CAND2/PMTR1 Is Required for Melatonin-Conferred Osmotic Stress Tolerance in Arabidopsis
Abstract
:1. Introduction
2. Results
2.1. Melatonin Confers Plant Osmotic Stress Tolerance
2.2. Melatonin Functions in Plant Osmotic Stress Tolerance through Regulating ROS Homeostasis
2.3. CAND2/PMTR1 Is an Osmotic Stress-Responsive Gene
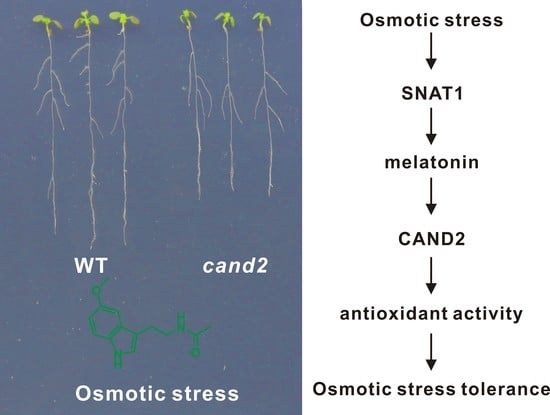
2.4. CAND2/PMTR1 Participates in Melatonin-Conferred Osmotic Stress Tolerance in Plants
3. Discussion
4. Materials and Methods
4.1. Plant Material and Growth Conditions
4.2. 3,3-diaminobenzidine (DAB) Staining and Nitrioblue Tetrazolium (NBT) Staining
4.3. Detection of Catalase (CAT) and Superoxide Dismutase (SOD) Activity
4.4. Quantitative Real-Time PCR
4.5. Melatonin Extraction and Assay
4.6. β-glucuronidase (GUS) Staining
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arnao, M.B.; Hernandez-Ruiz, J. Is Phytomelatonin a New Plant Hormone? Agronomy 2020, 10, 95. [Google Scholar] [CrossRef] [Green Version]
- Arnao, M.B.; Hernandez-Ruiz, J. Melatonin: A New Plant Hormone and/or a Plant Master Regulator? Trends Plant Sci. 2019, 24, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Liu, L.; Wang, L.; Li, B.; Jin, C.; Lin, X. Melatonin: A master regulator of plant development and stress responses. J. Integr. Plant Biol. 2021, 63, 126–145. [Google Scholar] [CrossRef] [PubMed]
- Bajwa, V.S.; Shukla, M.R.; Sherif, S.M.; Murch, S.J.; Saxena, P.K. Role of melatonin in alleviating cold stress in Arabidopsis thaliana. J. Pineal. Res. 2014, 56, 238–245. [Google Scholar] [CrossRef]
- Hernandez-Ruiz, J.; Cano, A.; Arnao, M.B. Melatonin acts as a growth-stimulating compound in some monocot species. J. Pineal. Res. 2005, 39, 137–142. [Google Scholar] [CrossRef]
- Wei, Y.; Bai, Y.; Cheng, X.; Zhu, B.; Reiter, R.J.; Shi, H. The dual roles of melatonin biosynthesis enzymes in the coordination of melatonin biosynthesis and autophagy in cassava. J. Pineal. Res. 2020, 69, e12652. [Google Scholar] [CrossRef]
- Wei, Y.; Bai, Y.; Cheng, X.; Reiter, R.J.; Yin, X.; Shi, H. Lighting the way: Advances in transcriptional regulation and integrative crosstalk of melatonin biosynthetic enzymes in cassava. J. Exp. Bot. 2021, 72, 161–166. [Google Scholar] [CrossRef]
- Yao, J.W.; Ma, Z.; Ma, Y.Q.; Zhu, Y.; Lei, M.Q.; Hao, C.Y.; Chen, L.Y.; Xu, Z.Q.; Huang, X. Role of melatonin in UV-B signaling pathway and UV-B stress resistance in Arabidopsis thaliana. Plant Cell Environ. 2021, 44, 114–129. [Google Scholar] [CrossRef]
- Lee, H.Y.; Back, K. Melatonin induction and its role in high light stress tolerance in Arabidopsis thaliana. J. Pineal. Res. 2018, 65, e12504. [Google Scholar] [CrossRef]
