Endoplasmic Reticulum‐Plasma Membrane Contact Sites as an Organizing Principle for Compartmentalized Calcium and cAMP Signaling
Abstract
:1. Introduction
2. STIM/Orai/TRPC-Mediated Store-Operated Ca2+ Entry
3. Ca2+ and cAMP Signaling Cascades form a Highly Interconnected Network
4. Compartmentalization of Ca2+/cAMP Signaling in Membrane Nanodomains at ER–PM Contact Sites
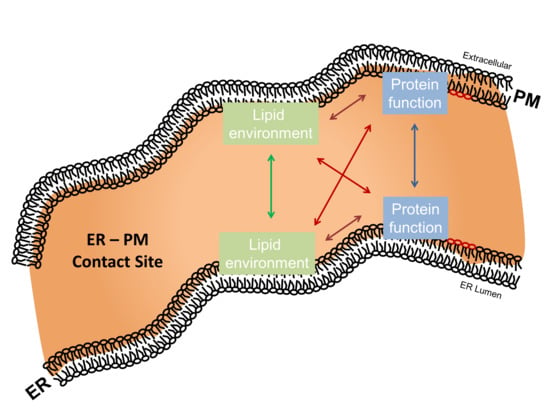
5. Regulatory Interplay between ER–PM Contact Site Lipid Species and Residing Proteins
5.1. ER–PM Contact Site-Residing Proteins Modulate Their Surrounding Lipid Environment
5.2. ER–PM Contact Site-Residing Lipid Species Modulate Protein Activity
6. Physiological Effects of ER–PM Contact Sites
7. Conclusions and Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Raffaello, A.; Mammucari, C.; Gherardi, G.; Rizzuto, R. Calcium at the Center of Cell Signaling: Interplay between Endoplasmic Reticulum, Mitochondria, and Lysosomes. Trends Biochem. Sci. 2016, 41, 1035–1049. [Google Scholar] [CrossRef] [Green Version]
- Berridge, M.J. The Inositol Trisphosphate/Calcium Signaling Pathway in Health and Disease. Physiol. Rev. 2016, 96, 1261–1296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gold, M.G.; Gonen, T.; Scott, J.D. Local CAMP Signaling in Disease at a Glance. J. Cell Sci. 2013, 126, 4537–4543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahuja, M.; Jha, A.; Maléth, J.; Park, S.; Muallem, S. CAMP and Ca2+ Signaling in Secretory Epithelia: Crosstalk and Synergism. Cell Calcium 2014, 55, 385–393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maléth, J.; Hegyi, P. Ca2+ Toxicity and Mitochondrial Damage in Acute Pancreatitis: Translational Overview. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2016, 371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madácsy, T.; Pallagi, P.; Maleth, J. Cystic Fibrosis of the Pancreas: The Role of CFTR Channel in the Regulation of Intracellular Ca2+ Signaling and Mitochondrial Function in the Exocrine Pancreas. Front. Physiol. 2018, 9, 1585. [Google Scholar] [CrossRef] [Green Version]
- Son, A.; Ahuja, M.; Schwartz, D.M.; Varga, A.; Swaim, W.; Kang, N.; Maleth, J.; Shin, D.M.; Muallem, S. Ca2+ Influx Channel Inhibitor SARAF Protects Mice from Acute Pancreatitis. Gastroenterology 2019, 157, 1660–1672.e2. [Google Scholar] [CrossRef] [Green Version]
- Pallagi, P.; Madácsy, T.; Varga, Á.; Maléth, J. Intracellular Ca2+ Signalling in the Pathogenesis of Acute Pancreatitis: Recent Advances and Translational Perspectives. Int. J. Mol. Sci. 2020, 21, 4005. [Google Scholar] [CrossRef] [PubMed]
- Parton, R.G.; del Pozo, M.A. Caveolae as Plasma Membrane Sensors, Protectors and Organizers. Nat. Rev. Mol. Cell Biol. 2013, 14, 98–112. [Google Scholar] [CrossRef]
- Sezgin, E.; Levental, I.; Mayor, S.; Eggeling, C. The Mystery of Membrane Organization: Composition, Regulation and Roles of Lipid Rafts. Nat. Rev. Mol. Cell Biol. 2017, 18, 361–374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, X.; Choi, S.; Maléth, J.J.; Park, S.; Ahuja, M.; Muallem, S. The ER/PM Microdomain, PI(4,5)P₂ and the Regulation of STIM1-Orai1 Channel Function. Cell Calcium 2015, 58, 342–348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prinz, W.A.; Toulmay, A.; Balla, T. The Functional Universe of Membrane Contact Sites. Nat. Rev. Mol. Cell Biol. 2020, 21, 7–24. [Google Scholar] [CrossRef]
- Valm, A.M.; Cohen, S.; Legant, W.R.; Melunis, J.; Hershberg, U.; Wait, E.; Cohen, A.R.; Davidson, M.W.; Betzig, E.; Lippincott-Schwartz, J. Applying Systems-Level Spectral Imaging and Analysis to Reveal the Organelle Interactome. Nature 2017, 546, 162–167. [Google Scholar] [CrossRef]
- Shai, N.; Yifrach, E.; van Roermund, C.W.T.; Cohen, N.; Bibi, C.; IJlst, L.; Cavellini, L.; Meurisse, J.; Schuster, R.; Zada, L.; et al. Systematic Mapping of Contact Sites Reveals Tethers and a Function for the Peroxisome-Mitochondria Contact. Nat. Commun. 2018, 9, 1761. [Google Scholar] [CrossRef]
- Schwarz, D.S.; Blower, M.D. The Endoplasmic Reticulum: Structure, Function and Response to Cellular Signaling. Cell Mol. Life Sci. 2016, 73, 79–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parekh, A.B.; Putney, J.W. Store-Operated Calcium Channels. Physiol. Rev. 2005, 85, 757–810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weis, W.I.; Kobilka, B.K. The Molecular Basis of G Protein-Coupled Receptor Activation. Annu. Rev. Biochem. 2018, 87, 897–919. [Google Scholar] [CrossRef] [PubMed]
- Lopez, J.J.; Jardin, I.; Sanchez-Collado, J.; Salido, G.M.; Smani, T.; Rosado, J.A. TRPC Channels in the SOCE Scenario. Cells 2020, 9, 126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, S.; Maleth, J.; Jha, A.; Lee, K.P.; Kim, M.S.; So, I.; Ahuja, M.; Muallem, S. The TRPCs-STIM1-Orai Interaction. Handb. Exp. Pharmacol. 2014, 223, 1035–1054. [Google Scholar] [CrossRef]
- Baba, Y.; Hayashi, K.; Fujii, Y.; Mizushima, A.; Watarai, H.; Wakamori, M.; Numaga, T.; Mori, Y.; Iino, M.; Hikida, M.; et al. Coupling of STIM1 to Store-Operated Ca2+ Entry through Its Constitutive and Inducible Movement in the Endoplasmic Reticulum. Proc. Natl. Acad. Sci. USA 2006, 103, 16704–16709. [Google Scholar] [CrossRef] [Green Version]
- Derler, I.; Fahrner, M.; Muik, M.; Lackner, B.; Schindl, R.; Groschner, K.; Romanin, C. A Ca2+ Release-Activated Ca2+ (CRAC) Modulatory Domain (CMD) within STIM1 Mediates Fast Ca2+-Dependent Inactivation of ORAI1 Channels. J. Biol. Chem. 2009, 284, 24933–24938. [Google Scholar] [CrossRef] [Green Version]
- Jardin, I.; Dionisio, N.; Frischauf, I.; Berna-Erro, A.; Woodard, G.E.; López, J.J.; Salido, G.M.; Rosado, J.A. The Polybasic Lysine-Rich Domain of Plasma Membrane-Resident STIM1 Is Essential for the Modulation of Store-Operated Divalent Cation Entry by Extracellular Calcium. Cell. Signal. 2013, 25, 1328–1337. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Lu, J.; Xu, P.; Xie, X.; Chen, L.; Xu, T. Mapping the Interacting Domains of STIM1 and Orai1 in Ca2+ Release-Activated Ca2+ Channel Activation. J. Biol. Chem. 2007, 282, 29448–29456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muik, M.; Frischauf, I.; Derler, I.; Fahrner, M.; Bergsmann, J.; Eder, P.; Schindl, R.; Hesch, C.; Polzinger, B.; Fritsch, R.; et al. Dynamic Coupling of the Putative Coiled-Coil Domain of ORAI1 with STIM1 Mediates ORAI1 Channel Activation. J. Biol. Chem. 2008, 283, 8014–8022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stathopulos, P.B.; Zheng, L.; Li, G.-Y.; Plevin, M.J.; Ikura, M. Structural and Mechanistic Insights into STIM1-Mediated Initiation of Store-Operated Calcium Entry. Cell 2008, 135, 110–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, J.P.; Zeng, W.; Dorwart, M.R.; Choi, Y.-J.; Worley, P.F.; Muallem, S. SOAR and the Polybasic STIM1 Domains Gate and Regulate Orai Channels. Nat. Cell Biol. 2009, 11, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Stathopulos, P.B.; Li, G.-Y.; Ikura, M. Biophysical Characterization of the EF-Hand and SAM Domain Containing Ca2+ Sensory Region of STIM1 and STIM2. Biochem. Biophys. Res. Commun. 2008, 369, 240–246. [Google Scholar] [CrossRef]
- Stathopulos, P.B.; Li, G.-Y.; Plevin, M.J.; Ames, J.B.; Ikura, M. Stored Ca2+ Depletion-Induced Oligomerization of Stromal Interaction Molecule 1 (STIM1) via the EF-SAM Region. J. Biol. Chem. 2006, 281, 35855–35862. [Google Scholar] [CrossRef] [Green Version]
- Ma, G.; Wei, M.; He, L.; Liu, C.; Wu, B.; Zhang, S.L.; Jing, J.; Liang, X.; Senes, A.; Tan, P.; et al. Inside-out Ca2+ Signalling Prompted by STIM1 Conformational Switch. Nat. Commun. 2015, 6. [Google Scholar] [CrossRef] [Green Version]
- Dong, H.; Fiorin, G.; Carnevale, V.; Treptow, W.; Klein, M.L. Pore Waters Regulate Ion Permeation in a Calcium Release-Activated Calcium Channel. Proc. Natl. Acad. Sci. USA 2013, 110, 17332–17337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirve, N.; Rajanikanth, V.; Hogan, P.G.; Gudlur, A. Coiled-Coil Formation Conveys a STIM1 Signal from ER Lumen to Cytoplasm. Cell Rep. 2018, 22, 72–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, G.N.; Zeng, W.; Kim, J.Y.; Yuan, J.P.; Han, L.; Muallem, S.; Worley, P.F. STIM1 Carboxyl-Terminus Activates Native SOC, Icrac and TRPC1 Channels. Nat. Cell Biol. 2006, 8, 1003–1010. [Google Scholar] [CrossRef] [PubMed]
- Muik, M.; Fahrner, M.; Derler, I.; Schindl, R.; Bergsmann, J.; Frischauf, I.; Groschner, K.; Romanin, C. A Cytosolic Homomerization and a Modulatory Domain within STIM1 C Terminus Determine Coupling to ORAI1 Channels. J. Biol. Chem. 2009, 284, 8421–8426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, X.; Jin, H.; Cai, X.; Li, S.; Shen, Y. Structural and Mechanistic Insights into the Activation of Stromal Interaction Molecule 1 (STIM1). Proc. Natl. Acad. Sci. USA 2012, 109, 5657–5662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, C.Y.; Hoover, P.J.; Mullins, F.M.; Bachhawat, P.; Covington, E.D.; Raunser, S.; Walz, T.; Garcia, K.C.; Dolmetsch, R.E.; Lewis, R.S. STIM1 Clusters and Activates CRAC Channels via Direct Binding of a Cytosolic Domain to Orai1. Cell 2009, 136, 876–890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muik, M.; Fahrner, M.; Schindl, R.; Stathopulos, P.; Frischauf, I.; Derler, I.; Plenk, P.; Lackner, B.; Groschner, K.; Ikura, M.; et al. STIM1 Couples to ORAI1 via an Intramolecular Transition into an Extended Conformation. EMBO J. 2011, 30, 1678–1689. [Google Scholar] [CrossRef]
