Selected Monocyclic Monoterpenes and Their Derivatives as Effective Anticancer Therapeutic Agents
Abstract
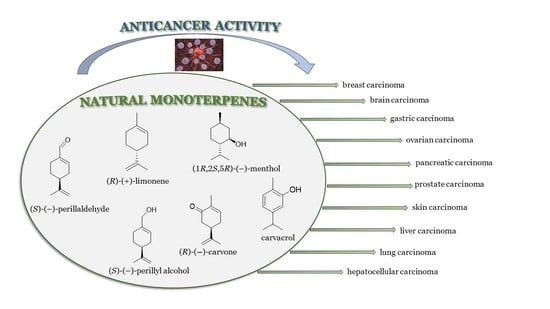
:1. Introduction
2. Carvone and Its Derivatives
3. Limonene and Its Derivatives
4. Perillyl Alcohol and Its Derivatives
5. Perillaldehyde and Its Derivatives
6. Carvacrol and Its Derivatives
7. Menthol and Its Derivatives
| No. | Base Unit: Type of the Monocyclic Monoterpenes | Name of Compound Structure | Cancer type Type of Human Tumor Cell Lines | Anticancer Activity Indexes IC50; EC50; GI; Concentration and Effect on Apoptotic Cell % Cell Viability % | Reference |
|---|---|---|---|---|---|
| 1/2 | carvone | (R)-(−)-carvone/(S)-(+)-carvone | cervical carcinoma (HeLa) | CC50 = 74.5 ± 13.1 μg/mL | [20] |
| 1/2 | carvone | (R)-(−)-carvone/(S)-(+)-carvone | Vero (healthy green monkey kidney cells) | CC50 > 200 μg/mL | [20] |
| 1/2 | carvone | (R)-(−)-carvone/(S)-(+)-carvone | murine mastocytoma (P815) | IC50 = 0.16 μM | [21] |
| 1/2 | carvone | (R)-(−)-carvone/(S)-(+)-carvone | acute lymphoblastic leukemia (CEM) | IC50 = 0.11 μM | [21] |
| 1/2 | carvone | (R)-(−)-carvone/(S)-(+)-carvone | myelogenous leukemia (K-562) | IC50 = 0.17 μM | [21] |
| 1/2 | carvone | (R)-(−)-carvone/(S)-(+)-carvone | breast (MCF-7) | IC50 = 0.63 μM | [21] |
| 1/2 | carvone | (R)-(−)-carvone/(S)-(+)-carvone | breast adenocarcinoma resistant to gemcitabine (MCF-7/gem) | IC50 = 0.91 μM | [21] |
| 1/2 | carvone | (R)-(−)-carvone/(S)-(+)-carvone | myeloma (KMS-5) | IC50 = 20 μM | [27] |
| 1 | (−)-carvone | (R)-(−)-carvone | colon (HT29) | IC50 = 325 μg/mL (MTT) IC50 = 169.5 μg/mL (NR) | [23] |
| 1 | (−)-carvone | (R)-(−)-carvone | CCD 841 CoTr (human healthy colon epithelial cells) | IC50 = 475 μg/mL (MTT) IC50 = 141.3 μg/mL (NR) | [23] |
| 1 | (−)-carvone | (R)-(−)-carvone | ovarian (OVCAR-8) | GI = 2.28 ± 1.38% at 25 μg/mL | [24] |
| 1 | (−)-carvone | (R)-(−)-carvone | colon (HCT-116) | GI = 11.94 ± 2.54% at 25 μg/mL | [24] |
| 1 | (−)-carvone | (R)-(−)-carvone | brain (SF-295) | GI = 12.28 ± 1.13 at 25 μg/mL | [24] |
| 2 | (+)-carvone |
(S)-(+)-carvone | colon (HT29) | IC50 nd (MTT) IC50 = 106.3 μg/mL (NR) | [23] |
| 2 | (+)-carvone | (S)-(+)-carvone | CCD 841 CoTr (human healthy colon epithelial cells) | IC50 = 310 μg/mL (MTT) IC50 = 111.2 μg/mL (NR) | [23] |
| 2 | (+)-carvone | (S)-(+)-carvone | ovarian (OVCAR-8) | GI = 48.07 ± 1.20% at 25 μg/mL | [24] |
| 2 | (+)-carvone | (S)-(+)-carvone | colon (HCT-116) | GI = 46.15 ± 2.46% at 25 μg/mL | [24] |
| 2 | (+)-carvone | (S)-(+)-carvone | brain (SF-295) | GI = 34.39 ± 3.47% at 25 μg/mL | [24] |
| 3a | (−)-carvone |
benzoic acid 2-(4-methyl-5-oxocyclohex-3- enyl)allyl ester  | prostate (LNCaP) | GI50 > 100 μM | [29] |
| 3b | (−)-carvone |
4-methylbenzoic acid 2-(4-methyl-5-oxocyclo- hex-3-enyl)allyl ester  | prostate (LNCaP) | GI50 = 57 μM | [29] |
| 3c | (−)-carvone |
4-fluorobenzoic acid 2-(4-methyl-5-oxocyclo- hex-3-enyl)allyl ester  | prostate (LNCaP) | GI50 >100 μM | [29] |
| 3d | (−)-carvone |
4-chlorobenzoic acid 2-(4-methyl-5-oxocyclo- hex-3-enyl)allyl ester  | prostate (LNCaP) | GI50 = 92 μM | [29] |
| 3e | (−)-carvone |
4-bromobenzoic acid 2-(4-methyl-5-oxocyclo- hex-3-enyl)allyl ester  | prostate (LNCaP) | GI50 = 80 μM | [29] |
| 3f | (−)-carvone |
4-methoxybenzoic acid 2-(4-methyl-5-oxocy- clohex-3-enyl)allyl ester  | prostate (LNCaP) | GI50 = 21 μM | [29] |
| 3g | (−)-carvone |
4-aminobenzoic acid 2-(4-methyl-5-oxocyclo- hex-3-enyl)allyl ester  | prostate (LNCaP) | GI50 = 45 μM | [29] |
| 4a | (−)-carvone |
2-methyl-5-{[1-(4-methylpiperazin-1-yl)meth- yl]vinyl}cyclohex-2-enone  | prostate (LNCaP) | GI50 > 100 μM | [29] |
| 4b | (−)-carvone |
5-[1-(4-ethylpiperazin-1-yl)methyl]vinyl-2- methylcyclohex-2-enone  | prostate (LNCaP) | GI50 > 100 μM | [29] |
| 4c | (−)-carvone |
5-[1-(4-isopropylpiperazin-1-yl)methyl]vinyl-2-methylcyclohex-2-enone | prostate (LNCaP) | GI50 > 100 μM | [29] |
| 4d | (−)-carvone |
5-[1-(4-isobutylpiperazin-1-yl)methyl]vinyl-2- methylcyclohex-2-enone  | prostate (LNCaP) | GI50 > 100 μM | [29] |
| 4e | (−)-carvone |
5-[1-(4-benzylpiperazin-1-yl)methyl]vinyl-2- methylcyclohex-2-enone  | prostate (LNCaP) | GI50 > 100 μM | [29] |
| 4f | (−)-carvone |
5-{1-[4-(4-methoxyphenyl)piperazin-1-yl]- methyl}vinyl-2-methylcyclohex-2-enone  | prostate (LNCaP) | GI50 = 45 μM | [29] |
| 4g | (−)-carvone |
5-{1-[4-(2-methoxyphenyl)piperazin-1-yl]- methyl}vinyl-2-methylcyclohex-2-enon  | prostate (LNCaP) | GI50 = 37 μM | [29] |
| 4h | (−)-carvone |
5-{1-[4-(2-chlorophenyl)piperazin-1-yl]meth- yl}vinyl-2-methylcyclohex-2-enone  | prostate (LNCaP) | GI50 = 19 μM | [29] |
| 5a | (−)-carvone |
2-methyl-5-[1-(pyrrolidin-1-ylmethyl)vinyl]- cyclohex-2-enone  | prostate (LNCaP) | GI50 > 100 μM | [29] |
| 5b | (−)-carvone |
2-methyl-5-[1-(piperidin-1-ylmethyl)vinyl]- cyclohex-2-enone  | prostate (LNCaP) | GI50 > 100 μM | [29] |
| 5c | (−)-carvone |
5-(1-cyclohexylaminomethyl)vinyl-2-methyl- cyclohex-2-enone  | prostate (LNCaP) | GI50 > 100 μM | [29] |
| 5d | (−)-carvone |
2-methyl-5-{1-[(2-thiophen-2-ylethylamino)- methyl]vinyl}cyclohex-2-enone  | prostate (LNCaP) | GI50 = 24 μM | [29] |
| 5e | (−)-carvone |
5-(1-dimethylaminomethyl)vinyl-2-methylcy- clohex-2-enone  | prostate (LNCaP) | GI50 = 75 μM | [29] |
| 5f | (−)-carvone |
5-[1-(adamantan-1-ylamino)methyl]vinyl-2- methylcyclohex-2-enone  | prostate (LNCaP) | GI50 = 83 μM | [29] |
| 7 | (+)-carvone | hydroisobenzofuran derivative of (+)-carvone (ester) | epithelial carcinoma (KB-3) | IC50 = 3 μM | [30] |
| 8 | (+)-carvone | hydroisobenzofuran derivative of (+)-carvone (diene) | epithelial carcinoma (KB-3) | IC50 = 1 μM | [30] |
| 8 | (+)-carvone | hydroisobenzofuran derivative of (+)-carvone (diene) | leukemia (RPMI-8226) |
GI50 = 0.148 μM LC50 = 9.36 μM | [30] |
| 8 | (+)-carvone | hydroisobenzofuran derivative of (+)-carvone (diene) | lung (HOP-92 |
GI50 = 0.552 μM LC50 = 26.8 μM | [30] |
| 9 | (+)-carvone | hydroisobenzofuran derivative of (+)-carvone (enone) | epithelial carcinoma (KB-3) | IC50 = 3 μM | [30] |
