Protective Effects of a Lutein Ester Prodrug, Lutein Diglutaric Acid, against H2O2-Induced Oxidative Stress in Human Retinal Pigment Epithelial Cells
Abstract
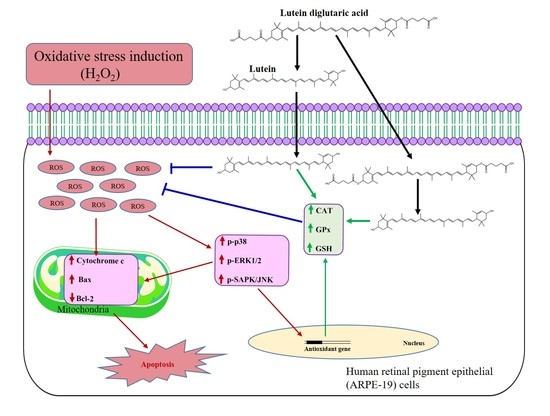
:1. Introduction
2. Results
2.1. Evaluation of Lut and Lut-DG Effect on Cell Viability of ARPE-19 Cells
2.2. Evaluation of H2O2 on Cell Viability of ARPE-19 Cells
2.3. Effect of Lut and Lut-DG against H2O2-Induced Oxidative Stress in ARPE-19 Cells
2.4. Effect of Lut and Lut-DG against H2O2-Induced Oxidative Stress via Modulation of the MAPKs Pathway
2.5. Lut and Lut-DG Inhibit Apoptosis by Modulation of Bax, Bcl-2 and Cytochrome c Expression in Oxidative Stressed ARPE-19 Cells
2.6. Lut and Lut-DG Exert Their Protective Effect against Oxidative Stress-Induced Cell Death via Modulation of Key Non-Enzymatic and Enzymatic Antioxidants in ARPE-19 Cells
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Synthesis of Lutein Diglutaric Acid (Lut-DG)
4.3. Cell Culture of ARPE-19 Cells
4.4. Cell Viability (MTT) Assay
4.5. Evaluation of Cytotoxicity of Lut and Lut-DG
4.6. Evaluation of Suitable H2O2 Concentration for Cytotoxicity Induction
4.7. Evaluation of the Protective Effect of Lut- and Lut-DG-Induced Oxidative Stress on ARPE-19 Cells
4.8. Evaluation of ROS Production
4.9. CAT, GPx and GSH Determination
4.10. Western Immunoblot Analysis
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khandhadia, S.; Cipriani, V.; Yates, J.R.; Lotery, A.J. Age-related macular degeneration and the complement system. Immunobiology 2012, 217, 127–146. [Google Scholar] [CrossRef] [PubMed]
- Ammar, M.J.; Hsu, J.; Chiang, A.; Ho, A.C.; Regillo, C.D. Age-related macular degeneration therapy: A review. Curr. Opin. Ophthalmol. 2020, 31, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Klein, R.; Cruickshanks, K.J.; Nash, S.D.; Krantz, E.M.; Nieto, F.J.; Huang, G.H.; Pankow, J.S.; Klein, B.E. The prevalence of age-related macular degeneration and associated risk factors. Arch. Ophthalmol. 2010, 128, 750–758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, W.L.; Su, X.; Li, X.; Cheung, C.M.; Klein, R.; Cheng, C.Y.; Wong, T.Y. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta-analysis. Lancet Glob. Health 2014, 2, e106–e116. [Google Scholar] [CrossRef] [Green Version]
- Nowak, J.Z. Age-related macular degeneration (AMD): Pathogenesis and therapy. Pharmacol. Rep. 2006, 58, 353–363. [Google Scholar] [PubMed]
- Salimiaghdam, N.; Riazi-Esfahani, M.; Fukuhara, P.S.; Schneider, K.; Kenney, C. Age-related macular degeneration (AMD): A review on its epidemiology and risk factors. Open Ophthalmol. J. 2019, 13, 90–99. [Google Scholar] [CrossRef]
- Ambati, J.; Fowler, B.J. Mechanisms of age-related macular degeneration. Neuron 2012, 75, 26–39. [Google Scholar] [CrossRef] [Green Version]
- Al Gwairi, O.; Thach, L.; Zheng, W.; Osman, N.; Little, P.J. Cellular and Molecular Pathology of Age-Related Macular Degeneration: Potential Role for Proteoglycans. J. Ophthalmol. 2016, 2016, 2913612. [Google Scholar] [CrossRef] [Green Version]
