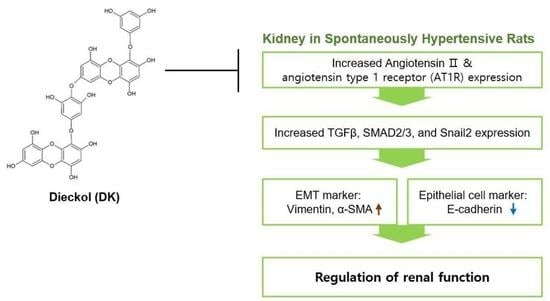
Attenuating Effects of Dieckol on Hypertensive Nephropathy in Spontaneously Hypertensive Rats
Abstract
:1. Introduction
2. Results
2.1. ECE and DK Reduced Systolic BP and Serum Level of Ang II in SHRs
2.2. ECE and DK Attenuated the Expression of AT1R in the Kidney of SHRs
2.3. ECE and DK Reduced the Expression of TGFβ, SMAD2/3, and Snail2 in the Kidney of SHRs
2.4. ECE and DK Reduced the EMT in the Kidney of the SHRs
2.5. ECE and DK Reduced the Renal Fibrosis and Glomerular Sclerosis in the Kidney of SHRs
2.6. ECE and DK Attenuated Renal Function Aggravation in SHRs
3. Discussion
4. Materials and Methods
4.1. Preparation of ECE and Isolation of DK
4.2. Hypertension Animal Model
4.3. Immunohistochemistry
4.4. RNA Extraction and Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
4.5. Enzyme-Linked Immunosorbent Assay (ELISA)
4.6. Histological Analysis
4.6.1. Masson’s Trichrome (MT) Stain
4.6.2. Periodic Acid–Schiff (PAS) Stain
- (1)
- Grade 0: normal glomeruli;
- (2)
- Grade 1: minimal sclerosis (sclerotic area up to 25%);
- (3)
- Grade 2: moderate sclerosis (sclerotic area 25% to 50%);
- (4)
- Grade 3: moderate–severe sclerosis (sclerotic area 50% to 75%);
- (5)
- Grade 4: severe sclerosis (sclerotic area 75% to 100%);
4.7. Systolic Blood Pressure, Diastolic Blood Pressure, and Mean Arterial Blood Pressure Measurements
4.8. In Vitro Modeling Using TCMK-1 Cells
4.9. Preparation of Protein and Western Blotting
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Whelton, P.K.; Carey, R.M.; Aronow, W.S.; Casey, D.E., Jr.; Collins, K.J.; Dennison Himmelfarb, C.; DePalma, S.M.; Gidding, S.; Jamerson, K.A.; Jones, D.W.; et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2018, 71, e127–e248. [Google Scholar] [CrossRef]
- Mennuni, S.; Rubattu, S.; Pierelli, G.; Tocci, G.; Fofi, C.; Volpe, M. Hypertension and kidneys: Unraveling complex molecular mechanisms underlying hypertensive renal damage. J. Hum. Hypertens. 2014, 28, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Breyer, M.D.; Susztak, K. The next generation of therapeutics for chronic kidney disease. Nat. Rev. Drug Discov. 2016, 15, 568–588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schelling, J.R. Tubular atrophy in the pathogenesis of chronic kidney disease progression. Pediatr. Nephrol. 2016, 31, 693–706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duggan, K.A.; Hodge, G.; Chen, J.; Trajanovska, S.; Hunter, T. Vasoactive intestinal peptide infusion reverses existing renal interstitial fibrosis via a blood pressure independent mechanism in the rat. Eur. J. Pharm. 2020, 873, 172979. [Google Scholar] [CrossRef]
- Chen, D.Q.; Feng, Y.L.; Cao, G.; Zhao, Y.Y. Natural Products as a Source for Antifibrosis Therapy. Trends Pharm. Sci. 2018, 39, 937–952. [Google Scholar] [CrossRef]
- Strutz, F. Pathogenesis of tubulointerstitial fibrosis in chronic allograft dysfunction. Clin. Transpl. 2009, 23, 26–32. [Google Scholar] [CrossRef]
- Hills, C.E.; Squires, P.E. TGF-beta1-induced epithelial-to-mesenchymal transition and therapeutic intervention in diabetic nephropathy. Am. J. Nephrol. 2010, 31, 68–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iwano, M.; Plieth, D.; Danoff, T.M.; Xue, C.; Okada, H.; Neilson, E.G. Evidence that fibroblasts derive from epithelium during tissue fibrosis. J. Clin. Investig. 2002, 110, 341–350. [Google Scholar] [CrossRef]