| 10 | (−)-carvone | (−)-8-hydroxycarvotanacetone | colon (HCT-116) |
GI = 75.2% | [24] |
| 10 | (−)-carvone | (−)-8-hydroxycarvotanacetone | ovarian (OVCAR-8) | GI = 94.01% | [24] |
| 10 | (−)-carvone | (−)-8-hydroxycarvotanacetone | brain (SF-295) | GI = 61.59% | [24] |
| 11 | (+)-carvone | (+)-8-hydroxycarvotanacetone | colon (HCT-116) | GI = 4.76% | [24] |
| 11 | (+)-carvone | (+)-8-hydroxycarvotanacetone | ovarian (OVCAR-8) | GI = 3.12% | [24] |
| 11 | (+)-carvone | (+)-8-hydroxycarvotanacetone | brain (SF-295) | GI = 16.36% | [24] |
| 12 | (−)-carvone | (−)-carvone epoxide | colon (HCT-116) | GI = 29.24% | [24] |
| 12 | (−)-carvone | (−)-carvone epoxide | ovarian (OVCAR-8) | GI = 8.21% | [24] |
| 12 | (−)-carvone | (−)-carvone epoxide | brain (SF-295) | GI = 10.93% | [24] |
| 13 | (−)-carvone | (−)-8-acetoxycarvotanacetone | colon (HCT-116) | GI = 10.36% | [24] |
| 13 | (−)-carvone | (−)-8-acetoxycarvotanacetone | ovarian (OVCAR-8) | GI = 1.62% | [24] |
| 13 | (−)-carvone | (−)-8-acetoxycarvotanacetone | brain (SF-295) | GI = 30.47% | [24] |
| 14 | (−)-carvone | (R,E)-2-(2-methyl-5-(prop-1-en-2-yl)cyclohex-2-en-1-one)thiosemicarbazone | larynx epidermoid carcinoma (Hep2) | the number of dead and apoptotic cells increased to about 20% at concentration 40 μM | [33] |
| 15 | (−)-carvone | complex Pd2L2Cl4; L = (R)-(−)-carvone  | larynx epidermoid carcinoma (Hep2) | IC50 = 30 μM | [33] |
| 17 | (+)-limonene | (R)-(+)-limonene | bladder | concentration 36 µM give 34.71% apoptotic cell percentage | [37,88] |
| 17 | (+)-limonene | (R)-(+)-limonene | lung (A549, H1299) | cells showed increase in the expression of autophagy- related genes lc3b, beclin1, atg3, atg5, atg7, atg12 and atg14 with enhanced protein expressions of the autophagy-related proteins LC3-II and Atg5 | [37,89] |
| 18 | (+)-limonene | (+)-limonene 1,2-epoxide | colon (HCT-116) | GI = 73.13% | [24] |
| 18 | (+)-limonene | (+)-limonene 1,2-epoxide | ovarian (OVCAR-8) | GI = 93.1% | [24] |
| 18 | (+)-limonene | (+)-limonene 1,2-epoxide | brain (SF-295) | GI = 58.48% | [24] |
| 18 | (+)-limonene | solid lipid nanoparticles (SLNs) formulation with (+)-limonene 1,2-epoxide 18 and glycerol monostearate (LIM-SLNs) | human skin (HaCaT) | cell viability 76.27 ± 1.63% | [39] |
| 19 | (+)-limonene | (+)-(1S,2S,4R)-limonene-1,2-diol | lung (A549) | IC50 = 1.53 – 0.04 mg/mL (48 h) | [40] |
| 19 | (+)-limonene | (+)-(1S,2S,4R)-limonene-1,2-diol | lung (H1264) | IC50 = 1.73 ± 0.04 mg/mL (48 h) | [40] |
| 19 | (+)-limonene | (+)-(1S,2S,4R)-limonene-1,2-diol | lung (H1299) | IC50 = 1.39 ± 0.06 mg/mL (48 h) | [40] |
| 19 | (+)-limonene | (+)-(1S,2S,4R)-limonene-1,2-diol | lung (Calu-6) | IC50 = 0.62 ± 0.02 mg/mL (48 h) | [40] |
| 20 | (−)-perillyl alcohol | POH/β-CD | sarcoma (S180) | GI = 60% | [46] |
| 22 | (−)-perillyl alcohol | [(4S)-4-prop-1-en-2-ylcyclohexen-1-yl]methyl N-(3-methyl-4-oxoimidazo[5,1-d][1,2,3,5]tetrazine-8-carbonyl)carbamate (NEO212) | lymphoma (HUT-78) | IC50 = 8 μM (24 h) IC50 ≤ 3 μM (48 h) | [47] |
| 22 | (−)-perillyl alcohol | [(4S)-4-prop-1-en-2-ylcyclohexen-1-yl]methyl N-(3-methyl-4-oxoimidazo[5,1-d][1,2,3,5]tetrazine-8-carbonyl)carbamate (NEO212) | lymphoma (HUT-102) | IC50 = 9 μM (72 h) IC50 = 3 μM (96 h) | [47] |
| 22 | (−)-perillyl alcohol | [(4S)-4-prop-1-en-2-ylcyclohexen-1-yl]methyl N-(3-methyl-4-oxoimidazo[5,1-d][1,2,3,5]tetrazine-8-carbonyl)carbamate (NEO212) | lymphoma (MyLa) | IC50 = 130 μM (72 h) IC50 = 85 μM (96 h) | [47] |
| 23 | (−)-perillyl alcohol | perillyl alcohol/temozolomide/linoleic acid conjugate (NEO412) | melanoma (A2058, M24) | IC50 = 5 μM | [48] |
| 23 | (−)-perillyl alcohol | perillyl alcohol/temozolomide/linoleic acid conjugate (NEO412) | melanoma (A375, M249) | IC50 = 25–35 μM | [48] |
| 24 | (−)-perillyl alcohol | 4-(prop-1-en-2-yl)-N-(3-(trifluoromethyl)phenyl)cyclohex-1-ene-1-carboxamide | glioblastoma (U251) | IC50 = 9.41 ± 0.38 μM | [49] |
| 24 | (−)-perillyl alcohol | 4-(prop-1-en-2-yl)-N-(3-(trifluoromethyl)phenyl)cyclohex-1-ene- 1-carboxamide | hepatocellular carcinoma (HepG2) | IC50 = 18.07 ± 0.10 μM | [49] |
| 25 | (−)-perillyl alcohol | N-(4-(4-amino-2-methylphenethyl)-3-methylphenyl)-4-(prop-1-en-2-yl)cyclohex-1-ene-1-carboxamide | glioblastoma (U251) | IC50 = 3.10 ± 0.12 μM | [49] |
| 25 | (−)-perillyl alcohol | N-(4-(4-amino-2-methylphenethyl)-3-methylphenyl)-4-(prop-1- en-2-yl)cyclohex-1-ene-1-carboxamide | hepatocellular carcinoma (HepG2) | IC50 = 1.49 ± 0.43 μM | [49] |
| 26a | (−)-perillyl alcohol | 4-{[(4S)-4-isopropenylcyclohex-1-en-1-yl]methyl}benzene-1,2-diol | breast (MCF-7) | IC50 = 25.9 ± 0.1 μM | [50] |
| 26a | (−)-perillyl alcohol | 4-{[(4S)-4-isopropenylcyclohex-1-en-1-yl]methyl}benzene-1,2-diol | prostate (PC-3) | IC50 = 12.2 ± 0.7 μM | [50] |
| 26a | (−)-perillyl alcohol | 4-{[(4S)-4-isopropenylcyclohex-1-en-1-yl]methyl}benzene-1,2-diol | colon (HT-29) | IC50 = 45.1 ± 0.2 μM | [50] |
| 26b | (−)-perillyl alcohol | 4-{[(4S)-4-isopropenylcyclohex-1-en-1-yl]methyl}benzene-1,3-diol | breast (MCF-7) | IC50 = 53.7 ± 0.4 μM | [50] |
| 26b | (−)-perillyl alcohol | 4-{[(4S)-4-isopropenylcyclohex-1-en-1-yl]methyl}benzene-1,3-diol | prostate (PC-3) | IC50 = 54.5 ± 0.5 μM | [50] |
| 26c | (−)-perillyl alcohol | 3-{[(4S)-4-isopropenylcyclohex-1-en-1-yl]methoxy}phenol | breast (MCF-7) | IC50 = 44.3 ± 0.7 μM | [50] |
| 26c | (−)-perillyl alcohol | 3-{[(4S)-4-isopropenylcyclohex-1-en-1-yl]methoxy}phenol | prostate (PC-3) | IC50 = 79.0 ± 0.2 μM | [50] |
| 27a | (−)-perillaldehyde | (S)-N-((4-(prop-1-en-2-yl)cyclohex-1-enyl)methyl)amantadine | lung (A549) | IC50 = 53.80 μM | [54] |
| 27a | (−)-perillaldehyde | (S)-N-((4-(prop-1-en-2-yl)cyclohex-1-enyl)methyl)amantadine | melanoma (A375-S2) | IC50 = 53.80 μM | [54] |
| 27a | (−)-perillaldehyde | (S)-N-((4-(prop-1-en-2-yl)cyclohex-1-enyl)methyl)amantadine | fibrosarcoma (HT1080) | IC50 = 56.17 μM | [54] |
| 27b | (−)-perillaldehyde | (S)-N-((4-(prop-1-en-2-yl)cyclohex-1-enyl)methyl)cyclohexanamine | lung (A549) | IC50 = 69.50 μM | [54] |