- Bhutto, I.; Lutty, G. Understanding age-related macular degeneration (AMD): Relationships between the photoreceptor/retinal pigment epithelium/Bruch’s membrane/choriocapillaris complex. Mol. Asp. Med. 2012, 33, 295–317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kay, P.; Yang, Y.C.; Paraoan, L. Directional protein secretion by the retinal pigment epithelium: Roles in retinal health and the development of age-related macular degeneration. J. Cell. Mol. Med. 2013, 17, 833–843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beatty, S.; Koh, H.; Phil, M.; Henson, D.; Boulton, M. The role of oxidative stress in the pathogenesis of age-related macular degeneration. Surv. Ophthalmol. 2000, 45, 115–134. [Google Scholar] [CrossRef] [Green Version]
- Bellezza, I. Oxidative Stress in Age-Related Macular Degeneration: Nrf2 as Therapeutic Target. Front. Pharmacol. 2018, 9, 1280. [Google Scholar] [CrossRef] [PubMed]
- Datta, S.; Cano, M.; Ebrahimi, K.; Wang, L.; Handa, J.T. The impact of oxidative stress and inflammation on RPE degeneration in non-neovascular AMD. Prog. Retin. Eye Res. 2017, 60, 201–218. [Google Scholar] [CrossRef] [PubMed]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef] [PubMed]
- Grune, T.; Jung, T.; Merker, K.; Davies, K.J. Decreased proteolysis caused by protein aggregates, inclusion bodies, plaques, lipofuscin, ceroid, and ‘aggresomes’ during oxidative stress, aging, and disease. Int. J. Biochem. Cell Biol. 2004, 36, 2519–2530. [Google Scholar] [CrossRef] [PubMed]
- Hecquet, C.; Lefevre, G.; Valtink, M.; Engelmann, K.; Mascarelli, F. Activation and Role of MAP Kinase-Dependent Pathways in Retinal Pigment Epithelial Cells: ERK and RPE Cell Proliferation. Investig. Ophthalmol. Vis. Sci. 2002, 43, 3091–3098. [Google Scholar]
- Garg, T.K.; Chang, J.Y. Oxidative stress causes ERK phosphorylation and cell death in cultured retinal pigment epithelium: Prevention of cell death by AG126 and 15-deoxy-delta 12, 14-PGJ2. BMC Ophthalmol. 2003, 3, 5. [Google Scholar] [CrossRef] [Green Version]
- Giansanti, V.; Rodriguez, G.E.V.; Savoldelli, M.; Gioia, R.; Forlino, A.; Mazzini, G.; Pennati, M.; Zaffaroni, N.; Scovassi, A.I.; Torriglia, A. Characterization of stress response in human retinal epithelial cells. J. Cell. Mol. Med. 2013, 17, 103–115. [Google Scholar] [CrossRef]
- Kopito, R.R. Aggresomes, inclusion bodies and protein aggregation. Trends Cell Biol. 2000, 10, 524–530. [Google Scholar] [CrossRef]
- Tokarz, P.; Kaarniranta, K.; Blasiak, J. Role of antioxidant enzymes and small molecular weight antioxidants in the pathogenesis of age-related macular degeneration (AMD). Biogerontology 2013, 14, 461–482. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.H.; Kim, D.-H.; Yang, S.G.; Kim, D.Y. Improved effect of a mitochondria-targeted antioxidant on hydrogen peroxide-induced oxidative stress in human retinal pigment epithelium cells. BMC Pharmacol. Toxicol. 2021, 22, 7. [Google Scholar] [CrossRef] [PubMed]