- Balakumar, P.; Sambathkumar, R.; Mahadevan, N.; Muhsinah, A.B.; Alsayari, A.; Venkateswaramurthy, N.; Jagadeesh, G. A potential role of the renin-angiotensin-aldosterone system in epithelial-to-mesenchymal transition-induced renal abnormalities: Mechanisms and therapeutic implications. Pharm. Res. 2019, 146, 104314. [Google Scholar] [CrossRef]
- Pan, J.; Zhang, J.; Zhang, X.; Zhou, X.; Lu, S.; Huang, X.; Shao, J.; Lou, G.; Yang, D.; Geng, Y.J. Role of microRNA-29b in angiotensin II-induced epithelial-mesenchymal transition in renal tubular epithelial cells. Int. J. Mol. Med. 2014, 34, 1381–1387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, F.; Huang, X.R.; Chung, A.C.; Hou, C.C.; Lai, K.N.; Lan, H.Y. Essential role for Smad3 in angiotensin II-induced tubular epithelial-mesenchymal transition. J. Pathol. 2010, 221, 390–401. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.S.; Kim, I.J.; Bae, E.H.; Ma, S.K.; Lee, J.; Kim, S.W. Angiotensin-(1-7) Attenuates Kidney Injury Due to Obstructive Nephropathy in Rats. PLoS ONE 2015, 10, e0142664. [Google Scholar] [CrossRef] [Green Version]
- Wijesekara, I.; Kim, S.K. Angiotensin-I-converting enzyme (ACE) inhibitors from marine resources: Prospects in the pharmaceutical industry. Mar. Drugs 2010, 8, 1080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Athukorala, Y.; Jeon, Y.J. Screening for angiotensin 1-converting enzyme inhibitory activity of Ecklonia cava. J. Food Sci. Nutr. 2005, 10, 134–139. [Google Scholar]
- Wijesinghe, W.A.; Ko, S.C.; Jeon, Y.J. Screening of Extracts from Red Algae in Jeju for Potentials MarineAngiotensin-I Converting Enzyme (ACE) Inhibitory Activity. Algae 2006, 21, 343–348. [Google Scholar]
- Wijesinghe, W.A.; Ko, S.C.; Jeon, Y.J. Effect of phlorotannins isolated from Ecklonia cava on angiotensin I-converting enzyme (ACE) inhibitory activity. Nutr. Res. Pract. 2011, 5, 93–100. [Google Scholar] [CrossRef] [Green Version]
- Gómez-Guzmán, M.; Rodríguez-Nogales, A.; Algieri, F.; Gálvez, J. Potential Role of Seaweed Polyphenols in Cardiovascular-Associated Disorders. Mar. Drugs 2018, 16, 250. [Google Scholar] [CrossRef] [Green Version]
- Jung, H.A.; Hyun, S.K.; Kim, H.R.; Choi, J.S. Angiotensin-converting enzyme I inhibitory activity of phlorotannins from Ecklonia stolonifera. Fish. Sci. 2006, 72, 1292–1299. [Google Scholar] [CrossRef]
- Son, M.; Oh, S.; Choi, C.H.; Park, K.Y.; Son, K.H.; Byun, K. Pyrogallol-Phloroglucinol-6,6-Bieckol from Ecklonia cava Attenuates Tubular Epithelial Cell (TCMK-1) Death in Hypoxia/Reoxygenation Injury. Mar. Drugs 2019, 17, 602. [Google Scholar] [CrossRef] [Green Version]
- Seccia, T.M.; Caroccia, B.; Calò, L.A. Hypertensive nephropathy. Moving from classic to emerging pathogenetic mechanisms. J. Hypertens. 2017, 35, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Shen, W.; Chen, Q.; Cao, Q.; Di, W.; Lan, R.; Chen, Z.; Bai, J.; Han, Z.; Xu, W. Inhibition of RAGE by FPS-ZM1 alleviates renal injury in spontaneously hypertensive rats. Eur. J. Pharm. 2020, 882, 173228. [Google Scholar] [CrossRef] [PubMed]
- Udani, S.; Lazich, I.; Bakris, G.L. Epidemiology of hypertensive kidney disease. Nat. Rev. Nephrol. 2011, 7, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Hill, G.S.; Heudes, D.; Bariéty, J. Morphometric study of arterioles and glomeruli in the aging kidney suggests focal loss of autoregulation. Kidney Int. 2003, 63, 1027–1036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hill, G.S. Hypertensive nephrosclerosis. Curr. Opin. Nephrol. Hypertens. 2008, 17, 266–270. [Google Scholar] [CrossRef]