| 27b | (−)-perillaldehyde | (S)-N-((4-(prop-1-en-2-yl)cyclohex-1-enyl)methyl)cyclohexanamine | melanoma (A375-S2) | IC50 = 72.77 μM | [54] |
| 27b | (−)-perillaldehyde | (S)-N-((4-(prop-1-en-2-yl)cyclohex-1-enyl)methyl)cyclohexanamine | fibrosarcoma (HT1080) | IC50 = 69.37 μM | [54] |
| 28 | (−)-perillaldehyde | (−)-perillaldehyde 1,2-epoxide | Colon (HCT-116) | GI = 99.46 ± 1.54% IC50 = 16.14 ± 1.86 μM | [55] |
| 28 | (−)-perillaldehyde | (−)-perillaldehyde 1,2-epoxide | ovarian (OVCAR-8) | GI = 99.37 ± 0.30% IC50 = 23.61 ± 1.13 μM | [55] |
| 28 | (−)-perillaldehyde | (−)-perillaldehyde 1,2-epoxide | glioblastoma (SF-295) | GI = 95.66 ± 5.06% IC50 = 21.99 ± 2.64 μM | [55] |
| 28 | (−)-perillaldehyde | (−)-perillaldehyde 1,2-epoxide | leukemia (HL-60) | GI = 99.71 ± 2.43% IC50 = 9.70 ± 1.01 μM | [55] |
| 28 | (−)-perillaldehyde | cSLNs loaded with perillaldehyde 1,2-epoxide | breast (MCF-7) | IC50 = 195.08 μg/mL | [56] |
| 29 | (−)-perillaldehyde | (−)-perillaldehyde 8,9-epoxide | colon (HCT-116) | GI = 98.64 ± 0.74% IC50 = 1.03 μM | [24] |
| 29 | (−)-perillaldehyde | (−)-perillaldehyde 8,9-epoxide | ovarian (OVCAR-8) | GI = 96.32 ± 1.51% IC50 = 1.15 μM | [24] |
| 29 | (−)-perillaldehyde | (−)-perillaldehyde 8,9-epoxide | glioblastoma (SF-295) | GI = 99.89 ± 0.24% IC50 = 1.75 μM | [24] |
| 29 | (−)-perillaldehyde | (−)-perillaldehyde 8,9-epoxide | leukemia (HL-60) | IC50 = 0.64 μM | [24] |
| 30 | carvacrol | carvacrol | metastatic breast (MDA-MB 231) | IC50 = 100 μM IC50 = 199 μM | [59] [60] |
| 30 | carvacrol | carvacrol | glioblastoma (U87) | IC50 = 322 μM | [60] |
| 30 | carvacrol | carvacrol | hepatocellular carcinoma (HepG2) | IC50 = 53.09 μg/mL IC50 = 0.4 mmol/L IC50 = 48.3 mg/L | [63] [64] [65] |
| 30 | carvacrol | carvacrol | L02 (human healthy epatocyte line) | cell viability is 100% with 0.4 mmol/L | [64] |
| 30 | carvacrol | carvacrol | HEK293 (healthy human renal cells) | IC50 = 90.5 mg/L | [65] |
| 30 | carvacrol | carvacrol | murine mastocytoma (P815) | IC50 = 0.067 μM | [21] |
| 30 | carvacrol | carvacrol | acute lymphoblastic leukemia (CEM) | IC50 = 0.042 μM | [21] |
| 30 | carvacrol | carvacrol | K-562 (human chronic myelogenous leukemia) | IC50 = 0.067 μM | [21] |
| 30 | carvacrol | carvacrol | breast (MCF-7) | IC50 = 0.125 μM | [21] |
| 30 | carvacrol | carvacrol | breast adenocarcinoma resistant to gemcitabine (MCF-7/gem) | 0.067 μM | [21] |
| 30 | carvacrol | carvacrol | choriocarcinoma ( JAR) | the cell viability decreased (76%) and increased the population of late apoptotic cells (23.8%) at concentration 300 µM | [70] |
| 30 | carvacrol | carvacrol | choriocarcinoma ( JEG3) | the cell viability decreased (49%) and increased the population of late apoptotic cells (1023%) at concentration 300 µM | [70] |
| 30 | carvacrol | carvacrol | colon (HCT-116) | IC50 = 92 µM (48 h) | [71] |
| 30 | carvacrol | carvacrol | colon (HT-29) | IC50 = 42 µM (48 h) | [71] |
| 30 | carvacrol | carvacrol nanoemulsion CANE | lung (A549) | 52.7% cell viability at concentration 100 µg/mL; 40.5% sub-G1 cell accumulation was observed at 100 µg/mL of CANE treatment | [72] |
| 30 | carvacrol | carvacrol nanoemulsion CANE | lung (PC-9) | dose-dependent cytotoxicity with 62.1 and 52.2% cell viability at 125 and 150 μg/mL concentrations | [72] |
| 30 | carvacrol | carvacrol nanoemulsion CANE | BEAS-2B (healthy bronchial epithelium cells) | no cytotoxicity up to 100 µg/ml | [72] |
| 30 | carvacrol | carvacrol nanoemulsion CANE | tumor in mice | 34.2 and 62.1% reduction in tumor weight in the mice treated with 50 and 100 mg/kg CANE | [72] |
| 30 | carvacrol | carvacrol | leukemia (KG1) | 60% cell viability at concentration 300 µg/mL (24 h) and 30% (48 h); the combination of car-vacrol/thymol (300 μM/50 μM): 10% cell viability | [73] |
| 30 | carvacrol | carvacrol | leukemia (HL60) | 80% cell viability at concentration 300 µg/mL (24 h) and 60% (48 h); the combination of car-vacrol/thymol (300 μM/50 μM): 5% cell viability | [73] |
| 30 | carvacrol | carvacrol | myelogenous leukemia (K562) | 80% cell viability at concentration 300 µg/mL (24 h); the combination of carvacrol/thymol (300 μM/50 μM): 30% cell viability | [73] |
| 30 | carvacrol | carvacrol | (PBMCs) peripheral blood mononuclear cell from healthy donors | 65% cell viability at concentration 300 µg/mL (48 h); the combination of carvacrol/thymol (300 μM/50 μM): 55% cell viability | [73] |
| 31 | carvacrol |
(E)-N-(2-hydroxy-6-isoproyl-3-methylbenzylindine)-2-(5-isopropyl-2-methylphenoxy) acetohydrazide |
colon (HCT-15) | GI50 = 80 µg/mL | [75] |
| 31 | carvacrol | (E)-N-(2-hydroxy-6-isoproyl-3-methylbenzylindine)-2-(5-isopropyl-2-methylphenoxy) acetohydrazide |
pancreatic (MIPaCa-2) | GI50 = 80 µg/mL | [75] |
| 32 | carvacrol |
(E)-N-(2-hydroxy-3-isoproyl-6-methylbenzylindine)-2-(5-isopropyl-2-methylphenoxy) acetohydrazide |
colon (HCT-15) | GI50 = 80 µg/mL | [75] |
| 32 | carvacrol | (E)-N-(2-hydroxy-3-isoproyl-6-methylbenzylindine)-2-(5-isopropyl-2-methylphenoxy) acetohydrazide |
pancreatic (MIPaCa-2) | GI50 = 10 µg/mL | [75] |
| 33 | carvacrol | (E)-N-(5-allyl-2-hydroxy-3-methoxybenzylindine)-2-(5-isopropyl-2-methylphenoxy) acetohydrazide | colon (HCT-15) | GI50 = 10 µg/mL | [75] |
| 33 | carvacrol | (E)-N-(5-allyl-2-hydroxy-3-methoxybenzylindine)-2-(5-isopropyl-2-methylphenoxy) acetohydrazide |
pancreatic (MIPaCa-2) | GI50 = 10 µg/mL | [75] |
| 34 | carvacrol | (E)-4-(2-hydroxy-6-isopropyl-3-methylbenzylideneamino)-2-isopropyl-5-methylphenol |
colon (HCT-15) | GI50 = 80 µg/mL | [76] |
| 34 | carvacrol | (E)-4-(2-hydroxy-6-isopropyl-3-methylbenzylideneamino)-2-isopropyl-5-methylphenol |
pancreatic (MIPaCa-2) | GI50 = 80 µg/mL | [76] |
| 35 | carvacrol | (E)-4-(2-hydroxy-3-isopropyl-6-methylbenzylideneamino)-5-isopropyl-2-methylphenol |
colon (HCT-15) | GI50 = 80 µg/mL | [76] |
| 35 | carvacrol | (E)-4-(2-hydroxy-3-isopropyl-6-methylbenzylideneamino)-5-isopropyl-2-methylphenol |
pancreatic (MIPaCa-2) | GI50 = 10.77 µg/mL | [76] |
| 36 | carvacrol | (E)-4-(2-hydroxy-6-isopropyl-3-methylbenzylideneamino)-5-isopropyl-2-methylphenol |