- Costa, V.; Moradas-Ferreira, P. Oxidative stress and signal transduction in Saccharomyces cerevisiae: Insights into ageing, apoptosis and diseases. Mol. Asp. Med. 2001, 22, 217–246. [Google Scholar] [CrossRef]
- Liles, M.R.; Newsome, D.A.; Oliver, P.D. Antioxidant enzymes in the aging human retinal pigment epithelium. Arch. Ophthalmol. 1991, 109, 1285–1288. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Dong, Y.; Liu, H.; Ren, H.; Cui, Z. Hesperetin protects against H(2)O(2)-triggered oxidative damage via upregulation of the Keap1-Nrf2/HO-1 signal pathway in ARPE-19 cells. Biomed Pharmacother 2017, 88, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Muangnoi, C.; Sharif, U.; Ratnatilaka Na Bhuket, P.; Rojsitthisak, P.; Paraoan, L. Protective Effects of Curcumin Ester Prodrug, Curcumin Diethyl Disuccinate against H2O2-Induced Oxidative Stress in Human Retinal Pigment Epithelial Cells: Potential Therapeutic Avenues for Age-Related Macular Degeneration. Int. J. Mol. Sci. 2019, 20, 3367. [Google Scholar] [CrossRef] [Green Version]
- Du, W.; An, Y.; He, X.; Zhang, D.; He, W. Protection of Kaempferol on Oxidative Stress-Induced Retinal Pigment Epithelial Cell Damage. Oxid. Med. Cell Longev. 2018, 2018, 1610751. [Google Scholar] [CrossRef]
- Oh, S.; Kim, Y.J.; Lee, E.K.; Park, S.W.; Yu, H.G. Antioxidative Effects of Ascorbic Acid and Astaxanthin on ARPE-19 Cells in an Oxidative Stress Model. Antioxidants 2020, 9, 833. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Liu, W.; Zhou, X.; Long, C.; Kuang, X.; Hu, J.; Tang, Y.; Liu, L.; He, J.; Huang, Z.; et al. Protective effect of lutein on ARPE-19 cells upon H2O2-induced G2/M arrest. Mol. Med. Rep. 2017, 16, 2069–2074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdel-Aal el, S.M.; Akhtar, H.; Zaheer, K.; Ali, R. Dietary sources of lutein and zeaxanthin carotenoids and their role in eye health. Nutrients 2013, 5, 1169–1185. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Gálvez, A.; Viera, I.; Roca, M. Carotenoids and Chlorophylls as Antioxidants. Antioxidants 2020, 9, 505. [Google Scholar] [CrossRef]
- Zhou, J.; Zhao, D.; Wang, N.; Zeng, Z.; Wang, C.; Hao, L.; Peng, X. Effects of lutein supplementation on inflammatory biomarkers and metabolic risk factors in adults with central obesity: Study protocol for a randomised controlled study. Trials 2020, 21, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nidhi, B.; Sharavana, G.; Ramaprasad, T.R.; Vallikannan, B. Lutein derived fragments exhibit higher antioxidant and anti-inflammatory properties than lutein in lipopolysaccharide induced inflammation in rats. Food Funct. 2015, 6, 450–460. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, Y.; Liu, X.; Wang, M.; Wang, P.; Yang, J.; Zhang, S. Lutein inhibits proliferation, invasion and migration of hypoxic breast cancer cells via downregulation of HES1. Int. J. Oncol. 2018, 52, 2119–2129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xavier, A.A.O.; Carvajal-Lérida, I.; Garrido-Fernández, J.; Pérez-Gálvez, A. In vitro bioaccessibility of lutein from cupcakes fortified with a water-soluble lutein esters formulation. J. Food Compos. Anal. 2018, 68, 60–64. [Google Scholar] [CrossRef]