- Kawasaki, T.; Lange, I.; Feske, S. A Minimal Regulatory Domain in the C Terminus of STIM1 Binds to and Activates ORAI1 CRAC Channels. Biochem. Biophys. Res. Commun. 2009, 385, 49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soboloff, J.; Rothberg, B.S.; Madesh, M.; Gill, D.L. STIM Proteins: Dynamic Calcium Signal Transducers. Nat. Rev. Mol. Cell Biol. 2012, 13, 549–565. [Google Scholar] [CrossRef] [Green Version]
- Fahrner, M.; Muik, M.; Schindl, R.; Butorac, C.; Stathopulos, P.; Zheng, L.; Jardin, I.; Ikura, M.; Romanin, C. A Coiled-Coil Clamp Controls Both Conformation and Clustering of Stromal Interaction Molecule 1 (STIM1). J. Biol. Chem. 2014, 289, 33231–33244. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Srinivasan, P.; Razavi, S.; Seymour, S.; Meraner, P.; Gudlur, A.; Stathopulos, P.B.; Ikura, M.; Rao, A.; Hogan, P.G. Initial Activation of STIM1, the Regulator of Store-Operated Calcium Entry. Nat. Struct. Mol. Biol. 2013, 20, 973–981. [Google Scholar] [CrossRef] [Green Version]
- Liou, J.; Fivaz, M.; Inoue, T.; Meyer, T. Live-Cell Imaging Reveals Sequential Oligomerization and Local Plasma Membrane Targeting of Stromal Interaction Molecule 1 after Ca2+ Store Depletion. Proc. Natl. Acad. Sci. USA 2007, 104, 9301–9306. [Google Scholar] [CrossRef] [Green Version]
- Zheng, S.; Zhou, L.; Ma, G.; Zhang, T.; Liu, J.; Li, J.; Nguyen, N.T.; Zhang, X.; Li, W.; Nwokonko, R.; et al. Calcium Store Refilling and STIM Activation in STIM- and Orai-Deficient Cell Lines. Pflug. Arch. 2018, 470, 1555–1567. [Google Scholar] [CrossRef] [Green Version]
- Wu, M.M.; Covington, E.D.; Lewis, R.S. Single-Molecule Analysis of Diffusion and Trapping of STIM1 and Orai1 at Endoplasmic Reticulum–Plasma Membrane Junctions. Mol. Biol. Cell 2014, 25, 3672–3685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, R.T.; Manji, S.S.M.; Parker, N.J.; Hancock, M.S.; van Stekelenburg, L.; Eid, J.-P.; Senior, P.V.; Kazenwadel, J.S.; Shandala, T.; Saint, R.; et al. Identification and Characterization of the STIM (Stromal Interaction Molecule) Gene Family: Coding for a Novel Class of Transmembrane Proteins. Biochem. J. 2001, 357, 673–685. [Google Scholar] [CrossRef]
- Lopez, E.; Jardin, I.; Berna-Erro, A.; Bermejo, N.; Salido, G.M.; Sage, S.O.; Rosado, J.A.; Redondo, P.C. STIM1 Tyrosine-Phosphorylation Is Required for STIM1-Orai1 Association in Human Platelets. Cell. Signal. 2012, 24, 1315–1322. [Google Scholar] [CrossRef]
- Lopez, E.; Frischauf, I.; Jardin, I.; Derler, I.; Muik, M.; Cantonero, C.; Salido, G.M.; Smani, T.; Rosado, J.A.; Redondo, P.C. STIM1 Phosphorylation at Y316 Modulates Its Interaction with SARAF and the Activation of SOCE and ICRAC. J. Cell Sci. 2019, 132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, R.T.; Senior, P.V.; Van Stekelenburg, L.; Layton, J.E.; Smith, P.J.; Dziadek, M.A. Stromal Interaction Molecule 1 (STIM1), a Transmembrane Protein with Growth Suppressor Activity, Contains an Extracellular SAM Domain Modified by N-Linked Glycosylation. Biochim. Et Biophys. Acta (BBA)-Protein Struct. Mol. Enzymol. 2002, 1596, 131–137. [Google Scholar] [CrossRef]
- Darbellay, B.; Arnaudeau, S.; Bader, C.R.; Konig, S.; Bernheim, L. STIM1L Is a New Actin-Binding Splice Variant Involved in Fast Repetitive Ca2+ Release. J. Cell Biol. 2011, 194, 335–346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horinouchi, T.; Higashi, T.; Higa, T.; Terada, K.; Mai, Y.; Aoyagi, H.; Hatate, C.; Nepal, P.; Horiguchi, M.; Harada, T.; et al. Different Binding Property of STIM1 and Its Novel Splice Variant STIM1L to Orai1, TRPC3, and TRPC6 Channels. Biochem. Biophys. Res. Commun. 2012, 428, 252–258. [Google Scholar] [CrossRef]
- Rana, A.; Yen, M.; Sadaghiani, A.M.; Malmersjö, S.; Park, C.Y.; Dolmetsch, R.E.; Lewis, R.S. Alternative Splicing Converts STIM2 from an Activator to an Inhibitor of Store-Operated Calcium Channels. J. Cell Biol. 2015, 209, 653–669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miederer, A.-M.; Alansary, D.; Schwär, G.; Lee, P.-H.; Jung, M.; Helms, V.; Niemeyer, B.A. A STIM2 Splice Variant Negatively Regulates Store-Operated Calcium Entry. Nat. Commun. 2015, 6, 6899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brandman, O.; Liou, J.; Park, W.S.; Meyer, T. STIM2 Is a Feedback Regulator That Stabilizes Basal Cytosolic and Endoplasmic Reticulum Ca2+ Levels. Cell 2007, 131, 1327–1339. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Mancarella, S.; Wang, Y.; Yue, C.; Ritchie, M.; Gill, D.L.; Soboloff, J. The Short N-Terminal Domains of STIM1 and STIM2 Control the Activation Kinetics of Orai1 Channels. J. Biol. Chem. 2009, 284, 19164–19168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berna-Erro, A.; Jardin, I.; Salido, G.M.; Rosado, J.A. Role of STIM2 in Cell Function and Physiopathology. J. Physiol. 2017, 595, 3111–3128. [Google Scholar] [CrossRef] [Green Version]
- Cai, X.; Zhou, Y.; Nwokonko, R.M.; Loktionova, N.A.; Wang, X.; Xin, P.; Trebak, M.; Wang, Y.; Gill, D.L. The Orai1 Store-Operated Calcium Channel Functions as a Hexamer. J. Biol. Chem. 2016, 291, 25764–25775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, X.; Pedi, L.; Diver, M.M.; Long, S.B. Crystal Structure of the Calcium Release-Activated Calcium Channel Orai. Science 2012, 338, 1308–1313. [Google Scholar] [CrossRef] [Green Version]
- Rothberg, B.S.; Wang, Y.; Gill, D.L. Orai Channel Pore Properties and Gating by STIM: Implications from the Orai Crystal Structure. Sci. Signal. 2013, 6, pe9. [Google Scholar] [CrossRef] [Green Version]
- Yen, M.; Lokteva, L.A.; Lewis, R.S. Functional Analysis of Orai1 Concatemers Supports a Hexameric Stoichiometry for the CRAC Channel. Biophys. J. 2016, 111, 1897–1907. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Cai, X.; Loktionova, N.A.; Wang, X.; Nwokonko, R.M.; Wang, X.; Wang, Y.; Rothberg, B.S.; Trebak, M.; Gill, D.L. The STIM1-Binding Site Nexus Remotely Controls Orai1 Channel Gating. Nat. Commun. 2016, 7. [Google Scholar] [CrossRef] [Green Version]
- Frischauf, I.; Muik, M.; Derler, I.; Bergsmann, J.; Fahrner, M.; Schindl, R.; Groschner, K.; Romanin, C. Molecular Determinants of the Coupling between STIM1 and Orai Channels: Differential activation of Orai1–3 Channels by a STIM1 coiled-coil mutant. J. Biol. Chem. 2009, 284, 21696–21706. [Google Scholar] [CrossRef] [Green Version]
- Frischauf, I.; Schindl, R.; Bergsmann, J.; Derler, I.; Fahrner, M.; Muik, M.; Fritsch, R.; Lackner, B.; Groschner, K.; Romanin, C. Cooperativeness of Orai Cytosolic Domains Tunes Subtype-Specific Gating. J. Biol. Chem. 2011, 286, 8577–8584. [Google Scholar] [CrossRef] [Green Version]
- Gudlur, A.; Quintana, A.; Zhou, Y.; Hirve, N.; Mahapatra, S.; Hogan, P.G. STIM1 Triggers a Gating Rearrangement at the Extracellular Mouth of the ORAI1 Channel. Nat. Commun. 2014, 5, 5164. [Google Scholar] [CrossRef] [Green Version]
- Niu, L.; Wu, F.; Li, K.; Li, J.; Zhang, S.L.; Hu, J.; Wang, Q. STIM1 Interacts with Termini of Orai Channels in a Sequential Manner. J. Cell Sci. 2020, 133. [Google Scholar] [CrossRef]
- Peckys, D.B.; Gaa, D.; Alansary, D.; Niemeyer, B.A.; de Jonge, N. Supra-Molecular Assemblies of ORAI1 at Rest Precede Local Accumulation into Puncta after Activation. Int. J. Mol. Sci. 2021, 22, 799. [Google Scholar] [CrossRef] [PubMed]
- McNally, B.A.; Somasundaram, A.; Jairaman, A.; Yamashita, M.; Prakriya, M. The C- and N-Terminal STIM1 Binding Sites on Orai1 Are Required for Both Trapping and Gating CRAC Channels. J. Physiol. 2013, 591, 2833–2850. [Google Scholar] [CrossRef]
- Palty, R.; Stanley, C.; Isacoff, E.Y. Critical Role for Orai1 C-Terminal Domain and TM4 in CRAC Channel Gating. Cell Res. 2015, 25, 963–980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, H.; Zhou, M.-H.; Hu, C.; Kuo, E.; Peng, X.; Hu, J.; Kuo, L.; Zhang, S.L. Differential Roles of the C and N Termini of Orai1 Protein in Interacting with Stromal Interaction Molecule 1 (STIM1) for Ca2+ Release-Activated Ca2+ (CRAC) Channel Activation*. J. Biol. Chem. 2013, 288, 11263–11272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baraniak, J.H.; Zhou, Y.; Nwokonko, R.M.; Gill, D.L. The Intricate Coupling between STIM Proteins and Orai Channels. Curr. Opin. Physiol. 2020, 17, 106–114. [Google Scholar] [CrossRef]
- Hou, X.; Outhwaite, I.R.; Pedi, L.; Long, S.B. Cryo-EM Structure of the Calcium Release-Activated Calcium Channel Orai in an Open Conformation. eLife 2020, 9. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wu, G.; Yu, Y.; Chen, X.; Ji, R.; Lu, J.; Li, X.; Zhang, X.; Yang, X.; Shen, Y. Molecular Understanding of Calcium Permeation through the Open Orai Channel. PLoS Biol. 2019, 17, e3000096. [Google Scholar] [CrossRef] [PubMed]