colon (HCT-15) | GI50 = 80 µg/mL | [76] |
| 36 | carvacrol | (E)-4-(2-hydroxy-6-isopropyl-3-methylbenzylideneamino)-5-isopropyl-2-methylphenol |
pancreatic (MIPaCa-2) | GI50 = 16.9 µg/mL | [76] |
| 37 | carvacrol | (E)-4-(5-allyl-2-hydroxy-3-methoxybenzylideneamino)-5-isopropyl-2-methylphenol |
colon (HCT-15) | GI50 = 80 µg/mL | [76] |
| 37 | carvacrol | (E)-4-(5-allyl-2-hydroxy-3-methoxybenzylideneamino)-5-isopropyl-2-methylphenol |
pancreatic (MIPaCa-2) | GI50 = 16.5 µg/ml | [76] |
| 38 | (−)-menthol | (−)-menthol | melanoma (G-361) | EC50 = 286 μM IC50 = 682 μM | [82] |
| 46a | (−)-menthol | 4-((1R,2S,5R)-2-isopropyl-5-methylcyclohexyl) 2-methyl (2S,4S,5R)-1-((2S,3R,5R)-5-(methoxycarbonyl)-1-((2R,3S,5S)-5-(methoxycarbonyl)-2-phenylpyrrolidine-3-carbonyl)-2-phenylpyrrolidine-3-carbonyl)-5-phenylpyrrolidine-2,4-dicarboxylate | prostate (PC-3) | GI50 = 6.4 µM | [83] |
| 46b | (−)-menthol | 4-((1R,2S,5R)-2-isopropyl-5-methylcyclohexyl) 2-methyl (2S,4S,5R)-5-(4-bromophenyl)-1-((2S,3R,5R)-2-(4-bromophenyl)-1-((2R,3S,5S)-2-(4-bromophenyl)-5-(methoxycarbonyl)-pyrrolidine-3-carbonyl)-5-(methoxycarbonyl)pyrrolidine-3-carbonyl)pyrrolidine-2,4-dicarboxylate | prostate (PC-3) | GI50 = 30.0 µM | [83] |
| 46c | (−)-menthol | 2-(tert-butyl) 4-((1R,2S,5R)-2-isopropyl-5-methylcyclohexyl) (2S,4S,5R)-1-((2S,3R,5R)-5-(tert-butoxycarbonyl)-1-((2R,3S,5S)-5-(tert-butoxycarbonyl)-2-phenylpyrrolidine-3-carbonyl)-2-phenylpyrrolidine-3-carbonyl)-5-phenylpyrrolidine-2,4-dicarboxylate | prostate (PC-3) | GI50 > 30.0 µM | [83] |
| 46d | (−)-menthol | 4-((1R,2S,5R)-2-isopropyl-5-methylcyclohexyl) 2-methyl (2S,4S,5R)-5-(3-chlorophenyl)-1-((2S,3R,5R)-5-(methoxycarbonyl)-1-((2R,3S,5S)-5-(methoxycarbonyl)-2-phenylpyrrolidine-3- carbonyl)-2-(4-methoxyphenyl)pyrrolidine-3-carbonyl)pyrrolidine-2,4-dicarboxylate | prostate (PC-3) | GI50 = 5.3 µM | [83] |
| 46e | (+)-menthol | 4-((1S,2R,5S)-2-isopropyl-5-methylcyclohexyl) 2-methyl (2R,4R,5S)-1-((2R,3S,5S)-5-(methoxycarbonyl)-1-((2S,3R,5R)-5-(methoxycarbonyl)-2-phenylpyrrolidine-3-carbonyl)-2- phenylpyrrolidine-3-carbonyl)-5-phenylpyrrolidine-2,4-dicarboxylate | prostate (PC-3) | GI50 = 4.4 μM | [83] |
| 46f | (+)-menthol | 4-((1S,2R,5S)-2-isopropyl-5-methylcyclohexyl) 2-methyl (2R,4R,5S)-5-(3-chlorophenyl)-1-((2R,3S,5S)-5-(methoxycarbonyl)-1-((2S,3R,5R)-5-(methoxycarbonyl)-2-phenylpyrrolidine-3-carbonyl)-2-(4-methoxyphenyl)pyrrolidine-3-carbonyl)pyrrolidine-2,4-dicarboxylate | prostate (PC-3) | GI50 = 6.0 μM | [83] |
| 47a | (−)-menthol | doxorubicin menthoxycarbonylacetylhydrazone | leukemia (HL-60) | IC50 = 0.57 ± 0.18 μM (24 h) IC50 = 0.26 ± 0.11 μM (48 h) IC50 = 0.23 ± 0.10 μM (72 h) | [84] |
| 47a | (−)-menthol | doxorubicin menthoxycarbonylacetylhydrazone | melanoma (518A2) | IC50 = 0.71 ± 0.52 μM (24 h) IC50 = 0.54 ± 0.02 μM (48 h) IC50 = 0.14 ± 0.01 μM (72 h) | [84] |
| 47a | (−)-menthol | doxorubicin menthoxycarbonylacetylhydrazone | breast (MCF-7/Topo) | IC50 = 8.4 ± 2.6 μM (24 h) IC50 = 3.8 ± 1.0 μM (48 h) IC50 = 2.7 ± 1.0 μM (72 h) | [84] |
| 47a | (−)-menthol | doxorubicin menthoxycarbonylacetylhydrazone | cervix (KB-V1/Vbl) | IC50 = 17.8 ± 3.8 μM (24 h) IC50 = 18.4 ± 3.0 μM (48 h) IC50 = 10.3 ± 2.1 μM (72 h) | [84] |
| 47b | (−)-menthol | doxorubicin 5-(menthoxycarbonyl)pentanoylhydrazone | leukemia (HL-60) | IC50 = 0.39 ± 0.18 μM (24 h) IC50 = 0.33 ± 0.11 μM (48 h) IC50 = 0.33 ± 0.05 μM (72 h) | [84] |
| 47b | (−)-menthol | doxorubicin 5-(menthoxycarbonyl)pentanoylhydrazone | melanoma (518A2) | IC50 = 1.2 ± 0.3 μM (24 h) IC50 = 0.42 ± 0.08 μM (48 h) IC50 = 0.32 ± 0.12 μM (72 h) | [84] |
| 47b | (−)-menthol | doxorubicin 5-(menthoxycarbonyl)pentanoylhydrazone | breast (MCF-7/Topo) | IC50 = 6.7 ± 0.9 μM (24 h) IC50 = 4.7 ± 1.2 μM (48 h) IC50 = 2.4 ± 1.0 μM (72 h) | [84] |
| 47b | (−)-menthol | doxorubicin 5-(menthoxycarbonyl)pentanoylhydrazone | cervix (KB-V1/Vbl) | IC50 > 100 μM (24 h) IC50 = 34.9 ± 15.7 μM (48 h) IC50 = 23.3 ± 12.5 μM (72 h) | [84] |
| 47c | (−)-menthol | doxorubicin 8-(menthoxycarbonyl)octanoylhydrazone | leukemia (HL-60) | IC50 = 0.40 ± 0.22 μM (24 h) IC50 = 0.49 ± 0.17 μM (48 h) IC50 = 0.37 ± 0.25 μM (72 h) | [84] |
| 47c | (−)-menthol | doxorubicin 8-(menthoxycarbonyl)octanoylhydrazone | melanoma (518A2) | IC50 = 0.82 ± 0.20 μM (24 h) IC50 = 0.51 ± 0.17 μM (48 h) IC50 = 0.46 ± 0.12 μM (72 h) | [84] |
| 47c | (−)-menthol | doxorubicin 8-(menthoxycarbonyl)octanoylhydrazone | breast (MCF-7/Topo) | IC50 = 10.2 ± 2.8 μM (24 h) IC50 = 7.2 ± 1.8 μM (48 h) IC50 = 4.3 ± 1.9 μM (72 h) | [84] |
| 47c | (−)-menthol | doxorubicin 8-(menthoxycarbonyl)octanoylhydrazone | cervix (KB-V1/Vbl) | IC50 = 79.6 ± 6.8 μM (24 h) IC50 = 21.5 ± 3.7 μM (48 h) IC50 = 21.8 ± 3.8 μM (72 h) | [84] |
| 47d | (−)-menthol | doxorubicin 11-(menthoxycarbonyl)undecanoylhydrazone | leukemia (HL-60) | IC50 = 0.30 ± 0.10 μM (24 h) IC50 = 0.25 ± 0.19 μM (48 h) IC50 = 0.11 ± 0.05 μM (72 h) | [84] |
| 47d | (−)-menthol | doxorubicin 11-(menthoxycarbonyl)undecanoylhydrazone | melanoma (518A2) | IC50 = 0.23 ± 0.06 μM (24 h) IC50 = 0.16 ± 0.05 μM (48 h) IC50 = 0.06 ± 0.01 μM (72 h) | [84] |
| 47d | (−)-menthol | doxorubicin 11-(menthoxycarbonyl)undecanoylhydrazone | breast (MCF-7/Topo) | IC50 = 7.1 ± 1.8 μM (24 h) IC50 = 2.6 ± 1.1 μM (48 h) IC50 = 2.6 ± 1.1 μM (72 h) | [84] |
| 47d | (−)-menthol | doxorubicin 11-(menthoxycarbonyl)undecanoylhydrazone | cervix (KB-V1/Vbl) | IC50 = 30.5 ± 4.6 μM (24 h) IC50 = 17.1 ± 2.5 μM (48 h) IC50 = 8.8 ± 0.7 μM (72 h) | [84] |
| 48a | (−)-menthol | (−)-menthyl[6-(aminomethyl)nicotinate]dichloridoplatinum(II) | melanoma (518A2) | IC50 = 7.4 ± 0.1 μM (24 h) | [85,90] |
| 48a | (−)-menthol | (−)-menthyl[6-(aminomethyl)nicotinate]dichloridoplatinum(II) | leukemia (HL-60) | IC50 = 8.0 ± 1.0 μM (24 h) | [85,90] |
| 48b | (−)-menthol | (−)-menthyl derivative [6-(aminomethyl)nicotinate]dichloridoplatinum(II) | melanoma (518A2) | IC50 ≥ 50 μM (24 h) | [85] |
| 48b | (−)-menthol | (−)-menthyl derivative [6-(aminomethyl)nicotinate]dichloridoplatinum(II) | leukemia (HL-60) | IC50 = n.d. (24 h) | [85] |