- Ochoa Becerra, M.; Mojica Contreras, L.; Hsieh Lo, M.; Mateos Díaz, J.; Castillo Herrera, G. Lutein as a functional food ingredient: Stability and bioavailability. J. Funct. Foods 2020, 66, 103771. [Google Scholar] [CrossRef]
- Rautio, J.; Kumpulainen, H.; Heimbach, T.; Oliyai, R.; Oh, D.; Järvinen, T.; Savolainen, J. Prodrugs: Design and clinical applications. Nat. Rev. Drug Discov. 2008, 7, 255. [Google Scholar] [CrossRef] [PubMed]
- Abet, V.; Filace, F.; Recio, J.; Alvarez-Builla, J.; Burgos, C. Prodrug approach: An overview of recent cases. Eur. J. Med. Chem. 2017, 127, 810–827. [Google Scholar] [CrossRef]
- Ratnatilaka Na Bhuket, P.; El-Magboub, A.; Haworth, I.S.; Rojsitthisak, P. Enhancement of Curcumin Bioavailability Via the Prodrug Approach: Challenges and Prospects. Eur. J. Drug Metab. Pharmacokinet. 2017, 42, 341–353. [Google Scholar] [CrossRef]
- Muangnoi, C.; Ratnatilaka Na Bhuket, P.; Jithavech, P.; Supasena, W.; Paraoan, L.; Patumraj, S.; Rojsitthisak, P. Curcumin diethyl disuccinate, a prodrug of curcumin, enhances anti-proliferative effect of curcumin against HepG2 cells via apoptosis induction. Sci. Rep. 2019, 9, 11718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muangnoi, C.; Ratnatilaka Na Bhuket, P.; Jithavech, P.; Wichitnithad, W.; Srikun, O.; Nerungsi, C.; Patumraj, S.; Rojsitthisak, P. Scale-Up Synthesis and In Vivo Anti-Tumor Activity of Curcumin Diethyl Disuccinate, an Ester Prodrug of Curcumin, in HepG2-Xenograft Mice. Pharmaceutics 2019, 11, 373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phumsuay, R.; Muangnoi, C.; Dasuni Wasana, P.W.; Hasriadi; Vajragupta, O.; Rojsitthisak, P.; Towiwat, P. Molecular Insight into the Anti-Inflammatory Effects of the Curcumin Ester Prodrug Curcumin Diglutaric Acid In Vitro and In Vivo. Int. J. Mol. Sci. 2020, 21, 5700. [Google Scholar] [CrossRef] [PubMed]
- Muangnoi, C.; Jithavech, P.; Ratnatilaka Na Bhuket, P.; Supasena, W.; Wichitnithad, W.; Towiwat, P.; Niwattisaiwong, N.; Haworth, I.S.; Rojsitthisak, P. A curcumin-diglutaric acid conjugated prodrug with improved water solubility and antinociceptive properties compared to curcumin. Biosci. Biotechnol. Biochem. 2018, 82, 1301–1308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhuang, S.; Yan, Y.; Daubert, R.A.; Han, J.; Schnellmann, R.G. ERK promotes hydrogen peroxide-induced apoptosis through caspase-3 activation and inhibition of Akt in renal epithelial cells. Am. J. Physiol. Renal Physiol. 2007, 292, F440–F447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.K.; Kim, H.J.; Kwon, C.H.; Kim, J.H.; Woo, J.S.; Jung, J.S.; Kim, J.M. Role of ERK activation in cisplatin-induced apoptosis in OK renal epithelial cells. J. Appl. Toxicol. 2005, 25, 374–382. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.; Yang, R.; Zheng, Z.; Zhou, Y.; Geng, Y.; Hu, Y.; Wu, S.; Wu, W. Sulforaphane-cysteine-induced apoptosis via phosphorylated ERK1/2-mediated maspin pathway in human non-small cell lung cancer cells. Cell Death Discov. 2017, 3, 17025. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative stress and antioxidant defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef] [Green Version]