- Tiffner, A.; Schober, R.; Hoeglinger, C.; Bonhenry, D.; Pandey, S.; Lunz, V.; Sallinger, M.; Frischauf, I.; Fahrner, M.; Lindinger, S.; et al. CRAC Channel Opening Is Determined by a Series of Orai1 Gating Checkpoints in the Transmembrane and Cytosolic Regions. J. Biol. Chem. 2020. [Google Scholar] [CrossRef]
- Pedersen, S.F.; Owsianik, G.; Nilius, B. TRP Channels: An Overview. Cell Calcium 2005, 38, 233–252. [Google Scholar] [CrossRef] [PubMed]
- Freichel, M.; Vennekens, R.; Olausson, J.; Stolz, S.; Philipp, S.E.; Weißgerber, P.; Flockerzi, V. Functional Role of TRPC Proteins in Native Systems: Implications from Knockout and Knock-down Studies. J. Physiol. 2005, 567, 59–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, J.; Zeng, W.; Huang, G.; Worley, P.; Muallem, S. STIM1 Heteromultimerizes TRPC Channels to Determine Their Function as Store-Operated Channels. Nat. Cell Biol. 2007, 9, 636–645. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Erxleben, C.; Abramowitz, J.; Flockerzi, V.; Zhu, M.X.; Armstrong, D.L.; Birnbaumer, L. Functional Interactions among Orai1, TRPCs, and STIM1 Suggest a STIM-Regulated Heteromeric Orai/TRPC Model for SOCE/Icrac Channels. Proc. Natl. Acad. Sci. USA 2008, 105, 2895–2900. [Google Scholar] [CrossRef] [Green Version]
- Liao, Y.; Erxleben, C.; Yildirim, E.; Abramowitz, J.; Armstrong, D.L.; Birnbaumer, L. Orai Proteins Interact with TRPC Channels and Confer Responsiveness to Store Depletion. Proc. Natl. Acad. Sci. USA 2007, 104, 4682–4687. [Google Scholar] [CrossRef] [Green Version]
- Liao, Y.; Plummer, N.W.; George, M.D.; Abramowitz, J.; Zhu, M.X.; Birnbaumer, L. A Role for Orai in TRPC-Mediated Ca2+ Entry Suggests That a TRPC:Orai Complex May Mediate Store and Receptor Operated Ca2+ Entry. Proc. Natl. Acad. Sci. USA 2009, 106, 3202–3206. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.S.; Zeng, W.; Yuan, J.P.; Shin, D.M.; Worley, P.F.; Muallem, S. Native Store-Operated Ca2+ Influx Requires the Channel Function of Orai1 and TRPC1. J. Biol. Chem. 2009, 284, 9733–9741. [Google Scholar] [CrossRef] [Green Version]
- Cheng, K.T.; Liu, X.; Ong, H.L.; Swaim, W.; Ambudkar, I.S. Local Ca2+ Entry via Orai1 Regulates Plasma Membrane Recruitment of TRPC1 and Controls Cytosolic Ca2+ Signals Required for Specific Cell Functions. PLoS Biol. 2011, 9, e1001025. [Google Scholar] [CrossRef] [Green Version]
- Ong, H.L.; Cheng, K.T.; Liu, X.; Bandyopadhyay, B.C.; Paria, B.C.; Soboloff, J.; Pani, B.; Gwack, Y.; Srikanth, S.; Singh, B.B.; et al. Dynamic Assembly of TRPC1-STIM1-Orai1 Ternary Complex Is Involved in Store-Operated Calcium Influx. J. Biol. Chem. 2007, 282, 9105–9116. [Google Scholar] [CrossRef] [Green Version]
- Zeng, W.; Yuan, J.P.; Kim, M.S.; Choi, Y.J.; Huang, G.N.; Worley, P.F.; Muallem, S. STIM1 Gates TRPC Channels, but Not Orai1, by Electrostatic Interaction. Mol. Cell 2008, 32, 439–448. [Google Scholar] [CrossRef] [Green Version]
- Albarran, L.; Lopez, J.J.; Jardin, I.; Sanchez-Collado, J.; Berna-Erro, A.; Smani, T.; Camello, P.J.; Salido, G.M.; Rosado, J.A. EFHB Is a Novel Cytosolic Ca2+ Sensor That Modulates STIM1-SARAF Interaction. Cell Physiol. Biochem. 2018, 51, 1164–1178. [Google Scholar] [CrossRef]
- Palty, R.; Raveh, A.; Kaminsky, I.; Meller, R.; Reuveny, E. SARAF Inactivates the Store Operated Calcium Entry Machinery to Prevent Excess Calcium Refilling. Cell 2012, 149, 425–438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albarran, L.; Regodón, S.; Salido, G.M.; Lopez, J.J.; Rosado, J.A. Role of STIM1 in the Surface Expression of SARAF. Channels 2016, 11, 84–88. [Google Scholar] [CrossRef]
- Jha, A.; Ahuja, M.; Maléth, J.; Moreno, C.M.; Yuan, J.P.; Kim, M.S.; Muallem, S. The STIM1 CTID Domain Determines Access of SARAF to SOAR to Regulate Orai1 Channel Function. J. Cell Biol. 2013, 202, 71–79. [Google Scholar] [CrossRef] [Green Version]
- Albarrán, L.; López, J.J.; Gómez, L.J.; Salido, G.M.; Rosado, J.A. SARAF Modulates TRPC1, but Not TRPC6, Channel Function in a STIM1-Independent Manner. Biochem. J. 2016, 473, 3581–3595. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wu, G.; Yang, Y.; Fu, S.; Liu, X.; Kang, H.; Yang, X.; Su, X.-C.; Shen, Y. Calmodulin Dissociates the STIM1-Orai1 Complex and STIM1 Oligomers. Nat. Commun. 2017, 8. [Google Scholar] [CrossRef]
- Albarran, L.; Lopez, J.J.; Amor, N.B.; Martin-Cano, F.E.; Berna-Erro, A.; Smani, T.; Salido, G.M.; Rosado, J.A. Dynamic Interaction of SARAF with STIM1 and Orai1 to Modulate Store-Operated Calcium Entry. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef]
- Bruce, J.I.E.; Straub, S.V.; Yule, D.I. Crosstalk between CAMP and Ca2+ Signaling in Non-Excitable Cells. Cell Calcium 2003, 34, 431–444. [Google Scholar] [CrossRef]
- Lee, K.P.; Yuan, J.P.; Hong, J.H.; So, I.; Worley, P.F.; Muallem, S. An Endoplasmic Reticulum/Plasma Membrane Junction: STIM1/Orai1/TRPCs. FEBS Lett. 2010, 584, 2022–2027. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, B.X.; Zhao, H.; Loessberg, P.; Muallem, S. Activation of the Plasma Membrane Ca2+ Pump during Agonist Stimulation of Pancreatic Acini. J. Biol. Chem. 1992, 267, 15419–15425. [Google Scholar] [CrossRef]
- Boyajian, C.L.; Garritsen, A.; Cooper, D.M. Bradykinin Stimulates Ca2+ Mobilization in NCB-20 Cells Leading to Direct Inhibition of Adenylylcyclase. A Novel Mechanism for Inhibition of CAMP Production. J. Biol. Chem. 1991, 266, 4995–5003. [Google Scholar] [CrossRef]
- Fagan, K.A.; Mahey, R.; Cooper, D.M. Functional Co-Localization of Transfected Ca(2+)-Stimulable Adenylyl Cyclases with Capacitative Ca2+ Entry Sites. J. Biol. Chem. 1996, 271, 12438–12444. [Google Scholar] [CrossRef] [Green Version]
- Halls, M.L.; Cooper, D.M.F. Regulation by Ca2+-Signaling Pathways of Adenylyl Cyclases. Cold Spring Harb. Perspect. Biol. 2011, 3, a004143. [Google Scholar] [CrossRef] [PubMed]
- Masada, N.; Ciruela, A.; MacDougall, D.A.; Cooper, D.M.F. Distinct Mechanisms of Regulation by Ca2+/Calmodulin of Type 1 and 8 Adenylyl Cyclases Support Their Different Physiological Roles. J. Biol. Chem. 2009, 284, 4451–4463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shuttleworth, T.J.; Thompson, J.L. Discriminating between Capacitative and Arachidonate-Activated Ca(2+) Entry Pathways in HEK293 Cells. J. Biol. Chem. 1999, 274, 31174–31178. [Google Scholar] [CrossRef] [Green Version]
- Watson, E.L.; Wu, Z.; Jacobson, K.L.; Storm, D.R.; Singh, J.C.; Ott, S.M. Capacitative Ca2+ Entry Is Involved in CAMP Synthesis in Mouse Parotid Acini. Am. J. Physiol. Cell Physiol. 1998, 274, C557–C565. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.C.L.; Cooper, D.M.F. Capacitative and 1-Oleyl-2-Acetyl-Sn-Glycerol-Activated Ca(2+) Entry Distinguished Using Adenylyl Cyclase Type 8. Mol. Pharm. 2006, 70, 769–777. [Google Scholar] [CrossRef] [Green Version]
- Willoughby, D.; Everett, K.L.; Halls, M.L.; Pacheco, J.; Skroblin, P.; Vaca, L.; Klussmann, E.; Cooper, D.M.F. Direct Binding Between Orai1 and AC8 Mediates Dynamic Interplay Between Ca2+ and CAMP Signaling. Sci. Signal. 2012, 5, ra29. [Google Scholar] [CrossRef]
- Willoughby, D.; Ong, H.L.; De Souza, L.B.; Wachten, S.; Ambudkar, I.S.; Cooper, D.M.F. TRPC1 Contributes to the Ca2+ -Dependent Regulation of Adenylate Cyclases. Biochem. J. 2014, 464, 73–84. [Google Scholar] [CrossRef] [Green Version]
- Michel, J.J.C.; Scott, J.D. AKAP Mediated Signal Transduction. Annu. Rev. Pharmacol. Toxicol. 2002, 42, 235–257. [Google Scholar] [CrossRef] [Green Version]
- Torres-Quesada, O.; Mayrhofer, J.E.; Stefan, E. The Many Faces of Compartmentalized PKA Signalosomes. Cell. Signal. 2017, 37, 1–11. [Google Scholar] [CrossRef]
- Johnstone, T.B.; Agarwal, S.R.; Harvey, R.D.; Ostrom, R.S. CAMP Signaling Compartmentation: Adenylyl Cyclases as Anchors of Dynamic Signaling Complexes. Mol. Pharm. 2018, 93, 270–276. [Google Scholar] [CrossRef] [Green Version]
- Müller, M.R.; Rao, A. NFAT, Immunity and Cancer: A Transcription Factor Comes of Age. Nat. Rev. Immunol. 2010, 10, 645–656. [Google Scholar] [CrossRef]
- Wu, H.; Peisley, A.; Graef, I.A.; Crabtree, G.R. NFAT Signaling and the Invention of Vertebrates. Trends Cell Biol. 2007, 17, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Kar, P.; Samanta, K.; Kramer, H.; Morris, O.; Bakowski, D.; Parekh, A.B. Dynamic Assembly of a Membrane Signaling Complex Enables Selective Activation of NFAT by Orai1. Curr. Biol. 2014, 24, 1361–1368. [Google Scholar] [CrossRef] [Green Version]
- Son, G.-Y.; Subedi, K.P.; Ong, H.L.; Noyer, L.; Saadi, H.; Zheng, C.; Bhardwaj, R.; Feske, S.; Ambudkar, I.S. STIM2 Targets Orai1/STIM1 to the AKAP79 Signaling Complex and Confers Coupling of Ca2+ Entry with NFAT1 Activation. Proc. Natl. Acad. Sci. USA 2020, 117, 16638–16648. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Zhao, A.Z.; Chan, G.C.; Baker, L.P.; Impey, S.; Beavo, J.A.; Storm, D.R. Phosphorylation and Inhibition of Olfactory Adenylyl Cyclase by CaM Kinase II in Neurons: A Mechanism for Attenuation of Olfactory Signals. Neuron 1998, 21, 495–504. [Google Scholar] [CrossRef] [Green Version]
