.P. Exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) renders influenza virus-specific CD8+ T cells hyporesponsive to antigen. Toxicol. Sci. 2003, 74, 74–84. [Google Scholar] [CrossRef] [Green Version]
- Teske, S.; Bohn, A.A.; Regal, J.F.; Neumiller, J.J.; Lawrence, B.P. Activation of the aryl hydrocarbon receptor increases pulmonary neutrophilia and diminishes host resistance to influenza A virus. Am. J. Physiol. Lung Cell Mol. Physiol. 2005, 289, L111–L124. [Google Scholar] [CrossRef]
- World Health Organization. Influenza (Seasonal). Fact Sheet No. 211. 2009. Available online: http://www.who.int/mediacentre/factsheets/fs211/en/ (accessed on 23 May 2020).
- Minchole, E.; Figueredo, A.L.; Omeñaca, M.; Panadero, C.; Royo, L.; Vengoechea, J.J.; Fandos, S.; de Pablo, F.; Bello, S. Seasonal Influenza A H1N1pdm09 Virus and Severe Outcomes: A Reason for Broader Vaccination in Non-Elderly, At-Risk People. PLoS ONE 2016, 11, e0165711. [Google Scholar]
- Viasus, D.; Paño-Pardo, J.R.; Pachón, J.; Campins, A.; López-Medrano, F.; Villoslada, A.; Fariñas, M.C.; Moreno, J.; Rodríguez-Baño, J.; Oteo, A.; et al. Factors associated with severe disease in hospitalized adults with pandemic (H1N1) 2009 in Spain. Clin. Microbiol. Infect. 2011, 17, 738–746. [Google Scholar] [CrossRef] [Green Version]
- Ji, J.; Song, L.; Wang, J.; Yang, Z.; Yan, H.; Li, T.; Yu, L.; Jian, L.; Jiang, F.; Li, J.; et al. Association between urinary per- and poly-fluoroalkyl substances and COVID-19 susceptibility. Environ. Int. 2021, 153, 106524. [Google Scholar] [CrossRef]


| Sample Size (n) | Sex (%) | Sample Collection | Total Comorbidity (%) | Major Comorbidities (%) | Clinical Outcomes (%) | References |
|---|---|---|---|---|---|---|
| 138 | Male (54.3) Female (45.7) | Zhongnan Hospital, Wuhan, China | 46.4 | Hypertension (31.2) Cardiovascular disease (14.5) Diabetes (10.1) Malignancy (7.2) Cerebrovascular disease (5.1) Lung disease (2.9) Kidney disease (2.9) Liver disease (2.9) | ICU patients (26.08) Non-ICU patients (73.91) | Wang et al. [9] |
| 41 | Male (73.0) Female (27.0) | Jin Yin-tan Hospital, Wuhan, China | 32 | Diabetes (20) Hypertension (15) Cardiovascular disease (15) Lung disease (2) Liver disease (2) Malignancy (2) | ICU patients (31.70) Non-ICUpatients (68.29) | Huang et al. [11] |
| 548 | Male (50.9) Female (49.08) | Tongji Hospital, Wuhan, China | 64.6 | Hypertension (30.3) Diabetes (15.1) Cardiovascular disease (6.2) Lund disease (5.6) Carcinoma (4.7) Kidney disease (1.8) Liver disease (0.9) | Death (16.5) Severe cases (49.08) Non-sever cases (50.91) | Li et al. [12] |
| 191 | Male (62) Female (38) | Jinyintan Hospital and Wuhan Pulmonary Hospital, China | 48 | Hypertension (30) Diabetes (19) Cardiovascular disease (8) Lung disease (3) Carcinoma (1) Kidney disease (1) | Survivor (70.72) Non-survivor (28.27) | Zhou et al. [13] |
| 663 | Male (48.4) Female (51.6) | Renmin Hospital, Wuhan, China | 37.3 | Lung disease (7.7) Cardiovascular disease (24.7) Gastrointestinal disease (4.7) Endocrine disease (10.1) Kidney disease (3.2) Carcinoma (2.1) Inflammatory diseases (0.9) | Death (67.1) Mild cases (38.31) Severe cases (47.51) | Zhang et al. [14] |
| 52 | Male (67) Female (33) | Yin-tan hospital, Wuhan, China | 40 | Diabetes (17) Brain disease (13.5) Cardiovascular disease (10) Lung disease (8) Malignancy (4) Dementia (2) Malnutrition (2) | Died (61.53) | Yang et al. [119] |
| NM | NM | North Carolina, USA | Cardiovascular diseases (14.0) Diabetes (11.0) Lung disease (10.0) Kidney disease (3.0) Others (5.0) | NM | NCDHHS [120] | |