- Li, D.; Wei, J.; Peng, Z.; Ma, W.; Yang, Q.; Song, Z.; Sun, W.; Yang, W.; Yuan, L.; Xu, X.; et al. Daily rhythms of phytomelatonin signaling modulate diurnal stomatal closure via regulating reactive oxygen species dynamics in Arabidopsis. J. Pineal. Res. 2020, 68, e12640. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Sun, X.; Li, C.; Wei, Z.W.; Liang, D.; Ma, F.W. Long-term exogenous application of melatonin delays drought-induced leaf senescence in apple. J. Pineal. Res. 2013, 54, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.K. Abiotic Stress Signaling and Responses in Plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, B.; Chen, Y.E.; Zhao, Y.Q.; Ding, C.B.; Liao, J.Q.; Hu, C.; Zhou, L.J.; Zhang, Z.W.; Yuan, S.; Yuan, M. Exogenous Melatonin Alleviates Oxidative Damages and Protects Photosystem II in Maize Seedlings Under Drought Stress. Front. Plant Sci. 2019, 10, 677. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Tan, D.X.; Liang, D.; Chang, C.; Jia, D.; Ma, F. Melatonin mediates the regulation of ABA metabolism, free-radical scavenging, and stomatal behaviour in two Malus species under drought stress. J. Exp. Bot. 2015, 66, 669–680. [Google Scholar] [CrossRef] [Green Version]
- Liang, D.; Ni, Z.; Xia, H.; Xie, Y.; Lv, X.; Wang, J.; Lin, L.; Deng, Q.; Luo, X. Exogenous melatonin promotes biomass accumulation and photosynthesis of kiwifruit seedlings under drought stress. Sci. Hortic. 2019, 246, 34–43. [Google Scholar] [CrossRef]
- Niu, X.; Deqing, C.; Liang, D. Effects of exogenous melatonin and abscisic acid on osmotic adjustment substances of ‘Summer Black’ grape under drought stress. Iop Conf. 2019, 295, 012012. [Google Scholar] [CrossRef]
- Wang, L.; Feng, C.; Zheng, X.; Guo, Y.; Zhou, F.; Shan, D.; Liu, X.; Kong, J. Plant mitochondria synthesize melatonin and enhance the tolerance of plants to drought stress. J. Pineal. Res. 2017, 63. [Google Scholar] [CrossRef]
- Yang, W.J.; Du, Y.T.; Zhou, Y.B.; Chen, J.; Xu, Z.S.; Ma, Y.Z.; Chen, M.; Min, D.H. Overexpression of TaCOMT Improves Melatonin Production and Enhances Drought Tolerance in Transgenic Arabidopsis. Int. J. Mol. Sci. 2019, 20, 652. [Google Scholar] [CrossRef] [Green Version]
- Zuo, B.; Zheng, X.; He, P.; Wang, L.; Lei, Q.; Feng, C.; Zhou, J.; Li, Q.; Han, Z.; Kong, J. Overexpression of MzASMT improves melatonin production and enhances drought tolerance in transgenic Arabidopsis thaliana plants. J. Pineal. Res. 2014, 57, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Liu, L.; Lu, B.; Ma, T.; Li, C. Exogenous melatonin promotes seed germination and osmotic regulation under salt stress in cotton (Gossypium hirsutum L.). PLoS ONE 2020, 15, e0228241. [Google Scholar]
- Choi, G.H.; Back, K. Suppression of Melatonin 2-Hydroxylase Increases Melatonin Production Leading to the Enhanced Abiotic Stress Tolerance against Cadmium, Senescence, Salt, and Tunicamycin in Rice Plants. Biomolecules 2019, 9, 589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, C.; Zheng, G.; Li, W.; Wang, Y.; Hu, B.; Wang, H.; Wu, H.; Qian, Y.; Zhu, X.G.; Tan, D.X.; et al. Melatonin delays leaf senescence and enhances salt stress tolerance in rice. J. Pineal. Res. 2015, 59, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.D.; Sun, X.S.; Liu, L.; Shi, H.D.; Chen, S.Y.; Zhao, D.K. Overexpression of the Melatonin Synthesis-Related Gene SlCOMT1 Improves the Resistance of Tomato to Salt Stress. Molecules 2019, 24, 1514. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, S.; David, A.; Yadav, S.; Baluska, F.; Bhatla, S.C. Salt stress-induced seedling growth inhibition coincides with differential distribution of serotonin and melatonin in sunflower seedling roots and cotyledons. Physiol. Plant 2014, 152, 714–728. [Google Scholar] [CrossRef]
- Zhao, G.; Yu, X.L.; Lou, W.; Wei, S.Q.; Wang, R.; Wan, Q.; Shen, W.B. Transgenic Arabidopsis overexpressing MsSNAT enhances salt tolerance via the increase in autophagy, and the reestablishment of redox and ion homeostasis. Environ. Exp. Bot. 2019, 164, 20–28. [Google Scholar] [CrossRef]
- Zheng, X.; Tan, D.X.; Allan, A.C.; Zuo, B.; Zhao, Y.; Reiter, R.J.; Wang, L.; Wang, Z.; Guo, Y.; Zhou, J.; et al. Chloroplastic biosynthesis of melatonin and its involvement in protection of plants from salt stress. Sci. Rep. 2017, 7, 41236. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Guo, Y. Elucidating the molecular mechanisms mediating plant salt-stress responses. New Phytol. 2018, 217, 523–539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, K.; Gao, J.; Sun, S.; Zhang, Z.; Yu, B.; Li, J.; Xie, C.; Li, G.; Wang, P.; Song, C.P.; et al. BONZAI Proteins Control Global Osmotic Stress Responses in Plants. Curr. Biol. 2020, 30, 4815–4825 e4814. [Google Scholar] [CrossRef] [PubMed]
- Yuan, F.; Yang, H.; Xue, Y.; Kong, D.; Ye, R.; Li, C.; Zhang, J.; Theprungsirikul, L.; Shrift, T.; Krichilsky, B.; et al. OSCA1 mediates osmotic-stress-evoked Ca2+ increases vital for osmosensing in Arabidopsis. Nature 2014, 514, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Stephan, A.B.; Kunz, H.H.; Yang, E.; Schroeder, J.I. Rapid hyperosmotic-induced Ca2+ responses in Arabidopsis thaliana exhibit sensory potentiation and involvement of plastidial KEA transporters. Proc. Natl. Acad. Sci. USA 2016, 113, E5242–E5249. [Google Scholar] [CrossRef] [Green Version]
- Wei, J.; Li, D.X.; Zhang, J.R.; Shan, C.; Rengel, Z.; Song, Z.B.; Chen, Q. Phytomelatonin receptor PMTR1-mediated signaling regulates stomatal closure in Arabidopsis thaliana. J. Pineal. Res. 2018, 65, e12500. [Google Scholar] [CrossRef] [PubMed]
- Pelagio-Flores, R.; Munoz-Parra, E.; Ortiz-Castro, R.; Lopez-Bucio, J. Melatonin regulates Arabidopsis root system architecture likely acting independently of auxin signaling. J. Pineal. Res. 2012, 53, 279–288. [Google Scholar] [CrossRef]
- Gao, F.; Xie, Y.; Shen, Y.; Lei, Z.; Wang, X.; Xia, H.; Liang, D.; University, S.A. Exogenous melatonin for NaCl stress with antioxidant enzymes and osmotic substances of Aclinidia deliciosa seedlings. J. Zhejiang A & F Univ. 2018, 35, 291–297. [Google Scholar]