- Motiani, R.K.; Tanwar, J.; Raja, D.A.; Vashisht, A.; Khanna, S.; Sharma, S.; Srivastava, S.; Sivasubbu, S.; Natarajan, V.T.; Gokhale, R.S. STIM1 Activation of Adenylyl Cyclase 6 Connects Ca2+ and CAMP Signaling during Melanogenesis. EMBO J. 2018, 37. [Google Scholar] [CrossRef]
- Maus, M.; Cuk, M.; Patel, B.; Lian, J.; Ouimet, M.; Kaufmann, U.; Yang, J.; Horvath, R.; Hornig-Do, H.-T.; Chrzanowska-Lightowlers, Z.; et al. Store-Operated Ca2+ Entry (SOCE) Controls Induction of Lipolysis and the Transcriptional Reprogramming to Lipid Metabolism. Cell Metab. 2017, 25, 698–712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lefkimmiatis, K.; Srikanthan, M.; Maiellaro, I.; Moyer, M.P.; Curci, S.; Hofer, A.M. Store-Operated Cyclic AMP Signalling Mediated by STIM1. Nat. Cell Biol. 2009, 11, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Hofer, A.M. Interactions between Calcium and CAMP Signaling. Curr. Med. Chem. 2012, 19, 5768–5773. [Google Scholar] [CrossRef] [PubMed]
- Spirli, C.; Locatelli, L.; Fiorotto, R.; Morell, C.M.; Fabris, L.; Pozzan, T.; Strazzabosco, M. Altered Store Operated Calcium Entry Increases Cyclic 3’,5’-Adenosine Monophosphate Production and Extracellular Signal-Regulated Kinases 1 and 2 Phosphorylation in Polycystin-2-Defective Cholangiocytes. Hepatology 2012, 55, 856–868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, G.; Tepikin, A.V.; Tengholm, A.; Gylfe, E. CAMP Induces Stromal Interaction Molecule 1 (STIM1) Puncta but Neither Orai1 Protein Clustering nor Store-Operated Ca2+ Entry (SOCE) in Islet Cells. J. Biol. Chem. 2012, 287, 9862–9872. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Alvarez, G.; Lu, B.; Yap, K.A.F.; Wong, L.C.; Thevathasan, J.V.; Lim, L.; Ji, F.; Tan, K.W.; Mancuso, J.J.; Tang, W.; et al. STIM2 Regulates PKA-Dependent Phosphorylation and Trafficking of AMPARs. Mol. Biol. Cell 2015, 26, 1141–1159. [Google Scholar] [CrossRef]
- Bender, A.T.; Beavo, J.A. Cyclic Nucleotide Phosphodiesterases: Molecular Regulation to Clinical Use. Pharm. Rev. 2006, 58, 488–520. [Google Scholar] [CrossRef] [PubMed]
- Jeon, K.-I.; Jono, H.; Miller, C.L.; Cai, Y.; Lim, S.; Liu, X.; Gao, P.; Abe, J.-I.; Li, J.-D.; Yan, C. Ca2+/Calmodulin-Stimulated PDE1 Regulates the Beta-Catenin/TCF Signaling through PP2A B56 Gamma Subunit in Proliferating Vascular Smooth Muscle Cells. FEBS J. 2010, 277, 5026–5039. [Google Scholar] [CrossRef]
- Kincaid, R.L.; Stith-Coleman, I.E.; Vaughan, M. Proteolytic Activation of Calmodulin-Dependent Cyclic Nucleotide Phosphodiesterase. J. Biol. Chem. 1985, 260, 9009–9015. [Google Scholar] [CrossRef]
- Sonnenburg, W.K.; Seger, D.; Kwak, K.S.; Huang, J.; Charbonneau, H.; Beavo, J.A. Identification of Inhibitory and Calmodulin-Binding Domains of the PDE1A1 and PDE1A2 Calmodulin-Stimulated Cyclic Nucleotide Phosphodiesterases. J. Biol. Chem. 1995, 270, 30989–31000. [Google Scholar] [CrossRef] [Green Version]
- Goraya, T.A.; Masada, N.; Ciruela, A.; Willoughby, D.; Clynes, M.A.; Cooper, D.M.F. Kinetic Properties of Ca2+/Calmodulin-Dependent Phosphodiesterase Isoforms Dictate Intracellular CAMP Dynamics in Response to Elevation of Cytosolic Ca2+. Cell. Signal. 2008, 20, 359–374. [Google Scholar] [CrossRef]
- Wagner, L.E.; Joseph, S.K.; Yule, D.I. Regulation of Single Inositol 1,4,5-Trisphosphate Receptor Channel Activity by Protein Kinase A Phosphorylation. J. Physiol. 2008, 586, 3577–3596. [Google Scholar] [CrossRef]
- Wagner, L.E.; Li, W.-H.; Yule, D.I. Phosphorylation of Type-1 Inositol 1,4,5-Trisphosphate Receptors by Cyclic Nucleotide-Dependent Protein Kinases: A Mutational Analysis of the Functionally Important Sites in the S2+ and S2- Splice Variants. J. Biol. Chem. 2003, 278, 45811–45817. [Google Scholar] [CrossRef] [Green Version]
- Wagner, L.E.; Li, W.-H.; Joseph, S.K.; Yule, D.I. Functional Consequences of Phosphomimetic Mutations at Key CAMP-Dependent Protein Kinase Phosphorylation Sites in the Type 1 Inositol 1,4,5-Trisphosphate Receptor. J. Biol. Chem. 2004, 279, 46242–46252. [Google Scholar] [CrossRef] [Green Version]
- Betzenhauser, M.J.; Fike, J.L.; Wagner, L.E.; Yule, D.I. Protein Kinase a Increases Type-2 Inositol 1,4,5-Trisphosphate Receptor Activity by Phosphorylation of Serine 937. J. Biol. Chem. 2009, 284, 25116–25125. [Google Scholar] [CrossRef] [Green Version]
- Park, S.; Shcheynikov, N.; Hong, J.H.; Zheng, C.; Suh, S.H.; Kawaai, K.; Ando, H.; Mizutani, A.; Abe, T.; Kiyonari, H.; et al. Irbit Mediates Synergy Between Ca2+ and CAMP Signaling Pathways During Epithelial Transport in Mice. Gastroenterology 2013, 145, 232–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Pathak, T.; Yoast, R.; Emrich, S.; Xin, P.; Nwokonko, R.M.; Johnson, M.; Wu, S.; Delierneux, C.; Gueguinou, M.; et al. A Calcium/CAMP Signaling Loop at the ORAI1 Mouth Drives Channel Inactivation to Shape NFAT Induction. Nat. Commun. 2019, 10. [Google Scholar] [CrossRef]
- Thompson, J.L.; Shuttleworth, T.J. Anchoring Protein AKAP79-Mediated PKA Phosphorylation of STIM1 Determines Selective Activation of the ARC Channel, a Store-Independent Orai Channel. J. Physiol. 2015, 593, 559–572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thompson, J.L.; Zhao, Y.; Stathopulos, P.B.; Grossfield, A.; Shuttleworth, T.J. Phosphorylation-Mediated Structural Changes within the SOAR Domain of Stromal Interaction Molecule 1 Enable Specific Activation of Distinct Orai Channels. J. Biol. Chem. 2018, 293, 3145–3155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cerra, M.C.; Imbrogno, S. Phospholamban and Cardiac Function: A Comparative Perspective in Vertebrates. Acta Physiol. 2012, 205, 9–25. [Google Scholar] [CrossRef]
- Bruce, J.I.E.; Yule, D.I.; Shuttleworth, T.J. Ca2+-Dependent Protein Kinase--a Modulation of the Plasma Membrane Ca2+-ATPase in Parotid Acinar Cells. J. Biol. Chem. 2002, 277, 48172–48181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stefan, C.J. Endoplasmic Reticulum–Plasma Membrane Contacts: Principals of Phosphoinositide and Calcium Signaling. Curr. Opin. Cell Biol. 2020, 63, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Vance, J.E. Inter-Organelle Membrane Contact Sites: Implications for Lipid Metabolism. Biol. Direct 2020, 15. [Google Scholar] [CrossRef] [PubMed]
- Scorrano, L.; De Matteis, M.A.; Emr, S.; Giordano, F.; Hajnóczky, G.; Kornmann, B.; Lackner, L.L.; Levine, T.P.; Pellegrini, L.; Reinisch, K.; et al. Coming Together to Define Membrane Contact Sites. Nat. Commun. 2019, 10. [Google Scholar] [CrossRef]
- Toulmay, A.; Prinz, W.A. A Conserved Membrane-Binding Domain Targets Proteins to Organelle Contact Sites. J. Cell Sci. 2012, 125, 49–58. [Google Scholar] [CrossRef] [Green Version]
- Min, S.-W.; Chang, W.-P.; Südhof, T.C. E-Syts, a Family of Membranous Ca2+-Sensor Proteins with Multiple C2 Domains. Proc. Natl. Acad. Sci. USA 2007, 104, 3823–3828. [Google Scholar] [CrossRef] [Green Version]
- Giordano, F.; Saheki, Y.; Idevall-Hagren, O.; Colombo, S.F.; Pirruccello, M.; Milosevic, I.; Gracheva, E.O.; Bagriantsev, S.N.; Borgese, N.; De Camilli, P. PI(4,5)P2-Dependent and Ca2+-Regulated ER-PM Interactions Mediated by the Extended Synaptotagmins. Cell 2013, 153, 1494–1509. [Google Scholar] [CrossRef] [Green Version]
- Schauder, C.M.; Wu, X.; Saheki, Y.; Narayanaswamy, P.; Torta, F.; Wenk, M.R.; De Camilli, P.; Reinisch, K.M. Structure of a Lipid-Bound Extended-Synaptotagmin Indicates a Role in Lipid Transfer. Nature 2014, 510, 552–555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, C.-L.; Hsieh, T.-S.; Yang, T.T.; Rothberg, K.G.; Azizoglu, D.B.; Volk, E.; Liao, J.-C.; Liou, J. Feedback Regulation of Receptor-Induced Ca2+ Signaling Mediated by E-Syt1 and Nir2 at Endoplasmic Reticulum-Plasma Membrane Junctions. Cell Rep. 2013, 5, 813–825. [Google Scholar] [CrossRef] [Green Version]
- Idevall-Hagren, O.; Lü, A.; Xie, B.; De Camilli, P. Triggered Ca2+ Influx Is Required for Extended Synaptotagmin 1-Induced ER-Plasma Membrane Tethering. EMBO J. 2015, 34, 2291–2305. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Busnadiego, R.; Saheki, Y.; De Camilli, P. Three-Dimensional Architecture of Extended Synaptotagmin-Mediated Endoplasmic Reticulum–Plasma Membrane Contact Sites. Proc. Natl. Acad. Sci. USA 2015, 112, E2004–E2013. [Google Scholar] [CrossRef] [Green Version]
- Saheki, Y.; Bian, X.; Schauder, C.M.; Sawaki, Y.; Surma, M.A.; Klose, C.; Pincet, F.; Reinisch, K.M.; De Camilli, P. Control of Plasma Membrane Lipid Homeostasis by the Extended Synaptotagmins. Nat. Cell Biol. 2016, 18, 504–515. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, T.-S.; Chen, Y.-J.; Chang, C.-L.; Lee, W.-R.; Liou, J. Cortical Actin Contributes to Spatial Organization of ER–PM Junctions. Mol. Biol. Cell 2017, 28, 3171–3180. [Google Scholar] [CrossRef] [Green Version]