| 48c | (−)-menthol | (−)-menthyl derivative [6-(aminomethyl)nicotinate]dichloridoplatinum(II) | melanoma (518A2) | IC50 = 3.0 ± 2.4 μM (24 h) | [85] |
| 48c | (−)-menthol | (−)-menthyl derivative [6-(aminomethyl)nicotinate]dichloridoplatinum(II) | leukemia (HL-60) | IC50 = 5 μM (24 h) | [85] |
| 48d | (−)-menthol | (−)-menthyl derivative [6-(aminomethyl)nicotinate]dichloridoplatinum(II) | melanoma (518A2) | IC50 = 10.0 ± 5.6 μM (24 h) | [85] |
| 48d | (−)-menthol | (−)-menthyl derivative [6-(aminomethyl)nicotinate]dichloridoplatinum(II) | leukemia (HL-60) | IC50 = 5.75 ± 1.8 μM (24 h) | [85] |
| 48e | (−)-menthol | (−)-menthyl derivative [6-(aminomethyl)nicotinate]dichloridoplatinum(II) | melanoma (518A2) | IC50 = 11.7 ± 4.0 μM (24 h) | [85] |
| 48e | (−)-menthol | (−)-menthyl derivative [6-(aminomethyl)nicotinate]dichloridoplatinum(II) | leukemia (HL-60) | IC50 = 7 μM (24 h) | [85] |
| 48f | (−)-menthol | (−)-menthyl derivative [6-(aminomethyl)nicotinate]dichloridoplatinum(II) | melanoma (518A2) | IC50 = 34 μM (24 h) | [85] |
| 48f | (−)-menthol | (−)-menthyl derivative [6-(aminomethyl)nicotinate]dichloridoplatinum(II) | leukemia (HL-60) | IC50 = n.d. (24 h) | [85] |
| 48g | (−)-menthol | (−)-menthyl derivative [6-(aminomethyl)nicotinate]dichloridoplatinum(II) | melanoma (518A2) | IC50 ≥ 50 μM (24 h) | [85] |
| 48g | (−)-menthol | (−)-menthyl derivative [6-(aminomethyl)nicotinate]dichloridoplatinum(II) | leukemia (HL-60) | IC50 = n.d. (24 h) | [85] |
| 48h | (+)-menthol | (+)-menthyl[6-(aminomethyl)nicotinate]dichloridoplatinum(II) | melanoma (518A2) | IC50 = 15.5 ± 0.9 μM (24 h) | [85,90] |
| 48h | (+)-menthol | (+)-menthyl[6-(aminomethyl)nicotinate]dichloridoplatinum(II) | leukemia (HL-60) | IC50 = 7.0 ± 0.2 μM (24 h) | [85,90] |
| 48i | (+)-menthol | (+)-menthyl derivative [6-(aminomethyl)nicotinate]dichloridoplatinum(II) | melanoma (518A2) | IC50 = 5.0 ± 2.0 μM (24 h) | [85] |
| 48i | (+)-menthol | (+)-menthyl derivative [6-(aminomethyl)nicotinate]dichloridoplatinum(II) | leukemia (HL-60) | IC50 = 15 μM (24 h) | [85] |
| 48j | (+)-menthol | (+)-menthyl derivative [6-(aminomethyl)nicotinate]dichloridoplatinum(II) | melanoma (518A2) | IC50 = 5.0 ± 1.4 μM (24 h) | [85] |
| 48j | (+)-menthol | (+)-menthyl derivative [6-(aminomethyl)nicotinate]dichloridoplatinum(II) | leukemia (HL-60) | IC50 = 13 μM (24 h) | [85] |
| 48k | (+)-neomenthol | (+)-neomenthyl[6-(aminomethyl)nicotinate]dichloridoplatinum(II) | melanoma (518A2) | IC50 = 8.3 ± 0.8 μM (24 h) | [85,90] |
| 48k | (+)-neomenthol | (+)-neomenthyl[6-(aminomethyl)nicotinate]dichloridoplatinum(II) | leukemia (HL-60) | IC50 = 9.8 ± 3.2 μM (24 h) | [85,90] |
| 48l | (+)-neomenthol | (+)-neomenthyl derivative [6-(aminomethyl)nicotinate]dichloridoplatinum(II) | melanoma (518A2) | IC50 = 2.7 ± 0.35 μM (24 h) | [85] |
| 48l | (+)-neomenthol | (+)-neomenthyl derivative [6-(aminomethyl)nicotinate]dichloridoplatinum(II) | leukemia (HL-60) | IC50 = 14 μM (24 h) | [85] |
| 48m | (+)-neomenthol | (+)-neomenthyl derivative [6-(aminomethyl)nicotinate]dichloridoplatinum(II) | melanoma (518A2) | IC50 = 5.0 ± 1.2 μM (24 h) | [85] |
| 48m | (+)-neomenthol | (+)-neomenthyl derivative [6-(aminomethyl)nicotinate]dichloridoplatinum(II) | leukemia (HL-60) | IC50 = 14 μM (24 h) | [85] |
| 48n | (−)-neomenthol | (−)-neomenthyl[6-(aminomethyl)nicotinate]dichloridoplatinum(II) | melanoma (518A2) | IC50 = 12.0 ± 7.1 μM (24 h) | [85] |
| 48n | (−)-neomenthol | (−)-neomenthyl[6-(aminomethyl)nicotinate]dichloridoplatinum(II) | leukemia (HL-60) | IC50 = n.d. (24 h) | [85] |
8. Therapeutic Deep Eutectic Solvents
9. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADR | Adriamycin |
| APIs | Active pharmaceutical ingredient |
| AML | Acute myeloid leukemia |
| AMPK | 5′ Adenosine monophosphate-activated protein kinase |
| APIs | Active pharmaceutical ingredients |
| BW | Body weight |
| BBB | Blood–brain barrier |
| CAM | Camphor |
| CAP | Capric acid |
| CV | Carvacrol |
| CVN | Carvone |
| CoN | Colon epithelial cells |
| COX-2 | Cyclooxygenase-2 |
| cSLNs | Cationic solid lipid nanoparticles |
| DEN | Diethylnitrosamine |
| DESs | Deep eutectic solvents |
| DMBA | 7,12-Dimethylbenz[a]anthracene |
| DMH | 1,2-Dimethylhydrazine |
| EC50 | Effective concentration of a drug or concentration of a drug which is calculated to inhibit virus induced cell death by 50% |
| ERK | Extracellular signal-regulated kinases |
| EU | Eucalyptol |
| GBA | Glioblastoma |
| GI50 | The 50% growth inhibition percentage |
| GI% | The cell growth inhibition percentage values |
| HBAs | Hydrogen bond acceptors |
| HC | Hepatocellular carcinoma |
| HDFs | Human dermal fibroblasts |
| HRPC | Hormone-refractory prostate cancer |
| IBU | Ibuprofen |
| IC50 | Concentration of an inhibitor where the response (or binding) is reduced by half |
| LIM | limonene |
| LIM-SLNs | Solid lipid nanoparticles formulation with (+)-limonene 1,2-epoxide and glycerol monostearate |
| LPO | Lipid peroxidation |
| MAPK | Mitogen-activated protein kinase |
| MA | Myristic acid |
| ME | Menthol |
| MTT | 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| MTT assay | The colorimetric assay based on the reduction of yellow tetrazolium salt (MTT) to purple formazan |
| NADESs | Natural deep eutectic systems |
| NEO100 | Highly pure perillyl alcohol |
| NR | 3-Amino-7-dimethyl-2-methylphenazine hydrochloride |
| NR assay | The neutral red (NR) dye uptake assay |
| NEO212 | Compound built of perillyl alcohol and temozolomide |
| p21waf1 | Cyclin-dependent kinase inhibitor |
| PAH | Perillaldehyde |
| 3-PCA | Pyrrolidine-3-carboxylic acid |
| PE | Perillaldehyde 1,2-epoxide |
| PLK1 | Polo-like kinase 1 |
| PM | Physical mixtures |
| POH | Perillyl alcohol |
| ROS | Reactive oxygen species |
| SLNs | Solid lipid nanoparticles |
| SRB | Sulforhodamine B |
| SAR | Structure-activity relationship |
| THEDES | Therapeutic deep eutectic system |
| THEDESs | Therapeutic deep eutectic solvents |
| TMZ | Temozolomide |
| TRPM8 | Transient receptor potential melastatin member 8 |
References