- Blasiak, J.; Barszczewska, G.; Gralewska, P.; Kaarniranta, K. Oxidative stress induces mitochondrial dysfunction and autophagy in ARPE-19 cells. Acta Ophthalmol. 2019, 97, 5425. [Google Scholar] [CrossRef]
- Barghi, N.; Hermisson, J.; Schlötterer, C. Polygenic adaptation: A unifying framework to understand positive selection. Nat. Rev. Genet. 2020, 21, 769–781. [Google Scholar] [CrossRef] [PubMed]
- Kaczara, P.; Sarna, T.; Burke, J.M. Dynamics of H2O2 availability to ARPE-19 cultures in models of oxidative stress. Free Radic. Biol. Med. 2010, 48, 1064–1070. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.R.; Yu, H.T.; Yang, Y.; Hang, L.; Yang, X.W.; Ding, S.H. Quercetin phospholipid complex significantly protects against oxidative injury in ARPE-19 cells associated with activation of Nrf2 pathway. Eur. J. Pharmacol. 2016, 770, 1–8. [Google Scholar] [CrossRef]
- Huang, S.Y.; Chang, S.F.; Chau, S.F.; Chiu, S.C. The Protective Effect of Hispidin against Hydrogen Peroxide-Induced Oxidative Stress in ARPE-19 Cells via Nrf2 Signaling Pathway. Biomolecules 2019, 9, 380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, S.; Qin, T.; Liu, Z.; Caceres, M.A.; Ronchi, C.F.; Chen, C.Y.O.; Yeum, K.-J.; Taylor, A.; Blumberg, J.B.; Liu, Y.; et al. Lutein and zeaxanthin supplementation reduces H2O2-induced oxidative damage in human lens epithelial cells. Mol. Vis. 2011, 17, 3180–3190. [Google Scholar]
- Li, S.-Y.; Lo, A.C.Y. Lutein Protects RGC-5 Cells Against Hypoxia and Oxidative Stress. Int. J. Mol. Sci. 2010, 11, 2109–2117. [Google Scholar] [CrossRef] [Green Version]
- Frede, K.; Ebert, F.; Kipp, A.P.; Schwerdtle, T.; Baldermann, S. Lutein Activates the Transcription Factor Nrf2 in Human Retinal Pigment Epithelial Cells. J. Agric. Food Chem. 2017, 65, 5944–5952. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Li, D.; Zhang, Y.; Zhang, L.; Liao, Z.; Aihemaitijiang, S.; Hou, Y.; Zhan, Z.; Xie, K.; Zhang, Z. Lutein protected the retina from light induced retinal damage by inhibiting increasing oxidative stress and inflammation. J. Funct. Foods 2020, 73, 104107. [Google Scholar] [CrossRef]
- Gombač, Z.; Osojnik Črnivec, I.G.; Skrt, M.; Istenič, K.; Knez Knafelj, A.; Pravst, I.; Poklar Ulrih, N. Stabilisation of Lutein and Lutein Esters with Polyoxyethylene Sorbitan Monooleate, Medium-Chain Triglyceride Oil and Lecithin. Foods 2021, 10, 500. [Google Scholar] [CrossRef] [PubMed]
- Subagio, A.; Wakaki, H.; Morita, N. Stability of Lutein and Its Myristate Esters. Biosci. Biotechnol. Biochem. 1999, 63, 1784–1786. [Google Scholar] [CrossRef] [PubMed]