- Seccia, T.M.; Maniero, C.; Belloni, A.S.; Guidolin, D.; Pothen, P.; Pessina, A.C.; Rossi, G.P. Role of angiotensin II, endothelin-1 and L-type calcium channel in the development of glomerular, tubulointerstitial and perivascular fibrosis. J. Hypertens. 2008, 26, 2022–2029. [Google Scholar] [CrossRef]
- Seccia, T.M.; Belloni, A.S.; Guidolin, D.; Sticchi, D.; Nussdorfer, G.G.; Pessina, A.C.; Rossi, G.P. The renal antifibrotic effects of angiotensin-converting enzyme inhibition involve bradykinin B2 receptor activation in angiotensin II-dependent hypertension. J. Hypertens. 2006, 24, 1419–1427. [Google Scholar] [CrossRef]
- Huang, S.; Susztak, K. Epithelial Plasticity versus EMT in Kidney Fibrosis. Trends Mol. Med. 2016, 22, 4–6. [Google Scholar] [CrossRef] [Green Version]
- Lovisa, S.; LeBleu, V.S.; Tampe, B.; Sugimoto, H.; Vadnagara, K.; Carstens, J.L.; Wu, C.C.; Hagos, Y.; Burckhardt, B.C.; Pentcheva-Hoang, T.; et al. Epithelial-to-mesenchymal transition induces cell cycle arrest and parenchymal damage in renal fibrosis. Nat. Med. 2015, 21, 998–1009. [Google Scholar] [CrossRef]
- Satou, R.; Penrose, H.; Navar, L.G. Inflammation as a Regulator of the Renin-Angiotensin System and Blood Pressure. Curr. Hypertens. Rep. 2018, 20, 100. [Google Scholar] [CrossRef]
- Urushihara, M.; Kagami, S. Role of the intrarenal renin-angiotensin system in the progression of renal disease. Pediatr. Nephrol. 2017, 32, 1471–1479. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Xu, C. Physiology and Pathophysiology of the Intrarenal Renin-Angiotensin System: An Update. J. Am. Soc. Nephrol. 2017, 28, 1040–1049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, X.M.; Nikolic-Paterson, D.J.; Lan, H.Y. TGF-β: The master regulator of fibrosis. Nat. Rev. Nephrol. 2016, 12, 325–338. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.M.; Yuan, H.Q.; Ren, Z.; Qu, S.L.; Liu, L.S.; Yin, K.; Fu, M.; Jiang, Z.S. Endothelial to mesenchymal transition in atherosclerotic vascular remodeling. Clin. Chim. Acta 2019, 490, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Guan, M.; Li, W.; Xu, L.; Zeng, Y.; Wang, D.; Zheng, Z.; Lyv, F.; Xue, Y. Metformin Improves Epithelial-to-Mesenchymal Transition Induced by TGF-β1 in Renal Tubular Epithelial NRK-52E Cells via Inhibiting Egr-1. J. Diabetes Res. 2018, 2018, 1031367. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Jia, Z.; Guo, X.; Yang, T. Aldosterone induces epithelial-mesenchymal transition via ROS of mitochondrial origin. Am. J. Physiol. Renal. Physiol. 2007, 293, F723–F731. [Google Scholar] [CrossRef]
- Leader, C.J.; Kelly, D.J.; Sammut, I.A.; Wilkins, G.T.; Walker, R.J. Spironolactone mitigates, but does not reverse, the progression of renal fibrosis in a transgenic hypertensive rat. Physiol. Rep. 2020, 8, e14448. [Google Scholar] [CrossRef] [PubMed]
- Buemi, M.; Nostro, L.; Di Pasquale, G.; Cavallaro, E.; Sturiale, A.; Floccari, F.; Aloisi, C.; Ruello, A.; Calapai, G.; Corica, F.; et al. Aquaporin-2 water channels in spontaneously hypertensive rats. Am. J. Hypertens. 2004, 17, 1170–1178. [Google Scholar] [CrossRef] [Green Version]
- Koo, H.; Hwang, S.; Kim, T.H.; Kang, S.W.; Oh, K.H.; Ahn, C.; Kim, Y.H. The ratio of urinary sodium and potassium and chronic kidney disease progression: Results from the KoreaN Cohort Study for Outcomes in Patients with Chronic Kidney Disease (KNOW-CKD). Medicine 2018, 97, e12820. [Google Scholar] [CrossRef]