- Dewick, P.M. Medicinal Natural Products: A Biosynthetic Approach, 3rd ed.; John Wiley & Sons Inc.: New York, NY, USA, 2009; ISBN 9780470741689. [Google Scholar]
- Christianson, D.W. Structural and chemical biology of terpenoid cyclases. Chem. Rev. 2017, 117, 11570–11648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salakhutdinov, N.F.; Volcho, K.P.; Yarovaya, O.I. Monoterpenes as a renewable source of biologically active compounds. Pure Appl. Chem. 2017, 89, 1105–1117. [Google Scholar] [CrossRef]
- Lima, P.S.S.; Lucchese, A.M.; Araújo-Filho, H.G.; Menezes, P.P.; Araújo, A.A.S.; Quintans-Júnior, L.J.; Quintans, J.S.S. Inclusion of terpenes in cyclodextrins: Preparation, characterization and pharmacological approaches. Carbohydr. Polym. 2016, 151, 965–987. [Google Scholar] [CrossRef] [PubMed]
- Neighbors, J.D. The mevalonate pathway and terpenes: A diversity of chemopreventatives. Curr. Pharmacol. Rep. 2018, 4, 157–169. [Google Scholar] [CrossRef]
- Wojtunik-Kulesza, K.A.; Kasprzak, K.; Oniszczuk, T.; Oniszczuk, A. Natural monoterpenes: Much more than only a scent. Chem. Biodivers. 2019, 16, e1900434. [Google Scholar] [CrossRef]
- Koziol, A.; Stryjewska, A.; Librowski, T.; Salat, K.; Gawel, M.; Moniczewski, A.; Lochynski, S. An overview of the pharmacological properties and potential applications of natural monoterpenes. Mini Rev. Med. Chem. 2014, 14, 1156–1168. [Google Scholar] [CrossRef]
- Koyama, S.; Heinbockel, T. The effects of essential oils and terpenes in relation to their routes of intake and application. Int. J. Mol. Sci. 2020, 21, 1558. [Google Scholar] [CrossRef] [Green Version]
- Kim, T.; Song, B.; Cho, K.S.; Lee, I.S. Therapeutic potential of volatile terpenes and terpenoids from forests for inflammatory diseases. Int. J. Mol. Sci. 2020, 21, 2187. [Google Scholar] [CrossRef] [Green Version]
- de Mesquita, L.S.S.; Luz, T.R.S.A.; de Mesquita, J.W.C.; Coutinho, D.F.; do Amaral, F.M.M.; de Sousa Ribeiro, M.N.; Malik, S. Exploring the anticancer properties of essential oils from family Lamiaceae. Food Rev. Int. 2019, 35, 105–131. [Google Scholar] [CrossRef]
- Spisni, E.; Petrocelli, G.; Imbesi, V.; Spigarelli, R.; Azzinnari, D.; Sarti, M.D.; Campieri, M.; Valerii, M.C. Antioxidant, anti-inflammatory, and microbial-modulating activities of essential oils: Implications in colonic pathophysiology. Int. J. Mol. Sci. 2020, 21, 4152. [Google Scholar] [CrossRef]
- Yang, W.; Chen, X.; Li, Y.; Guo, S.; Wang, Z.; Yu, X. Advances in pharmacological activities of terpenoids. Nat. Prod. Commun. 2020, 15, 1934578X20903555. [Google Scholar] [CrossRef] [Green Version]
- Zielińska-Błajet, M.; Feder-Kubis, J. Monoterpenes and their derivatives—Recent development in biological and medical applications. Int. J. Mol. Sci. 2020, 21, 7078. [Google Scholar] [CrossRef] [PubMed]
- Sobral, M.V.; Xavier, A.L.; Lima, T.C.; De Sousa, D.P. Antitumor activity of monoterpenes found in essential oils. Sci. World J. 2014, 2014, 953451. [Google Scholar] [CrossRef] [PubMed]
- Hansen, B.B.; Spittle, S.; Chen, B.; Poe, D.; Zhang, Y.; Klein, J.M.; Horton, A.; Adhikari, L.; Zelovich, T.; Doherty, B.W.; et al. Deep dutectic solvents: A review of fundamentals and applications. Chem. Rev. 2021, 121, 1232–1285. [Google Scholar] [CrossRef]
- Zhang, Q.; De Oliveira Vigier, K.; Royer, S.; Jérôme, F. Deep eutectic solvents: Syntheses, properties and applications. Chem. Soc. Rev. 2012, 41, 7108–7146. [Google Scholar] [CrossRef]
- Liu, Y.; Friesen, J.B.; McAlpine, J.B.; Lankin, D.C.; Chen, S.N.; Pauli, G.F. Natural deep eutectic solvents: Properties, applications, and perspectives. J. Nat. Prod. 2018, 81, 679–690. [Google Scholar] [CrossRef] [PubMed]
- Bicas, J.L.; Neri-Numa, I.A.; Ruiz, A.L.T.G.; De Carvalho, J.E.; Pastore, G.M. Evaluation of the antioxidant and antiproliferative potential of bioflavors. Food Chem. Toxicol. 2011, 49, 1610–1615. [Google Scholar] [CrossRef]
- Morcia, C.; Malnati, M.; Terzi, V. In vitro antifungal activity of terpinen-4-ol, eugenol, carvone, 1,8-cineole (eucalyptol) and thymol against mycotoxigenic plant pathogens. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2012, 29, 415–422. [Google Scholar] [CrossRef] [Green Version]
- Mesa-Arango, A.C.; Montiel-Ramos, J.; Zapata, B.; Durán, C.; Betancur-Galvis, L.; Stashenko, E. Citral and carvone chemotypes from the essential oils of Colombian Lippia alba (Mill.) N.E. Brown: Composition, cytotoxicity and antifungal activity. Mem. Inst. Oswaldo Cruz 2009, 104, 878–884. [Google Scholar] [CrossRef] [Green Version]
- Jaafari, A.; Tilaoui, M.; Mouse, H.A.; M’Bark, L.A.; Aboufatima, R.; Chait, A.; Lepoivre, M.; Zyad, A. Comparative study of the antitumor effect of natural monoterpenes: Relationship to cell cycle analysis. Braz. J. Pharmacogn. 2012, 22, 534–540. [Google Scholar] [CrossRef] [Green Version]
- Aydın, E.; Türkez, H.; Keleş, M.S. Potential anticancer activity of carvone in N2a neuroblastoma cell line. Toxicol. Ind. Health 2015, 31, 764–772. [Google Scholar] [CrossRef]
- Paduch, R.; Trytek, M.; Król, S.K.; Kud, J.; Frant, M.; Kandefer-Szerszeń, M.; Fiedurek, J. Biological activity of terpene compounds produced by biotechnological methods. Pharm. Biol. 2016, 54, 1096–1107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrade, L.N.; Lima, T.C.; Amaral, R.G.; Do Ó Pessoa, C.; De Moraes Filho, M.O.; Soares, B.M.; Do Nascimento, L.G.; Carvalho, A.A.C.; De Sousa, D.P. Evaluation of the cytotoxicity of structurally correlated p-menthane derivatives. Molecules 2015, 20, 13264–13280. [Google Scholar] [CrossRef] [Green Version]
- Vinothkumar, R.; Sudha, M.; Viswanathan, P.; Kabalimoorthy, J.; Balasubramanian, T.; Nalini, N. Modulating effect of D-carvone on 1,2-dimethylhydrazine-induced pre-neoplastic lesions, oxidative stress and biotransforming enzymes, in an experimental model of rat colon carcinogenesis. Cell Prolif. 2013, 46, 705–720. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, T.; Ganapathy, S.; Veeran, V.; Namasivayam, N. Preventive effect of D-carvone during DMBA induced mouse skin tumorigenesis by modulating xenobiotic metabolism and induction of apoptotic events. Biomed. Pharmacother. 2019, 111, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Chen, H. Anticancer effects of Carvone in myeloma cells is mediated through the inhibition of p38 MAPK signalling pathway, apoptosis induction and inhibition of cell invasion. JBUON 2018, 23, 747–751. [Google Scholar] [PubMed]