- Kang, K.; Lee, K.; Park, S.; Kim, Y.S.; Back, K. Enhanced production of melatonin by ectopic overexpression of human serotonin N-acetyltransferase plays a role in cold resistance in transgenic rice seedlings. J. Pineal. Res. 2010, 49, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Moustafa-Farag, M.; Almoneafy, A.; Mahmoud, A.; Elkelish, A.; Arnao, M.B.; Li, L.; Ai, S. Melatonin and Its Protective Role against Biotic Stress Impacts on Plants. Biomolecules 2019, 10, 54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.R.; Li, D.X.; Wei, J.; Ma, W.N.; Kong, X.Y.; Rengel, Z.; Chen, Q. Melatonin alleviates aluminum-induced root growth inhibition by interfering with nitric oxide production in Arabidopsis. Environ. Exp. Bot. 2019, 161, 157–165. [Google Scholar] [CrossRef]
- Zhang, Y.; Ji, T.T.; Li, T.T.; Tian, Y.Y.; Wang, L.F.; Liu, W.C. Jasmonic acid promotes leaf senescence through MYC2-mediated repression of CATALASE2 expression in Arabidopsis. Plant Sci. 2020, 299. [Google Scholar] [CrossRef]
- Zhang, Y.; Tian, Y.Y.; Wang, L.F.; Li, Y.H.; Li, T.T.; Liu, W.C. WDR5a functions in cadmium-inhibited root meristem growth by regulating nitric oxide accumulation in Arabidopsis. Planta 2020, 252. [Google Scholar] [CrossRef]
- Li, T.T.; Liu, W.C.; Wang, F.F.; Ma, Q.B.; Lu, Y.T.; Yuan, T.T. SORTING NEXIN 1 Functions in Plant Salt Stress Tolerance through Changes of NO Accumulation by Regulating NO Synthase-Like Activity. Front. Plant. Sci. 2018, 9. [Google Scholar] [CrossRef]
- Giannopolitis, C.N.; Ries, S.K. Superoxide dismutases: I. Occurrence in higher plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Methods Enzymol 1984, 105, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.C.; Li, Y.H.; Yuan, H.M.; Zhang, B.L.; Zhai, S.; Lu, Y.T. WD40-REPEAT 5a functions in drought stress tolerance by regulating nitric oxide accumulation in Arabidopsis. Plant Cell Environ. 2017, 40, 543–552. [Google Scholar] [CrossRef] [PubMed]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.-F.; Li, T.-T.; Zhang, Y.; Guo, J.-X.; Lu, K.-K.; Liu, W.-C. CAND2/PMTR1 Is Required for Melatonin-Conferred Osmotic Stress Tolerance in Arabidopsis. Int. J. Mol. Sci. 2021, 22, 4014. https://doi.org/10.3390/ijms22084014
Wang L-F, Li T-T, Zhang Y, Guo J-X, Lu K-K, Liu W-C. CAND2/PMTR1 Is Required for Melatonin-Conferred Osmotic Stress Tolerance in Arabidopsis. International Journal of Molecular Sciences. 2021; 22(8):4014. https://doi.org/10.3390/ijms22084014
Chicago/Turabian StyleWang, Lin-Feng, Ting-Ting Li, Yu Zhang, Jia-Xing Guo, Kai-Kai Lu, and Wen-Cheng Liu. 2021. "CAND2/PMTR1 Is Required for Melatonin-Conferred Osmotic Stress Tolerance in Arabidopsis" International Journal of Molecular Sciences 22, no. 8: 4014. https://doi.org/10.3390/ijms22084014
APA StyleWang, L. -F., Li, T. -T., Zhang, Y., Guo, J. -X., Lu, K. -K., & Liu, W. -C. (2021). CAND2/PMTR1 Is Required for Melatonin-Conferred Osmotic Stress Tolerance in Arabidopsis. International Journal of Molecular Sciences, 22(8), 4014. https://doi.org/10.3390/ijms22084014