- He, J.; Mills, K.T.; Appel, L.J.; Yang, W.; Chen, J.; Lee, B.T.; Rosas, S.E.; Porter, A.; Makos, G.; Weir, M.R.; et al. Urinary Sodium and Potassium Excretion and CKD Progression. J. Am. Soc. Nephrol. 2016, 27, 1202–1212. [Google Scholar] [CrossRef]
- Inker, L.A.; Astor, B.C.; Fox, C.H.; Isakova, T.; Lash, J.P.; Peralta, C.A.; Kurella Tamura, M.; Feldman, H.I. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for the evaluation and management of CKD. Am. J. Kidney Dis. 2014, 63, 713–735. [Google Scholar] [CrossRef] [Green Version]
- Inoue, B.H.; Arruda-Junior, D.F.; Campos, L.C.; Barreto, A.L.; Rodrigues, M.V.; Krieger, J.E.; Girardi, A.C. Progression of microalbuminuria in SHR is associated with lower expression of critical components of the apical endocytic machinery in the renal proximal tubule. Am. J. Physiol. Renal. Physiol. 2013, 305, F216–F226. [Google Scholar] [CrossRef]
- Russo, L.M.; Osicka, T.M.; Brammar, G.C.; Candido, R.; Jerums, G.; Comper, W.D. Renal processing of albumin in diabetes and hypertension in rats: Possible role of TGF-beta1. Am. J. Nephrol. 2003, 23, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.H.; Son, B.S.; Kim, B.K.; Chee, H.Y.; Song, K.S.; Lee, B.H.; Shin, H.C.; Lee, K.B. Antihypertensive effect of Ecklonia cava extract. Korean J. Pharm. 2006, 37, 200–205. [Google Scholar]
- Son, M.; Oh, S.; Lee, H.S.; Ryu, B.; Jiang, Y.; Jang, J.T.; Jeon, Y.J.; Byun, K. Pyrogallol-Phloroglucinol-6,6′-Bieckol from Ecklonia cava Improved Blood Circulation in Diet-Induced Obese and Diet-Induced Hypertension Mouse Models. Mar. Drugs 2019, 17, 272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maric, C.; Sandberg, K.; Hinojosa-Laborde, C. Glomerulosclerosis and tubulointerstitial fibrosis are attenuated with 17beta-estradiol in the aging Dahl salt sensitive rat. J. Am. Soc. Nephrol. 2004, 15, 1546–1556. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Wen, Y.; Lv, L.L.; Liu, H.; Tang, R.N.; Ma, K.L.; Liu, B.C. Involvement of endoplasmic reticulum stress in angiotensin II-induced NLRP3 inflammasome activation in human renal proximal tubular cells in vitro. Acta Pharm. Sin. 2015, 36, 821–830. [Google Scholar] [CrossRef] [PubMed] [Green Version]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Son, M.; Oh, S.; Choi, J.; Jang, J.T.; Son, K.H.; Byun, K. Attenuating Effects of Dieckol on Hypertensive Nephropathy in Spontaneously Hypertensive Rats. Int. J. Mol. Sci. 2021, 22, 4230. https://doi.org/10.3390/ijms22084230
Son M, Oh S, Choi J, Jang JT, Son KH, Byun K. Attenuating Effects of Dieckol on Hypertensive Nephropathy in Spontaneously Hypertensive Rats. International Journal of Molecular Sciences. 2021; 22(8):4230. https://doi.org/10.3390/ijms22084230
Chicago/Turabian StyleSon, Myeongjoo, Seyeon Oh, Junwon Choi, Ji Tae Jang, Kuk Hui Son, and Kyunghee Byun. 2021. "Attenuating Effects of Dieckol on Hypertensive Nephropathy in Spontaneously Hypertensive Rats" International Journal of Molecular Sciences 22, no. 8: 4230. https://doi.org/10.3390/ijms22084230
APA StyleSon, M., Oh, S., Choi, J., Jang, J. T., Son, K. H., & Byun, K. (2021). Attenuating Effects of Dieckol on Hypertensive Nephropathy in Spontaneously Hypertensive Rats. International Journal of Molecular Sciences, 22(8), 4230. https://doi.org/10.3390/ijms22084230