- Mochizuki, S.; Miki, H.; Zhou, R.; Kido, Y.; Nishimura, W.; Kikuchi, M.; Noda, Y. Oxysterol-Binding Protein-Related Protein (ORP) 6 Localizes to the ER and ER-Plasma Membrane Contact Sites and Is Involved in the Turnover of PI4P in Cerebellar Granule Neurons. Exp. Cell Res. 2018, 370, 601–612. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.; Torta, F.; Masai, K.; Lucast, L.; Czapla, H.; Tanner, L.B.; Narayanaswamy, P.; Wenk, M.R.; Nakatsu, F.; De Camilli, P. Intracellular transport. PI4P/Phosphatidylserine Countertransport at ORP5- and ORP8-Mediated ER-Plasma Membrane contacts. Science 2015, 349, 428–432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moser von Filseck, J.; Čopič, A.; Delfosse, V.; Vanni, S.; Jackson, C.L.; Bourguet, W.; Drin, G. Intracellular transport. Phosphatidylserine Transport by ORP/Osh Proteins Is Driven by Phosphatidylinositol 4-Phosphate. Science 2015, 349, 432–436. [Google Scholar] [CrossRef] [PubMed]
- Moser von Filseck, J.M.; Vanni, S.; Mesmin, B.; Antonny, B.; Drin, G. A Phosphatidylinositol-4-Phosphate Powered Exchange Mechanism to Create a Lipid Gradient between Membranes. Nat. Commun. 2015, 6, 6671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schulz, T.A.; Choi, M.-G.; Raychaudhuri, S.; Mears, J.A.; Ghirlando, R.; Hinshaw, J.E.; Prinz, W.A. Lipid-Regulated Sterol Transfer between Closely Apposed Membranes by Oxysterol-Binding Protein Homologues. J. Cell Biol. 2009, 187, 889–903. [Google Scholar] [CrossRef] [Green Version]
- Tahirovic, S.; Schorr, M.; Mayinger, P. Regulation of Intracellular Phosphatidylinositol-4-Phosphate by the Sac1 Lipid Phosphatase. Traffic 2005, 6, 116–130. [Google Scholar] [CrossRef]
- Naito, T.; Ercan, B.; Krshnan, L.; Triebl, A.; Koh, D.H.Z.; Wei, F.-Y.; Tomizawa, K.; Torta, F.T.; Wenk, M.R.; Saheki, Y. Movement of Accessible Plasma Membrane Cholesterol by the GRAMD1 Lipid Transfer Protein Complex. eLife 2019, 8, e51401. [Google Scholar] [CrossRef]
- Nishimura, T.; Gecht, M.; Covino, R.; Hummer, G.; Surma, M.A.; Klose, C.; Arai, H.; Kono, N.; Stefan, C.J. Osh Proteins Control Nanoscale Lipid Organization Necessary for PI(4,5)P2 Synthesis. Mol. Cell 2019, 75, 1043–1057.e8. [Google Scholar] [CrossRef] [Green Version]
- Sharma, S.; Quintana, A.; Findlay, G.M.; Mettlen, M.; Baust, B.; Jain, M.; Nilsson, R.; Rao, A.; Hogan, P.G. An SiRNA Screen for NFAT Activation Identifies Septins as Coordinators of Store-Operated Ca2+ Entry. Nature 2013, 499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deb, B.K.; Hasan, G. SEPT7-Mediated Regulation of Ca2+ Entry through Orai Channels Requires Other Septin Subunits. Cytoskeleton 2019, 76, 104–114. [Google Scholar] [CrossRef] [Green Version]
- Deb, B.K.; Pathak, T.; Hasan, G. Store-Independent Modulation of Ca2+ Entry through Orai by Septin 7. Nat. Commun. 2016, 7. [Google Scholar] [CrossRef] [Green Version]
- Katz, Z.B.; Zhang, C.; Quintana, A.; Lillemeier, B.F.; Hogan, P.G. Septins Organize Endoplasmic Reticulum-Plasma Membrane Junctions for STIM1-ORAI1 Calcium Signalling. Sci. Rep. 2019, 9. [Google Scholar] [CrossRef] [PubMed]
- Deb, B.K.; Chakraborty, P.; Gopurappilly, R.; Hasan, G. SEPT7 Regulates Ca2+ Entry through Orai Channels in Human Neural Progenitor Cells and Neurons. Cell Calcium 2020, 90, 102252. [Google Scholar] [CrossRef] [PubMed]
- Maass, K.; Fischer, M.A.; Seiler, M.; Temmerman, K.; Nickel, W.; Seedorf, M. A Signal Comprising a Basic Cluster and an Amphipathic α-Helix Interacts with Lipids and Is Required for the Transport of Ist2 to the Yeast Cortical ER. J. Cell Sci. 2009, 122, 625–635. [Google Scholar] [CrossRef] [Green Version]
- Whitlock, J.M.; Hartzell, H.C. Anoctamins/TMEM16 Proteins: Chloride Channels Flirting with Lipids and Extracellular Vesicles. Annu. Rev. Physiol. 2017, 79, 119–143. [Google Scholar] [CrossRef] [Green Version]
- Dang, S.; Feng, S.; Tien, J.; Peters, C.J.; Bulkley, D.; Lolicato, M.; Zhao, J.; Zuberbühler, K.; Ye, W.; Qi, L.; et al. Cryo-EM Structures of the TMEM16A Calcium-Activated Chloride Channel. Nature 2017, 552, 426–429. [Google Scholar] [CrossRef]
- Paulino, C.; Kalienkova, V.; Lam, A.K.M.; Neldner, Y.; Dutzler, R. Activation Mechanism of the Calcium-Activated Chloride Channel TMEM16A Revealed by Cryo-EM. Nature 2017, 552, 421–425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, W.C.; Xiao, S.; Huang, F.; Harfe, B.D.; Jan, Y.N.; Jan, L.Y. Calcium-Activated Chloride Channels (CaCCs) Regulate Action Potential and Synaptic Response in Hippocampal Neurons. Neuron 2012, 74, 179–192. [Google Scholar] [CrossRef] [Green Version]
- Stöhr, H.; Heisig, J.B.; Benz, P.M.; Schöberl, S.; Milenkovic, V.M.; Strauss, O.; Aartsen, W.M.; Wijnholds, J.; Weber, B.H.F.; Schulz, H.L. TMEM16B, a Novel Protein with Calcium-Dependent Chloride Channel Activity, Associates with a Presynaptic Protein Complex in Photoreceptor Terminals. J. Neurosci. 2009, 29, 6809–6818. [Google Scholar] [CrossRef]
- Suzuki, J.; Umeda, M.; Sims, P.J.; Nagata, S. Calcium-Dependent Phospholipid Scrambling by TMEM16F. Nature 2010, 468, 834–838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brunner, J.D.; Lim, N.K.; Schenck, S.; Duerst, A.; Dutzler, R. X-Ray Structure of a Calcium-Activated TMEM16 Lipid Scramblase. Nature 2014, 516, 207–212. [Google Scholar] [CrossRef]
- Alvadia, C.; Lim, N.K.; Clerico Mosina, V.; Oostergetel, G.T.; Dutzler, R.; Paulino, C. Cryo-EM Structures and Functional Characterization of the Murine Lipid Scramblase TMEM16F. eLife 2019, 8. [Google Scholar] [CrossRef] [PubMed]
- Jha, A.; Chung, W.Y.; Vachel, L.; Maleth, J.; Lake, S.; Zhang, G.; Ahuja, M.; Muallem, S. Anoctamin 8 Tethers Endoplasmic Reticulum and Plasma Membrane for Assembly of Ca2+ Signaling Complexes at the ER/PM Compartment. EMBO J. 2019, 38. [Google Scholar] [CrossRef] [PubMed]
- Akhmanova, A.; Steinmetz, M.O. Microtubule +TIPs at a Glance. J. Cell Sci. 2010, 123, 3415–3419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asanov, A.; Sherry, R.; Sampieri, A.; Vaca, L. A Relay Mechanism between EB1 and APC Facilitate STIM1 Puncta Assembly at Endoplasmic Reticulum–Plasma Membrane Junctions. Cell Calcium 2013, 54, 246–256. [Google Scholar] [CrossRef]
- Grigoriev, I.; Gouveia, S.M.; van der Vaart, B.; Demmers, J.; Smyth, J.T.; Honnappa, S.; Splinter, D.; Steinmetz, M.O.; Putney, J.W.; Hoogenraad, C.C.; et al. STIM1 Is a Microtubule plus End Tracking Protein Involved in Remodeling of the Endoplasmic Reticulum. Curr. Biol. 2008, 18, 177–182. [Google Scholar] [CrossRef] [Green Version]
- Honnappa, S.; Gouveia, S.M.; Weisbrich, A.; Damberger, F.F.; Bhavesh, N.S.; Jawhari, H.; Grigoriev, I.; van Rijssel, F.J.A.; Buey, R.M.; Lawera, A.; et al. An EB1-Binding Motif Acts as a Microtubule Tip Localization Signal. Cell 2009, 138, 366–376. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.-L.; Chen, Y.-J.; Quintanilla, C.G.; Hsieh, T.-S.; Liou, J. EB1 Binding Restricts STIM1 Translocation to ER–PM Junctions and Regulates Store-Operated Ca2+ Entry. J. Cell Biol. 2018, 217, 2047–2058. [Google Scholar] [CrossRef] [Green Version]
- Balla, T.; Kim, Y.J.; Alvarez-Prats, A.; Pemberton, J. Lipid Dynamics at Contact Sites between the Endoplasmic Reticulum and Other Organelles. Annu. Rev. Cell Dev. Biol. 2019, 35, 85–109. [Google Scholar] [CrossRef]
- Dell’Acqua, M.L.; Faux, M.C.; Thorburn, J.; Thorburn, A.; Scott, J.D. Membrane-Targeting Sequences on AKAP79 Bind Phosphatidylinositol-4, 5-Bisphosphate. EMBO J. 1998, 17, 2246–2260. [Google Scholar] [CrossRef] [Green Version]
- Blunsom, N.J.; Cockcroft, S. CDP-Diacylglycerol Synthases (CDS): Gateway to Phosphatidylinositol and Cardiolipin Synthesis. Front. Cell Dev. Biol. 2020, 8, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Q.; Gabelli, S.B.; Raben, D.M. Diacylglycerol Kinases: Relationship to Other Lipid Kinases. Adv. Biol. Regul. 2019, 71, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Collado, J.; Kalemanov, M.; Campelo, F.; Bourgoint, C.; Thomas, F.; Loewith, R.; Martínez-Sánchez, A.; Baumeister, W.; Stefan, C.J.; Fernández-Busnadiego, R. Tricalbin-Mediated Contact Sites Control ER Curvature to Maintain Plasma Membrane Integrity. Dev. Cell 2019, 51, 476–487.e7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Besprozvannaya, M.; Dickson, E.; Li, H.; Ginburg, K.S.; Bers, D.M.; Auwerx, J.; Nunnari, J. GRAM Domain Proteins Specialize Functionally Distinct ER-PM Contact Sites in Human Cells. eLife 2018, 7. [Google Scholar] [CrossRef] [PubMed]
- Sandhu, J.; Li, S.; Fairall, L.; Pfisterer, S.G.; Gurnett, J.E.; Xiao, X.; Weston, T.A.; Vashi, D.; Ferrari, A.; Orozco, J.L.; et al. Aster Proteins Facilitate Nonvesicular Plasma Membrane to ER Cholesterol Transport in Mammalian Cells. Cell 2018, 175, 514–529.e20. [Google Scholar] [CrossRef] [Green Version]
- Johnson, B.; Leek, A.N.; Solé, L.; Maverick, E.E.; Levine, T.P.; Tamkun, M.M. Kv2 Potassium Channels Form Endoplasmic Reticulum/Plasma Membrane Junctions via Interaction with VAPA and VAPB. Proc. Natl. Acad. Sci. USA 2018, 115, E7331–E7340. [Google Scholar] [CrossRef] [Green Version]
- Johnson, B.; Leek, A.N.; Tamkun, M.M. Kv2 Channels Create Endoplasmic Reticulum/Plasma Membrane Junctions: A Brief History of Kv2 Channel Subcellular Localization. Channels 2019, 13, 88–101. [Google Scholar] [CrossRef] [Green Version]