- Sorasitthiyanukarn, F.N.; Muangnoi, C.; Rojsitthisak, P.; Rojsitthisak, P. Chitosan-alginate nanoparticles as effective oral carriers to improve the stability, bioavailability, and cytotoxicity of curcumin diethyl disuccinate. Carbohydr. Polym. 2021, 256, 117426. [Google Scholar] [CrossRef] [PubMed]
- Tong, W.G.; Ding, X.Z.; Talamonti, M.S.; Bell, R.H.; Adrian, T.E. LTB4 stimulates growth of human pancreatic cancer cells via MAPK and PI-3 kinase pathways. Biochem. Biophys. Res. Commun. 2005, 335, 949–956. [Google Scholar] [CrossRef]
- Lee, W.J.; Hsiao, M.; Chang, J.L.; Yang, S.F.; Tseng, T.H.; Cheng, C.W.; Chow, J.M.; Lin, K.H.; Lin, Y.W.; Liu, C.C.; et al. Quercetin induces mitochondrial-derived apoptosis via reactive oxygen species-mediated ERK activation in HL-60 leukemia cells and xenograft. Arch. Toxicol. 2015, 89, 1103–1117. [Google Scholar] [CrossRef]
- Wada, T.; Penninger, J.M. Mitogen-activated protein kinases in apoptosis regulation. Oncogene 2004, 23, 2838–2849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Y.; Liu, W.-Z.; Liu, T.; Feng, X.; Yang, N.; Zhou, H.-F. Signaling pathway of MAPK/ERK in cell proliferation, differentiation, migration, senescence and apoptosis. J. Recept. Signal Transduct. 2015, 35, 600–604. [Google Scholar] [CrossRef] [PubMed]
- Bhujade, A.; Gupta, G.; Talmale, S.; Das, S.K.; Patil, M.B. Induction of apoptosis in A431 skin cancer cells by Cissus quadrangularis Linn stem extract by altering Bax-Bcl-2 ratio, release of cytochrome c from mitochondria and PARP cleavage. Food Funct. 2013, 4, 338–346. [Google Scholar] [CrossRef] [PubMed]
- Yue, J.; López, J.M. Understanding MAPK Signaling Pathways in Apoptosis. Int. J. Mol. Sci. 2020, 21, 2346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fung, F.K.C.; Law, B.Y.K.; Lo, A.C.Y. Lutein Attenuates Both Apoptosis and Autophagy upon Cobalt (II) Chloride-Induced Hypoxia in Rat Műller Cells. PLoS ONE 2016, 11, e0167828. [Google Scholar] [CrossRef]
- Chucair, A.J.; Rotstein, N.P.; SanGiovanni, J.P.; During, A.; Chew, E.Y.; Politi, L.E. Lutein and Zeaxanthin Protect Photoreceptors from Apoptosis Induced by Oxidative Stress: Relation with Docosahexaenoic Acid. Investig. Ophthalmol. Vis. Sci. 2007, 48, 5168–5177. [Google Scholar] [CrossRef] [PubMed]
- Trevithick-Sutton, C.C.; Foote, C.S.; Collins, M.; Trevithick, J.R. The retinal carotenoids zeaxanthin and lutein scavenge superoxide and hydroxyl radicals: A chemiluminescence and ESR study. Mol. Vis. 2006, 12, 1127–1135. [Google Scholar]
- Kamoshita, M.; Toda, E.; Osada, H.; Narimatsu, T.; Kobayashi, S.; Tsubota, K.; Ozawa, Y. Lutein acts via multiple antioxidant pathways in the photo-stressed retina. Sci. Rep. 2016, 6, 30226. [Google Scholar] [CrossRef] [PubMed]
- Kamiya, T.; Courtney, M.; Laukkanen, M.O. Redox-Activated Signal Transduction Pathways Mediating Cellular Functions in Inflammation, Differentiation, Degeneration, Transformation, and Death. Oxid. Med. Cell Longev. 2016, 2016, 8479718. [Google Scholar] [CrossRef] [PubMed]