| 305 | Male (49.5) Female (50.5) | A hospital in Georgia, USA | 94.09 | Diabetes (39.7) Cardiovascular disease (53.8) Lung disease (36) Obesity (12.7) Kidney disease (5.2) Liver disease (2.3) | Died (17.1) ICU patients (39) Non-ICU patients (61) | Gold et al. [121] |
| 1099 | Male (58.1) Female (41.9) | Wuhan Jinyintan Hospital, Wuhan, China | 23.7 | Hypertension (15.0) Diabetes (7.4) Cardiovascular disease (2.5) Liver disease (2.1) Lung disease (1.1) Carcinoma (0.9) Kidney disease (0.7) | Died (1.4) Non-severe cases (84.25) Severe cases (15.74) | Guan et al. [122] |
| 74 | Male (50.0) Female (50.0) | Hospitals in the Zhejiang Province, China | 33.78 | Hypertension (16.22) Diabetes (9.46) Liver disease (10.81) | Severe cases (22.97) Mild cases (77.97) | Jin et al. [123] |
| 1590 | Male (57.3) Female (42.7) | Throughout China | 25.09 | Diabetes (8.2) Hypertension (16.9) Chronic obstructive pulmonary disease (COPD) (NM) Carcinoma (NM) | Death (3.1) ICU patients (6.2) | Guan et al. [124] |
| 99 | Male (68) Female (32) | Jinyintan Hospital, Wuhan, China | 51% | Cardiovascular disease (40) Digestive system disorder (11) Endocrine disorders (13) Carcinoma (1) Lung disease (1) Nervous system disease (1) | Died (11) Severe cases (58) Non-severe cases (31) | Chen et al. [125] |
| 44,672 | NM | Hubei Province, China | NM | Cardiovascular disease (10.5) Diabetes (7.3) Lung disease (6.3) Hypertension (6.0) Carcinoma (5.6) | Died (2.3) Mild cases (81.0) Severe cases (14.0) Critical cases (5.0) | Wu and McGooga [126] |
| 140 | Female (49.3) Male (50.7) | Hospitals in Wuhan, China | 64.3 | Hypertension (30.0) Diabetes (12.1) Liver disease (5.7) Gastrointestinal disease (5.0) Cardiovascular disease (8.5) Hyperlipidemia 7 (5.0) Endocrine diseases (3.6) Liver disease (1.4) Lung disease (1.4) | Non-severe cases (58.57) Severe cases (41.42) | |
| 7162 | NM | USA | 37.6 | Diabetes (10.9) Lung disease (9.2) Cardiovascular disease (9.0) Autoimmune disease (3.7) Kidney disease (3.0) Liver disease (0.6) | ICU patient (14) Non-ICU patient (28.55) | CDC [127] |
| 202 | Male (57.4) Female (42.6) | Jiangsu province, China | 27.2 | Hypertension (14.4) Diabetes (9.4) Lung disease (3.5) Liver diseases (2.0) Cardiovascular diseases (2.5) Cerebrovascular diseases (1.5) Carcinoma (1.0) | Death (0) Non-severe cases (88.61) Severe cases (11.38) | Huang et al. [128] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adegoke, E.O.; Rahman, M.S.; Park, Y.-J.; Kim, Y.J.; Pang, M.-G. Endocrine-Disrupting Chemicals and Infectious Diseases: From Endocrine Disruption to Immunosuppression. Int. J. Mol. Sci. 2021, 22, 3939. https://doi.org/10.3390/ijms22083939
Adegoke EO, Rahman MS, Park Y-J, Kim YJ, Pang M-G. Endocrine-Disrupting Chemicals and Infectious Diseases: From Endocrine Disruption to Immunosuppression. International Journal of Molecular Sciences. 2021; 22(8):3939. https://doi.org/10.3390/ijms22083939
Chicago/Turabian StyleAdegoke, Elikanah Olusayo, Md Saidur Rahman, Yoo-Jin Park, Young Ju Kim, and Myung-Geol Pang. 2021. "Endocrine-Disrupting Chemicals and Infectious Diseases: From Endocrine Disruption to Immunosuppression" International Journal of Molecular Sciences 22, no. 8: 3939. https://doi.org/10.3390/ijms22083939
APA StyleAdegoke, E. O., Rahman, M. S., Park, Y. -J., Kim, Y. J., & Pang, M. -G. (2021). Endocrine-Disrupting Chemicals and Infectious Diseases: From Endocrine Disruption to Immunosuppression. International Journal of Molecular Sciences, 22(8), 3939. https://doi.org/10.3390/ijms22083939