- Amarilio, R.; Ramachandran, S.; Sabanay, H.; Lev, S. Differential Regulation of Endoplasmic Reticulum Structure through VAP-Nir Protein Interaction. J. Biol. Chem. 2005, 280, 5934–5944. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.J.; Guzman-Hernandez, M.-L.; Wisniewski, E.; Balla, T. Phosphatidylinositol-Phosphatidic Acid Exchange by Nir2 at ER-PM Contact Sites Maintains Phosphoinositide Signaling Competence. Dev. Cell 2015, 33, 549–561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.; Kedan, A.; Marom, M.; Gavert, N.; Keinan, O.; Selitrennik, M.; Laufman, O.; Lev, S. The Phosphatidylinositol-Transfer Protein Nir2 Binds Phosphatidic Acid and Positively Regulates Phosphoinositide Signalling. EMBO Rep. 2013, 14, 891–899. [Google Scholar] [CrossRef] [PubMed]
- Vig, M.; Peinelt, C.; Beck, A.; Koomoa, D.L.; Rabah, D.; Koblan-Huberson, M.; Kraft, S.; Turner, H.; Fleig, A.; Penner, R.; et al. CRACM1 Is a Plasma Membrane Protein Essential for Store-Operated Ca2+ Entry. Science 2006, 312, 1220–1223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peinelt, C.; Vig, M.; Koomoa, D.L.; Beck, A.; Nadler, M.J.S.; Koblan-Huberson, M.; Lis, A.; Fleig, A.; Penner, R.; Kinet, J.-P. Amplification of CRAC Current by STIM1 and CRACM1 (Orai1). Nat. Cell Biol. 2006, 8, 771–773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soboloff, J.; Spassova, M.A.; Tang, X.D.; Hewavitharana, T.; Xu, W.; Gill, D.L. Orai1 and STIM Reconstitute Store-Operated Calcium Channel Function*. J. Biol. Chem. 2006, 281, 20661–20665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mercer, J.C.; DeHaven, W.I.; Smyth, J.T.; Wedel, B.; Boyles, R.R.; Bird, G.S.; Putney, J.W. Large store-operated calcium-selective currents due to co-expression of orai1 or orai2 with the intracellular calcium sensor, STIM1. J. Biol. Chem. 2006, 281, 24979–24990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wyles, J.P.; McMaster, C.R.; Ridgway, N.D. Vesicle-Associated Membrane Protein-Associated Protein-A (VAP-A) Interacts with the Oxysterol-Binding Protein to Modify Export from the Endoplasmic Reticulum. J. Biol. Chem. 2002, 277, 29908–29918. [Google Scholar] [CrossRef] [Green Version]
- Antonny, B.; Bigay, J.; Mesmin, B. The Oxysterol-Binding Protein Cycle: Burning off PI(4)P to Transport Cholesterol. Annu. Rev. Biochem. 2018, 87, 809–837. [Google Scholar] [CrossRef]
- Lehto, M.; Hynynen, R.; Karjalainen, K.; Kuismanen, E.; Hyvärinen, K.; Olkkonen, V.M. Targeting of OSBP-Related Protein 3 (ORP3) to Endoplasmic Reticulum and Plasma Membrane Is Controlled by Multiple Determinants. Exp. Cell Res. 2005, 310, 445–462. [Google Scholar] [CrossRef] [PubMed]
- Gulyás, G.; Sohn, M.; Kim, Y.J.; Várnai, P.; Balla, T. ORP3 Phosphorylation Regulates Phosphatidylinositol 4-Phosphate and Ca2+ Dynamics at Plasma Membrane–ER Contact Sites. J. Cell Sci. 2020, 133. [Google Scholar] [CrossRef]
- Du, X.; Kumar, J.; Ferguson, C.; Schulz, T.A.; Ong, Y.S.; Hong, W.; Prinz, W.A.; Parton, R.G.; Brown, A.J.; Yang, H. A Role for Oxysterol-Binding Protein-Related Protein 5 in Endosomal Cholesterol Trafficking. J. Cell Biol. 2011, 192, 121–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, D.; Mäyränpää, M.I.; Wong, J.; Perttilä, J.; Lehto, M.; Jauhiainen, M.; Kovanen, P.T.; Ehnholm, C.; Brown, A.J.; Olkkonen, V.M. OSBP-Related Protein 8 (ORP8) Suppresses ABCA1 Expression and Cholesterol Efflux from Macrophages. J. Biol. Chem. 2008, 283, 332–340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maeda, K.; Anand, K.; Chiapparino, A.; Kumar, A.; Poletto, M.; Kaksonen, M.; Gavin, A.-C. Interactome Map Uncovers Phosphatidylserine Transport by Oxysterol-Binding Proteins. Nature 2013, 501, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Ghai, R.; Du, X.; Wang, H.; Dong, J.; Ferguson, C.; Brown, A.J.; Parton, R.G.; Wu, J.-W.; Yang, H. ORP5 and ORP8 Bind Phosphatidylinositol-4, 5-Biphosphate (PtdIns(4,5)P2) and Regulate Its Level at the Plasma Membrane. Nat. Commun. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Sohn, M.; Korzeniowski, M.; Zewe, J.P.; Wills, R.C.; Hammond, G.R.V.; Humpolickova, J.; Vrzal, L.; Chalupska, D.; Veverka, V.; Fairn, G.D.; et al. PI(4,5)P2 Controls Plasma Membrane PI4P and PS Levels via ORP5/8 Recruitment to ER-PM Contact Sites. J. Cell Biol. 2018, 217, 1797–1813. [Google Scholar] [CrossRef]
- Heilmeyer, L.M.G.; Vereb, G.; Vereb, G.; Kakuk, A.; Szivák, I. Mammalian Phosphatidylinositol 4-Kinases. IUBMB Life 2003, 55, 59–65. [Google Scholar] [CrossRef]
- Kanaho, Y.; Kobayashi-Nakano, A.; Yokozeki, T. The Phosphoinositide Kinase PIP5K That Produces the Versatile Signaling Phospholipid PI4,5P(2). Biol. Pharm. Bull. 2007, 30, 1605–1609. [Google Scholar] [CrossRef] [Green Version]
- Blunsom, N.J.; Cockcroft, S. Phosphatidylinositol Synthesis at the Endoplasmic Reticulum. Biochim. Et Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2020, 1865, 158471. [Google Scholar] [CrossRef]
- Lefkimmiatis, K.; Zaccolo, M. CAMP Signaling in Subcellular Compartments. Pharmacol. Ther. 2014, 143, 295–304. [Google Scholar] [CrossRef] [Green Version]
- Kadamur, G.; Ross, E.M. Mammalian Phospholipase C. Annu. Rev. Physiol. 2013, 75, 127–154. [Google Scholar] [CrossRef] [Green Version]
- Del Bel, L.M.; Brill, J.A. Sac1, a Lipid Phosphatase at the Interface of Vesicular and Nonvesicular Transport. Traffic 2018, 19, 301–318. [Google Scholar] [CrossRef]
- Maléth, J.; Choi, S.; Muallem, S.; Ahuja, M. Translocation between PI(4,5)P2-Poor and PI(4,5)P2-Rich Microdomains during Store Depletion Determines STIM1 Conformation and Orai1 Gating. Nat. Commun. 2014, 5, 5843. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Kong, C.; Xie, H.; McPherson, P.S.; Grinstein, S.; Trimble, W.S. Phosphatidylinositol Polyphosphate Binding to the Mammalian Septin H5 Is Modulated by GTP. Curr. Biol. 1999, 9, 1458–1467. [Google Scholar] [CrossRef] [Green Version]
- Vandecaetsbeek, I.; Vangheluwe, P.; Raeymaekers, L.; Wuytack, F.; Vanoevelen, J. The Ca2+ Pumps of the Endoplasmic Reticulum and Golgi Apparatus. Cold Spring Harb. Perspect. Biol. 2011, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verboomen, H.; Mertens, L.; Eggermont, J.; Wuytack, F.; Van Den Bosch, L. Modulation of SERCA2 Activity: Regulated Splicing and Interaction with Phospholamban. Biosci. Rep. 1995, 15, 307. [Google Scholar] [CrossRef]
- Liou, J.; Kim, M.L.; Do Heo, W.; Jones, J.T.; Myers, J.W.; Ferrell, J.E.; Meyer, T. STIM Is a Ca2+ Sensor Essential for Ca2+-Store-Depletion-Triggered Ca2+ Influx. Curr. Biol. 2005, 15, 1235–1241. [Google Scholar] [CrossRef] [Green Version]
- Roos, J.; DiGregorio, P.J.; Yeromin, A.V.; Ohlsen, K.; Lioudyno, M.; Zhang, S.; Safrina, O.; Kozak, J.A.; Wagner, S.L.; Cahalan, M.D.; et al. STIM1, an Essential and Conserved Component of Store-Operated Ca2+ Channel Function. J. Cell Biol. 2005, 169, 435–445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ercan, E.; Momburg, F.; Engel, U.; Temmerman, K.; Nickel, W.; Seedorf, M. A Conserved, Lipid-Mediated Sorting Mechanism of Yeast Ist2 and Mammalian STIM Proteins to the Peripheral ER. Traffic 2009, 10, 1802–1818. [Google Scholar] [CrossRef]
- Wang, Q.-C.; Wang, X.; Tang, T.-S. EB1 Traps STIM1 and Regulates Local Store-Operated Ca2+ Entry. J. Cell Biol. 2018, 217, 1899–1900. [Google Scholar] [CrossRef]
- López, J.J.; Salido, G.M.; Pariente, J.A.; Rosado, J.A. Interaction of STIM1 with Endogenously Expressed Human Canonical TRP1 upon Depletion of Intracellular Ca2+ Stores. J. Biol. Chem. 2006, 281, 28254–28264. [Google Scholar] [CrossRef] [Green Version]
- Lees, J.A.; Messa, M.; Sun, E.W.; Wheeler, H.; Torta, F.; Wenk, M.R.; De Camilli, P.; Reinisch, K.M. Lipid Transport by TMEM24 at ER-Plasma Membrane Contacts Regulates Pulsatile Insulin Secretion. Science 2017, 355. [Google Scholar] [CrossRef] [Green Version]
- Sun, E.W.; Guillén-Samander, A.; Bian, X.; Wu, Y.; Cai, Y.; Messa, M.; De Camilli, P. Lipid Transporter TMEM24/C2CD2L Is a Ca2+-Regulated Component of ER–Plasma Membrane Contacts in Mammalian Neurons. Proc. Natl. Acad. Sci. USA 2019, 116, 5775–5784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wes, P.D.; Chevesich, J.; Jeromin, A.; Rosenberg, C.; Stetten, G.; Montell, C. TRPC1, a Human Homolog of a Drosophila Store-Operated Channel. Proc. Natl. Acad. Sci. USA 1995, 92, 9652–9656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, X.; Chu, P.B.; Peyton, M.; Birnbaumer, L. Molecular Cloning of a Widely Expressed Human Homologue for the Drosophila Trp Gene. FEBS Lett. 1995, 373, 193–198. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.P.; Choi, S.; Hong, J.H.; Ahuja, M.; Graham, S.; Ma, R.; So, I.; Shin, D.M.; Muallem, S.; Yuan, J.P. Molecular Determinants Mediating Gating of Transient Receptor Potential Canonical (TRPC) Channels by Stromal Interaction Molecule 1 (STIM1). J. Biol. Chem. 2014, 289, 6372–6382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murphy, S.E.; Levine, T.P. VAP, a Versatile Access Point for the Endoplasmic Reticulum: Review and Analysis of FFAT-like Motifs in the VAPome. Biochim. Biophys. Acta 2016, 1861, 952–961. [Google Scholar] [CrossRef]