- Wang, J.; Hu, Y.; Wang, Y.; Yang, Y.; Li, S.; Hou, Y.; Zhuang, Z.; Wu, F. D-carvone attenuates biochemical and molecular expression via oncogenic signaling in aryl hydrocarbon-induced hamster mucosal carcinogenesis. Pharmacogn. Mag. 2020, 16, 303–310. [Google Scholar] [CrossRef]
- Chen, J.; Lu, M.; Jing, Y.; Dong, J. The synthesis of L-carvone and limonene derivatives with increased antiproliferative effect and activation of ERK pathway in prostate cancer cells. Bioorg. Med. Chem. 2006, 14, 6539–6547. [Google Scholar] [CrossRef] [PubMed]
- Bateman, T.D.; Joshi, A.L.; Moon, K.; Galitovskaya, E.N.; Upreti, M.; Chambers, T.C.; McIntosh, M.C. Synthesis and anticancer activity of sclerophytin-inspired hydroisobenzofurans. Bioorg. Med. Chem. Lett. 2009, 19, 6898–6901. [Google Scholar] [CrossRef] [Green Version]
- Dilworth, J.R.; Hueting, R. Metal complexes of thiosemicarbazones for imaging and therapy. Inorg. Chim. Acta 2012, 389, 3–15. [Google Scholar] [CrossRef]
- Liu, Y.-H.; Shao, J.; Xie, C.-Z.; Song, X.-Q.; Bao, W.-G.; Xu, J.-Y. Four Cu(II) complexes based on antitumor chelators: Synthesis, structure, DNA binding/damage, HSA interaction and enhanced cytotoxicity. Dalt. Trans. 2016, 45, 8036–8048. [Google Scholar] [CrossRef]
- Kokina, T.E.; Sheludyakova, L.A.; Eremina, Y.A.; Vorontsova, E.V.; Glinskaya, L.A.; Piryazev, D.A.; Lider, E.V.; Tkachev, A.V.; Larionov, S.V. Complexes of Cu(I) and Pd(II) with (+)-camphor and (–)-carvone thiosemicarbazones: Synthesis, structure, and cytotoxicity of the Pd(II) complex. Russ. J. Gen. Chem. 2017, 87, 1674–1684. [Google Scholar] [CrossRef]
- Chi, G.; Wei, M.; Xie, X.; Soromou, L.W.; Liu, F.; Zhao, S. Suppression of MAPK and NF-κB pathways by limonene contributes to attenuation of lipopolysaccharide-induced inflammatory responses in acute lung injury. Inflammation 2013, 36, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.A.; Pappan, K.; Thompson, P.A.; Want, E.J.; Siskos, A.P.; Keun, H.C.; Wulff, J.; Hu, C.; Lang, J.E.; Chow, H.H.S. Plasma metabolomic profiles of breast cancer patients after short-term limonene intervention. Cancer Prev. Res. 2015, 8, 86–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vieira, A.J.; Beserra, F.P.; Souza, M.C.; Totti, B.M.; Rozza, A.L. Limonene: Aroma of innovation in health and disease. Chem. Biol. Interact. 2018, 283, 97–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anandakumar, P.; Kamaraj, S.; Vanitha, M.K. D-limonene: A multifunctional compound with potent therapeutic effects. J. Food Biochem. 2021, 45, e13566. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, T.S.; Marques, M.R.; do Ó Pessoa, C.; Lotufo, L.V.C.; Magalhães, H.I.F.; de Moraes, M.O.; de Lima, D.P.; Tininis, A.G.; de Oliveira, J.E. In vitro cytotoxic activity of brazilian middle west plant extracts. Braz. J. Pharmacogn. 2011, 21, 456–464. [Google Scholar] [CrossRef] [Green Version]
- Souto, E.B.; Zielinska, A.; Souto, S.B.; Durazzo, A.; Lucarini, M.; Santini, A.; Silva, A.M.; Atanasov, A.G.; Marques, C.; Andrade, L.N.; et al. (+)-Limonene 1,2-epoxide-loaded SLNs: Evaluation of drug release, antioxidant activity, and cytotoxicity in an HaCaT cell line. Int. J. Mol. Sci. 2020, 21, 1449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, T.K.; Roh, H.S.; Yu, J.S.; Baek, J.; Lee, S.; Ra, M.; Kim, S.Y.; Baek, K.H.; Kim, K.H. Pinecone of Pinus koraiensis inducing apoptosis in human lung cancer cells by activating caspase-3 and its chemical constituents. Chem. Biodivers. 2017, 14, e1600412. [Google Scholar] [CrossRef] [PubMed]
- Gelb, M.H.; Tamanoi, F.; Yokoyama, K.; Ghomashchi, F.; Esson, K.; Gould, M.N. The inhibition of protein prenyltransferases by oxygenated metabolites of limonene and perillyl alcohol. Cancer Lett. 1995, 91, 169–175. [Google Scholar] [CrossRef]
- Shojaei, S.; Kiumarsi, A.; Moghadam, A.R.; Alizadeh, J.; Marzban, H.; Ghavami, S. Perillyl alcohol (monoterpene alcohol), limonene. In Enzymes; Academic Press: Cambridge, MA, USA, 2014; Volume 36, pp. 7–32. [Google Scholar]
- Nuutinen, T. Medicinal properties of terpenes found in Cannabis sativa and Humulus lupulus. Eur. J. Med. Chem. 2018, 157, 198–228. [Google Scholar] [CrossRef]
- Chen, T.C.; da Fonseca, C.O.; Schönthal, A.H. Intranasal perillyl alcohol for glioma therapy: Molecular mechanisms and clinical development. Int. J. Mol. Sci. 2018, 19, 3905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, T.C.; Da Fonseca, C.O.; Schönthal, A.H. Perillyl alcohol and its drug-conjugated derivatives as potential novel methods of treating brain metastases. Int. J. Mol. Sci. 2016, 17, 1463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rezende, A.A.; Santos, R.S.; Andrade, L.N.; Amaral, R.G.; Pereira, M.M.; Bani, C.; Chen, M.; Priefer, R.; da Silva, C.F.; de Albuquerque Júnior, R.L.C.; et al. Anti-tumor efficiency of perillylalcohol/β-cyclodextrin inclusion complexes in a sarcoma S180-induced mice model. Pharmaceutics 2021, 13, 245. [Google Scholar] [CrossRef]
- Silva-Hirschberg, C.; Hartman, H.; Stack, S.; Swenson, S.; Minea, R.O.; Davitz, M.A.; Chen, T.C.; Schönthal, A.H. Cytotoxic impact of a perillyl alcohol–temozolomide conjugate, NEO212, on cutaneous T-cell lymphoma in vitro. Ther. Adv. Med. Oncol. 2019, 11, 1758835919891567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swenson, S.; Silva-Hirschberg, C.; Wang, W.; Singh, A.; Hofman, F.M.; Chen, K.L.; Schönthal, A.H.; Chen, T.C. Neo412: A temozolomide analog with transdermal activity in melanoma in vitro and in vivo. Oncotarget 2018, 9, 37026–37041. [Google Scholar] [CrossRef] [Green Version]