- Ishii, T.; Itoh, K.; Yamamoto, M. Roles of Nrf2 in Activation of Antioxidant Enzyme Genes via Antioxidant Responsive Elements. Methods Enzymol. 2002, 348, 182–190. [Google Scholar]
- Nguyen, T.; Nioi, P.; Pickett, C.B. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J. Biol. Chem. 2009, 284, 13291–13295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paraoan, L.; Ratnayaka, A.; Spiller, D.G.; Hiscott, P.; White, M.R.; Grierson, I. Unexpected intracellular localization of the AMD-associated cystatin C variant. Traffic 2004, 5, 884–895. [Google Scholar] [CrossRef] [PubMed]








| Antibodies | Dilution |
|---|---|
| Anti-Phospho-ERK1/2 (Cell Signalling, Danvers, USA) | 1:1000 |
| Anti-Phospho-p38 (Cell Signalling, Danvers, USA) | 1:1000 |
| Anti-Phospho-SAPK/JNK (Cell Signalling, Danvers, USA) | 1:1000 |
| Anti-ERK1/2 (Cell Signalling, Danvers, USA) | 1:1000 |
| Anti- p38 (Cell Signalling, Danvers, USA) | 1:1000 |
| Anti-SAPK/JNK (Cell Signalling, Danvers, USA) | 1:1000 |
| Anti-cytochrome C (Cell Signalling, Danvers, USA) | 1:1000 |
| Anti-Bax (Cell Signalling, Danvers, USA) | 1:1000 |
| Anti-Bcl-2 (Cell Signalling, Danvers, USA) | 1:1000 |
| Anti-beta actin (Sigma-Aldrich, Dorset, UK) | 1:5000 |
| Secondary horseradish peroxidase (HRP)-conjugated anti-rabbit (Cell Signalling, Hertfordshire, UK) | 1:2000 |
| Secondary horseradish peroxidase (HRP)-conjugated anti-mouse (Cell Signalling, Hertfordshire, UK) | 1:2000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muangnoi, C.; Phumsuay, R.; Jongjitphisut, N.; Waikasikorn, P.; Sangsawat, M.; Rashatasakhon, P.; Paraoan, L.; Rojsitthisak, P. Protective Effects of a Lutein Ester Prodrug, Lutein Diglutaric Acid, against H2O2-Induced Oxidative Stress in Human Retinal Pigment Epithelial Cells. Int. J. Mol. Sci. 2021, 22, 4722. https://doi.org/10.3390/ijms22094722
Muangnoi C, Phumsuay R, Jongjitphisut N, Waikasikorn P, Sangsawat M, Rashatasakhon P, Paraoan L, Rojsitthisak P. Protective Effects of a Lutein Ester Prodrug, Lutein Diglutaric Acid, against H2O2-Induced Oxidative Stress in Human Retinal Pigment Epithelial Cells. International Journal of Molecular Sciences. 2021; 22(9):4722. https://doi.org/10.3390/ijms22094722
Chicago/Turabian StyleMuangnoi, Chawanphat, Rianthong Phumsuay, Nattapong Jongjitphisut, Pasin Waikasikorn, Monsin Sangsawat, Paitoon Rashatasakhon, Luminita Paraoan, and Pornchai Rojsitthisak. 2021. "Protective Effects of a Lutein Ester Prodrug, Lutein Diglutaric Acid, against H2O2-Induced Oxidative Stress in Human Retinal Pigment Epithelial Cells" International Journal of Molecular Sciences 22, no. 9: 4722. https://doi.org/10.3390/ijms22094722
APA StyleMuangnoi, C., Phumsuay, R., Jongjitphisut, N., Waikasikorn, P., Sangsawat, M., Rashatasakhon, P., Paraoan, L., & Rojsitthisak, P. (2021). Protective Effects of a Lutein Ester Prodrug, Lutein Diglutaric Acid, against H2O2-Induced Oxidative Stress in Human Retinal Pigment Epithelial Cells. International Journal of Molecular Sciences, 22(9), 4722. https://doi.org/10.3390/ijms22094722