- Yu, H.; Liu, Y.; Gulbranson, D.R.; Paine, A.; Rathore, S.S.; Shen, J. Extended Synaptotagmins Are Ca2+-Dependent Lipid Transfer Proteins at Membrane Contact Sites. Proc. Natl. Acad. Sci. USA 2016, 113, 4362–4367. [Google Scholar] [CrossRef] [Green Version]
- Saheki, Y.; De Camilli, P. The Extended-Synaptotagmins. Biochim. Biophys. Acta 2017, 1864, 1490–1493. [Google Scholar] [CrossRef]
- Corradi, V.; Sejdiu, B.I.; Mesa-Galloso, H.; Abdizadeh, H.; Noskov, S.Y.; Marrink, S.J.; Tieleman, D.P. Emerging Diversity in Lipid–Protein Interactions. Chem. Rev. 2019, 119, 5775–5848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grouleff, J.; Irudayam, S.J.; Skeby, K.K.; Schiøtt, B. The Influence of Cholesterol on Membrane Protein Structure, Function, and Dynamics Studied by Molecular Dynamics Simulations. Biochim. Et Biophys. Acta (BBA)-Biomembr. 2015, 1848, 1783–1795. [Google Scholar] [CrossRef] [Green Version]
- Muller, M.P.; Jiang, T.; Sun, C.; Lihan, M.; Pant, S.; Mahinthichaichan, P.; Trifan, A.; Tajkhorshid, E. Characterization of Lipid-Protein Interactions and Lipid-Mediated Modulation of Membrane Protein Function Through Molecular Simulation. Chem. Rev. 2019, 119, 6086–6161. [Google Scholar] [CrossRef]
- Bieberich, E. Sphingolipids and Lipid Rafts: Novel Concepts and Methods of Analysis. Chem. Phys. Lipids 2018, 216, 114–131. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.M.; Fairn, G.D. Mesoscale Organization of Domains in the Plasma Membrane—beyond the Lipid Raft. Crit. Rev. Biochem. Mol. Biol. 2018, 53, 192–207. [Google Scholar] [CrossRef]
- Rheinstädter, M.C.; Mouritsen, O.G. Small-Scale Structure in Fluid Cholesterol–Lipid Bilayers. Curr. Opin. Colloid Interface Sci. 2013, 18, 440–447. [Google Scholar] [CrossRef]
- Jardin, I.; Salido, G.M.; Rosado, J.A. Role of Lipid Rafts in the Interaction between HTRPC1, Orai1 and STIM1. Channels 2008, 2, 401–403. [Google Scholar] [CrossRef] [Green Version]
- Galan, C.; Woodard, G.E.; Dionisio, N.; Salido, G.M.; Rosado, J.A. Lipid Rafts Modulate the Activation but Not the Maintenance of Store-Operated Ca2+ Entry. Biochim. Et Biophys. Acta (BBA)-Mol. Cell Res. 2010, 1803, 1083–1093. [Google Scholar] [CrossRef] [Green Version]
- Lockwich, T.P.; Liu, X.; Singh, B.B.; Jadlowiec, J.; Weiland, S.; Ambudkar, I.S. Assembly of Trp1 in a Signaling Complex Associated with Caveolin-Scaffolding Lipid Raft Domains. J. Biol. Chem. 2000, 275, 11934–11942. [Google Scholar] [CrossRef] [Green Version]
- Murata, T.; Lin, M.I.; Stan, R.V.; Bauer, P.M.; Yu, J.; Sessa, W.C. Genetic Evidence Supporting Caveolae Microdomain Regulation of Calcium Entry in Endothelial Cells. J. Biol. Chem. 2007, 282, 16631–16643. [Google Scholar] [CrossRef] [Green Version]
- Prakash, Y.S.; Thompson, M.A.; Vaa, B.; Matabdin, I.; Peterson, T.E.; He, T.; Pabelick, C.M. Caveolins and Intracellular Calcium Regulation in Human Airway Smooth Muscle. Am. J. Physiol. Lung Cell. Mol. Physiol. 2007, 293, L1118–L1126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pani, B.; Liu, X.; Bollimuntha, S.; Cheng, K.T.; Niesman, I.R.; Zheng, C.; Achen, V.R.; Patel, H.H.; Ambudkar, I.S.; Singh, B.B. Impairment of TRPC1–STIM1 Channel Assembly and AQP5 Translocation Compromise Agonist-Stimulated Fluid Secretion in Mice Lacking Caveolin1. J. Cell Sci. 2013, 126, 667–675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rathor, N.; Chung, H.K.; Wang, S.R.; Wang, J.; Turner, D.J.; Rao, J.N. Caveolin-1 Enhances Rapid Mucosal Restitution by Activating TRPC1-mediated Ca2+ Signaling. Physiol. Rep. 2014, 2. [Google Scholar] [CrossRef] [Green Version]
- Alicia, S.; Angélica, Z.; Carlos, S.; Alfonso, S.; Vaca, L. STIM1 Converts TRPC1 from a Receptor-Operated to a Store-Operated Channel: Moving TRPC1 in and out of Lipid Rafts. Cell Calcium 2008, 44, 479–491. [Google Scholar] [CrossRef] [PubMed]
- Pani, B.; Ong, H.L.; Liu, X.; Rauser, K.; Ambudkar, I.S.; Singh, B.B. Lipid Rafts Determine Clustering of STIM1 in Endoplasmic Reticulum-Plasma Membrane Junctions and Regulation of Store-Operated Ca2+ Entry (SOCE). J. Biol. Chem. 2008, 283, 17333–17340. [Google Scholar] [CrossRef] [Green Version]
- Pani, B.; Ong, H.L.; Brazer, S.W.; Liu, X.; Rauser, K.; Singh, B.B.; Ambudkar, I.S. Activation of TRPC1 by STIM1 in ER-PM Microdomains Involves Release of the Channel from Its Scaffold Caveolin-1. Proc. Natl. Acad. Sci. USA 2009, 106, 20087–20092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sathish, V.; Abcejo, A.J.; Thompson, M.A.; Sieck, G.C.; Prakash, Y.S.; Pabelick, C.M. Caveolin-1 Regulation of Store-Operated Ca2+ Influx in Human Airway Smooth Muscle. Eur. Respir. J. 2012, 40, 470–478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, F.; Sun, L.; Machaca, K. Constitutive Recycling of the Store-Operated Ca2+ Channel Orai1 and Its Internalization during Meiosis. J. Cell Biol. 2010, 191, 523–535. [Google Scholar] [CrossRef] [Green Version]
- Fridolfsson, H.N.; Roth, D.M.; Insel, P.A.; Patel, H.H. Regulation of Intracellular Signaling and Function by Caveolin. FASEB J. 2014, 28, 3823–3831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeh, Y.-C.; Parekh, A.B. Distinct Structural Domains of Caveolin-1 Independently Regulate Ca2+ Release-Activated Ca2+ Channels and Ca2+ Microdomain-Dependent Gene Expression. Mol. Cell Biol. 2015, 35, 1341–1349. [Google Scholar] [CrossRef] [Green Version]
- Gwozdz, T.; Dutko-Gwozdz, J.; Schafer, C.; Bolotina, V.M. Overexpression of Orai1 and STIM1 Proteins Alters Regulation of Store-Operated Ca2+ Entry by Endogenous Mediators. J. Biol. Chem. 2012, 287, 22865–22872. [Google Scholar] [CrossRef] [Green Version]
- Dionisio, N.; Galán, C.; Jardín, I.; Salido, G.M.; Rosado, J.A. Lipid Rafts Are Essential for the Regulation of SOCE by Plasma Membrane Resident STIM1 in Human Platelets. Biochim. Et Biophys. Acta (BBA)-Mol. Cell Res. 2011, 1813, 431–437. [Google Scholar] [CrossRef] [Green Version]
- Derler, I.; Jardin, I.; Stathopulos, P.B.; Muik, M.; Fahrner, M.; Zayats, V.; Pandey, S.K.; Poteser, M.; Lackner, B.; Absolonova, M.; et al. Cholesterol Modulates Orai1 Channel Function. Sci. Signal. 2016, 9, ra10. [Google Scholar] [CrossRef] [Green Version]
- Bóhorquez-Hernández, A.; Gratton, E.; Pacheco, J.; Asanov, A.; Vaca, L. Cholesterol Modulates the Cellular Localization of Orai1 Channels and Its Disposition among Membrane Domains. Biochim. Biophys. Acta 2017, 1862, 1481–1490. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, J.; Dominguez, L.; Bohórquez-Hernández, A.; Asanov, A.; Vaca, L. A Cholesterol-Binding Domain in STIM1 Modulates STIM1-Orai1 Physical and Functional Interactions. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doktorova, M.; Symons, J.; Levental, I. Structural and Functional Consequences of Reversible Lipid Asymmetry in Living Membranes. Nat. Chem. Biol. 2020, 16, 1321–1330. [Google Scholar] [CrossRef] [PubMed]
- Collins, S.R.; Meyer, T. Evolutionary Origins of STIM1 and STIM2 within Ancient Ca2+ Signaling Systems. Trends Cell Biol. 2011, 21, 202–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heo, W.D.; Inoue, T.; Park, W.S.; Kim, M.L.; Park, B.O.; Wandless, T.J.; Meyer, T. PI(3,4,5)P3 and PI(4,5)P2 Lipids Target Proteins with Polybasic Clusters to the Plasma Membrane. Science 2006, 314, 1458–1461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhardwaj, R.; Müller, H.-M.; Nickel, W.; Seedorf, M. Oligomerization and Ca2+/Calmodulin Control Binding of the ER Ca2+-Sensors STIM1 and STIM2 to Plasma Membrane Lipids. Biosci. Rep. 2013, 33. [Google Scholar] [CrossRef]
- Korzeniowski, M.K.; Popovic, M.A.; Szentpetery, Z.; Varnai, P.; Stojilkovic, S.S.; Balla, T. Dependence of STIM1/Orai1-Mediated Calcium Entry on Plasma Membrane Phosphoinositides. J. Biol. Chem. 2009, 284, 21027–21035. [Google Scholar] [CrossRef] [Green Version]
- Calloway, N.; Owens, T.; Corwith, K.; Rodgers, W.; Holowka, D.; Baird, B. Stimulated Association of STIM1 and Orai1 Is Regulated by the Balance of PtdIns(4,5)P2 between Distinct Membrane Pools. J. Cell Sci. 2011, 124, 2602–2610. [Google Scholar] [CrossRef] [Green Version]
- Imai, Y.; Itsuki, K.; Okamura, Y.; Inoue, R.; Mori, M.X. A Self-Limiting Regulation of Vasoconstrictor-Activated TRPC3/C6/C7 Channels Coupled to PI(4,5)P2-Diacylglycerol Signalling. J. Physiol. 2012, 590, 1101–1119. [Google Scholar] [CrossRef] [Green Version]
- Itsuki, K.; Imai, Y.; Hase, H.; Okamura, Y.; Inoue, R.; Mori, M.X. PLC-Mediated PI(4,5)P2 Hydrolysis Regulates Activation and Inactivation of TRPC6/7 Channels. J. Gen. Physiol. 2014, 143, 183–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.; Jeon, J.-P.; Hong, C.; Kim, J.; Myeong, J.; Jeon, J.-H.; So, I. An Essential Role of PI(4,5)P2 for Maintaining the Activity of the Transient Receptor Potential Canonical (TRPC)4β. Pflug. Arch. Eur. J. Physiol. 2013, 465, 1011–1021. [Google Scholar] [CrossRef]
- Shi, J.; Birnbaumer, L.; Large, W.A.; Albert, A.P. Myristoylated Alanine-Rich C Kinase Substrate Coordinates Native TRPC1 Channel Activation by Phosphatidylinositol 4,5-Bisphosphate and Protein Kinase C in Vascular Smooth Muscle. FASEB J. 2014, 28, 244–255. [Google Scholar] [CrossRef] [Green Version]
- Bodnar, D.; Chung, W.Y.; Yang, D.; Hong, J.H.; Jha, A.; Muallem, S. STIM-TRP Pathways and Microdomain Organization: Ca2+ Influx Channels: The Orai-STIM1-TRPC Complexes. In Store-Operated Ca2+ Entry (SOCE) Pathways: Emerging Signaling Concepts in Human (Patho)physiology; Groschner, K., Graier, W.F., Romanin, C., Eds.; Advances in Experimental Medicine and Biology; Springer International Publishing: Cham, Switzerland, 2017; pp. 139–157. ISBN 978-3-319-57732-6. [Google Scholar]
- Ando, H.; Mizutani, A.; Matsu-ura, T.; Mikoshiba, K. IRBIT, a Novel Inositol 1,4,5-Trisphosphate (IP3) Receptor-Binding Protein, Is Released from the IP3 Receptor upon IP3 Binding to the Receptor. J. Biol. Chem. 2003, 278, 10602–10612. [Google Scholar] [CrossRef] [Green Version]
- Cooper, D.M.F. Store-Operated Ca2+-Entry and Adenylyl Cyclase. Cell Calcium 2015, 58, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Pagano, M.; Clynes, M.A.; Masada, N.; Ciruela, A.; Ayling, L.-J.; Wachten, S.; Cooper, D.M.F. Insights into the Residence in Lipid Rafts of Adenylyl Cyclase AC8 and Its Regulation by Capacitative Calcium Entry. Am. J. Physiol. Cell Physiol. 2009, 296, C607–C619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tabbasum, V.G.; Cooper, D.M.F. Structural and Functional Determinants of AC8 Trafficking, Targeting and Responsiveness in Lipid Raft Microdomains. J. Membr. Biol. 2019, 252, 159–172. [Google Scholar] [CrossRef] [Green Version]
- Ayling, L.J.; Briddon, S.J.; Halls, M.L.; Hammond, G.R.V.; Vaca, L.; Pacheco, J.; Hill, S.J.; Cooper, D.M.F. Adenylyl Cyclase AC8 Directly Controls Its Micro-Environment by Recruiting the Actin Cytoskeleton in a Cholesterol-Rich Milieu. J. Cell Sci. 2012, 125, 869–886. [Google Scholar] [CrossRef] [Green Version]
- Delint-Ramirez, I.; Willoughby, D.; Hammond, G.R.V.; Hammond, G.V.R.; Ayling, L.J.; Cooper, D.M.F. Palmitoylation Targets AKAP79 Protein to Lipid Rafts and Promotes Its Regulation of Calcium-Sensitive Adenylyl Cyclase Type 8. J. Biol. Chem. 2011, 286, 32962–32975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cornelius, F.; Habeck, M.; Kanai, R.; Toyoshima, C.; Karlish, S.J.D. General and Specific Lipid–Protein Interactions in Na,K-ATPase. Biochim. Et Biophys. Acta (BBA)-Biomembr. 2015, 1848, 1729–1743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Denning, E.J.; Beckstein, O. Influence of Lipids on Protein-Mediated Transmembrane Transport. Chem. Phys. Lipids 2013, 169, 57–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Locke, D.; Harris, A.L. Connexin Channels and Phospholipids: Association and Modulation. BMC Biol. 2009, 7, 52. [Google Scholar] [CrossRef] [Green Version]
- Shuttleworth, T.J. Selective Activation of Distinct Orai Channels by STIM1. Cell Calcium 2017, 63, 40–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hille, B.; Dickson, E.J.; Kruse, M.; Vivas, O.; Suh, B.-C. Phosphoinositides Regulate Ion Channels. Biochim. Biophys. Acta 2015, 1851, 844–856. [Google Scholar] [CrossRef] [PubMed]
- Aharonovitz, O.; Zaun, H.C.; Balla, T.; York, J.D.; Orlowski, J.; Grinstein, S. Intracellular Ph Regulation by Na+/H+ Exchange Requires Phosphatidylinositol 4,5-Bisphosphate. J. Cell Biol. 2000, 150, 213–224. [Google Scholar] [CrossRef] [Green Version]
- Hong, J.H.; Yang, D.; Shcheynikov, N.; Ohana, E.; Shin, D.M.; Muallem, S. Convergence of IRBIT, Phosphatidylinositol (4,5) Bisphosphate, and WNK/SPAK Kinases in Regulation of the Na+-HCO3− Cotransporters Family. Proc. Natl. Acad. Sci. USA 2013, 110, 4105–4110. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; McNicholas, C.M.; Bevensee, M.O. Phosphatidylinositol 4,5-Bisphosphate (PIP2) Stimulates the Electrogenic Na/HCO3 Cotransporter NBCe1-A Expressed in Xenopus Oocytes. Proc. Natl. Acad. Sci. USA 2009, 106, 14150–14155. [Google Scholar] [CrossRef] [Green Version]
- Lopreiato, R.; Giacomello, M.; Carafoli, E. The Plasma Membrane Calcium Pump: New Ways to Look at an Old Enzyme. J. Biol. Chem. 2014, 289, 10261–10268. [Google Scholar] [CrossRef] [Green Version]
- Hansen, S.B.; Tao, X.; MacKinnon, R. Structural Basis of PIP2 Activation of the Classical Inward Rectifier K+ Channel Kir2.2. Nature 2011, 477, 495–498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whorton, M.R.; MacKinnon, R. Crystal Structure of the Mammalian GIRK2 K+ Channel and Gating Regulation by G Proteins, PIP2, and Sodium. Cell 2011, 147, 199–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, K.C.; Sanders, C.R. Regulation of KCNQ/Kv7 Family Voltage-Gated K+ Channels by Lipids. Biochim. Biophys. Acta 2017, 1859, 586–597. [Google Scholar] [CrossRef]
- Katona, M.; Vizvári, E.; Németh, L.; Facskó, A.; Venglovecz, V.; Rakonczay, Z.; Hegyi, P.; Tóth-Molnár, E. Experimental Evidence of Fluid Secretion of Rabbit Lacrimal Gland Duct Epithelium. Investig. Ophthalmol. Vis. Sci. 2014, 55, 4360–4367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vizvári, E.; Katona, M.; Orvos, P.; Berczeli, O.; Facskó, A.; Rárosi, F.; Venglovecz, V.; Rakonczay, Z.; Hegyi, P.; Ding, C.; et al. Characterization of Na+-K+-2Cl- Cotransporter Activity in Rabbit Lacrimal Gland Duct Cells. Investig. Ophthalmol. Vis. Sci. 2016, 57, 3828–3835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berczeli, O.; Vizvári, E.; Katona, M.; Török, D.; Szalay, L.; Rárosi, F.; Németh, I.; Rakonczay, Z.; Hegyi, P.; Ding, C.; et al. Novel Insight into the Role of CFTR in Lacrimal Gland Duct Function in Mice. Investig. Ophthalmol. Vis. Sci. 2018, 59, 54–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szarka, D.; Elekes, G.; Berczeli, O.; Vizvári, E.; Szalay, L.; Ding, C.; Tálosi, L.; Tóth-Molnár, E. Alpha-Adrenergic Agonists Stimulate Fluid Secretion in Lacrimal Gland Ducts. Investig. Ophthalmol. Vis. Sci. 2020, 61, 3. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.G.; Ohana, E.; Park, H.W.; Yang, D.; Muallem, S. Molecular Mechanism of Pancreatic and Salivary Gland Fluid and HCO3 Secretion. Physiol. Rev. 2012, 92, 39–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hegyi, P.; Maléth, J.; Venglovecz, V.; Rakonczay, Z. Pancreatic Ductal Bicarbonate Secretion: Challenge of the Acinar Acid Load. Front. Physiol. 2011, 2, 36. [Google Scholar] [CrossRef] [Green Version]



| Proteome | Localization | Trigger | Interaction | Function |
|---|---|---|---|---|
| AKAP79 | PM (PI(4,5)P2) [172] | PKA [101] | Regulates positioning of STIM1/STIM2/Orai1/PKA [107] | |
| ANO8 | PI(4,5)P2 [165] | Mediates Orai1 inactivation through Recruitment of ER-localized SERCA [165] | ||
| CDS | ER [173] | PA→CDP-DAG [173] | ||
| DGK | ER [174] | DAG→PA [174] | ||
| E-Syt1 |
| Ca2+ [136] |
| |
|
| |||
| F-actin | Stabilizes Nir2-containing ER-PM MCS [142] | |||
| GRAMD2 | PM (PI(4,5)P2) [176] |
| ||
| ||||
| IP3R | ER [2] | IP3 [2] | Outward-rectifying Ca2+ channel [2] | |
| Kv2 | ER (VAP) [178] | Phosphorylation [178] | VAP [178] | Delayed rectifier potassium channel [179] |
| Nir2 |
| VAP [180] |
| |
|
| |||
| Orai1 | PM [183] | STIM1 [184,185,186] | PM-localized inward-rectifying Ca2+ channel [183] | |
| ORP | ER (VAP) [187] | VAP [187] |
| |
| ||||
| ORP3 |
|
| VAP [189] |
|
|
|
| ||
| ||||
| ORP5/8 | ||||
| ||||
| ORP6 | ER (VAP) [143] | VAP [143] | PI4P→PI transfer (PM→ER) [143] | |
| PI4K | PM [196] | PI→PI4P [196] | ||
| PIP5K | PM [197] |
| PI4P→PI(4,5)P2 [197] | |
| PIS | ER [198] | CDP-DAG→PI [198] | ||
| PKA | PM (AKAP) [172] | cAMP [199] | AKAP [101] | |
| PLC | PM [200] | Ca2+ [200] | PI(4,5)P2→DAG + IP3 [200] | |
| Sac1 | ER [148] | PI4P phosphatase [201] | ||
| SARAF |
|
| Attenuates SOCE [83,202] | |
|
| |||
| ||||
| Septin4 | PM (PI(4,5)P2) [203] | Promote STIM1—Orai1 assembly formation [154] | ||
| Septin7 | PM (PI(4,5)P2) [203] | Prevents STIM1—Orai1 assembly formation [152,155] | ||
| SERCA | ER [204] |
| Inward-rectifying Ca2+ channel [204] | |
| ||||
| STIM1 |
| ER-localized Ca2+ sensor [206,207] | ||
|
|
| ||
| ||||
| TMEM24 |
| PI transfer ER→PM [211] | ||
|
| |||
| TRPC1 | PM [213,214] |
| PM-localized non-selective cation channels | |
| VAP | ER [187] |
| Anchoring protein [216] | |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crul, T.; Maléth, J. Endoplasmic Reticulum‐Plasma Membrane Contact Sites as an Organizing Principle for Compartmentalized Calcium and cAMP Signaling. Int. J. Mol. Sci. 2021, 22, 4703. https://doi.org/10.3390/ijms22094703
Crul T, Maléth J. Endoplasmic Reticulum‐Plasma Membrane Contact Sites as an Organizing Principle for Compartmentalized Calcium and cAMP Signaling. International Journal of Molecular Sciences. 2021; 22(9):4703. https://doi.org/10.3390/ijms22094703
Chicago/Turabian StyleCrul, Tim, and József Maléth. 2021. "Endoplasmic Reticulum‐Plasma Membrane Contact Sites as an Organizing Principle for Compartmentalized Calcium and cAMP Signaling" International Journal of Molecular Sciences 22, no. 9: 4703. https://doi.org/10.3390/ijms22094703
APA StyleCrul, T., & Maléth, J. (2021). Endoplasmic Reticulum‐Plasma Membrane Contact Sites as an Organizing Principle for Compartmentalized Calcium and cAMP Signaling. International Journal of Molecular Sciences, 22(9), 4703. https://doi.org/10.3390/ijms22094703