- Mukhtar, Y.M.; Wang, K.; Li, R.; Deng, W.; Adu-Frimpong, M.; Zhang, H.; Zhang, K.; Gu, C.; Xu, X.; Yu, J. Novel: N-arylamide derivatives of (S)-perillic acid ((S)-PA): In vitro and in vivo cytotoxicity and antitumor evaluation. RSC Adv. 2019, 9, 19973–19982. [Google Scholar] [CrossRef] [Green Version]
- Said, B.; Montenegro, I.; Valenzuela, M.; Olguín, Y.; Caro, N.; Werner, E.; Godoy, P.; Villena, J.; Madrid, A. Synthesis and antiproliferative activity of new cyclodiprenyl phenols against select cancer cell lines. Molecules 2018, 23, 2323. [Google Scholar] [CrossRef] [Green Version]
- Malu, T.J.; Banerjee, N.; Singh, A.K.; Kannadasan, S.; Ethiraj, K.R. A study of antioxidant potential of perilladehyde. IOP Conf. Ser. Mater. Sci. Eng. 2017, 263. [Google Scholar] [CrossRef]
- Tian, J.; Zeng, X.; Lü, A.; Zhu, A.; Peng, X.; Wang, Y. Perillaldehyde, a potential preservative agent in foods: Assessment of antifungal activity against microbial spoilage of cherry tomatoes. LWT Food Sci. Technol. 2015, 60, 63–70. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, S.; Feng, Q.; Huang, X.; Wang, X.; Peng, Y.; Zhao, Z.; Liu, Z. Perilaldehyde activates AMP-activated protein kinase to suppress the growth of gastric cancer via induction of autophagy. J. Cell. Biochem. 2019, 120, 1716–1725. [Google Scholar] [CrossRef] [PubMed]
- Hui, Z.; Zhang, M.; Cong, L.; Xia, M.; Dong, J. Synthesis and antiproliferative effects of amino-modified perillyl alcohol derivatives. Molecules 2014, 19, 6671–6682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luciana, N.A.; Patrícia, S.; Ricardo, G.A.; Grace, A.A.D.; Andressa, d.S.; Melina, A.; Ricardo, L.C.A.J.; Maria, C.S.L.; Claudia, O.P.; Adriana, A.C.; et al. Evaluation of cytotoxic and antitumor activity of perillaldehyde 1,2-epoxide. J. Med. Plants Res. 2018, 12, 590–600. [Google Scholar] [CrossRef]
- Souto, E.B.; Souto, S.B.; Zielinska, A.; Durazzo, A.; Lucarini, M.; Santini, A.; Horbańczuk, O.K.; Atanasov, A.G.; Marques, C.; Andrade, L.N.; et al. Perillaldehyde 1,2-epoxide loaded SLN-tailored mAb: Production, physicochemical characterization and in vitro cytotoxicity profile in MCF-7 cell lines. Pharmaceutics 2020, 12, 161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suntres, Z.E.; Coccimiglio, J.; Alipour, M. The bioactivity and toxicological actions of carvacrol. Crit. Rev. Food Sci. Nutr. 2015, 55, 304–318. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, M.; Varoni, E.M.; Iriti, M.; Martorell, M.; Setzer, W.N.; del Mar Contreras, M.; Salehi, B.; Soltani-Nejad, A.; Rajabi, S.; Tajbakhsh, M.; et al. Carvacrol and human health: A comprehensive review. Phyther. Res. 2018, 32, 1675–1687. [Google Scholar] [CrossRef]
- Arunasree, K.M. Anti-proliferative effects of carvacrol on a human metastatic breast cancer cell line, MDA-MB 231. Phytomedicine 2010, 17, 581–588. [Google Scholar] [CrossRef]
- Baranauskaite, J.; Kubiliene, A.; Marksa, M.; Petrikaite, V.; Vitkevičius, K.; Baranauskas, A.; Bernatoniene, J. The influence of different oregano species on the antioxidant activity determined using HPLC postcolumn DPPH method and anticancer activity of carvacrol and rosmarinic acid. Biomed Res. Int. 2017, 2017, 1681392. [Google Scholar] [CrossRef] [Green Version]
- Rojas-Armas, J.P.; Arroyo-Acevedo, J.L.; Palomino-Pacheco, M.; Herrera-Calderón, O.; Ortiz-Sánchez, J.M.; Rojas-Armas, A.; Calva, J.; Castro-Luna, A.; Hilario-Vargas, J. The essential oil of Cymbopogon citratus stapt and carvacrol: An approach of the antitumor effect on 7,12-dimethylbenz-[α]-anthracene (DMBA)-induced breast cancer in female rats. Molecules 2020, 25, 3284. [Google Scholar] [CrossRef]
- Li, L.; He, L.; Wu, Y.; Zhang, Y. Carvacrol affects breast cancer cells through TRPM7 mediated cell cycle regulation. Life Sci. 2021, 266, 118894. [Google Scholar] [CrossRef]
- Özkan, A.; Erdoğan, A. A comparative evaluation of antioxidant and anticancer activity of essential oil from Origanum onites (Lamiaceae) and its two major phenolic componentstle. Turk. J. Biol. 2011, 35, 735–742. [Google Scholar] [CrossRef]
- Yin, Q.; Yan, F.; Zu, X.-Y.; Wu, Y.; Wu, X.; Liao, M.; Deng, S.; Yin, L.; Zhuang, Y. Anti-proliferative and pro-apoptotic effect of carvacrol on human hepatocellular carcinoma cell line HepG-2. Cytotechnology 2012, 64, 43–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elshafie, H.S.; Armentano, M.F.; Carmosino, M.; Bufo, S.A.; De Feo, V.; Camele, I. Cytotoxic activity of origanum vulgare L. on hepatocellular carcinoma cell line HepG2 and evaluation of its biological activity. Molecules 2017, 22, 1435. [Google Scholar] [CrossRef] [Green Version]
- Jayakumar, S.; Madankumar, A.; Asokkumar, S.; Raghunandhakumar, S.; Gokula Dhas, K.; Kamaraj, S.; Josephine Divya, M.G.; Devaki, T. Potential preventive effect of carvacrol against diethylnitrosamine-induced hepatocellular carcinoma in rats. Mol. Cell. Biochem. 2012, 360, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Mu, Z.-y.; Huang, Y.; Fu, C. Anti-prostate cancer effect of carvacrol via MAPK signaling pathway. Acad. J. Second Mil. Med. Univ. 2014, 35, 285–290. [Google Scholar] [CrossRef]
- Khan, F.; Khan, I.; Farooqui, A.; Ansari, I.A. Carvacrol induces Reactive Oxygen Species (ROS)-mediated apoptosis along with cell cycle arrest at G 0/G 1 in human prostate cancer cells. Nutr. Cancer 2017, 69, 1075–1087. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Singh, V.K.; Saeed, M.; Kausar, M.A.; Ansari, I.A. Carvacrol induced program cell death and cell cycle arrest in androgen-independent human prostate cancer cells via inhibition of Notch signaling. Anticancer. Agents Med. Chem. 2019, 19, 1588–1608. [Google Scholar] [CrossRef] [PubMed]
- Lim, W.; Ham, J.; Bazer, F.W.; Song, G. Carvacrol induces mitochondria-mediated apoptosis via disruption of calcium homeostasis in human choriocarcinoma cells. J. Cell. Physiol. 2019, 234, 1803–1815. [Google Scholar] [CrossRef]
- Pakdemirli, A.; Karaca, C.; Sever, T.; Daskin, E.; Leblebici, A.; Yiğitbaşi, T.; Başbinar, Y. Carvacrol alters soluble factors in HCT-116 and HT-29 cell lines. Turk. J. Med. Sci. 2020, 50, 271–276. [Google Scholar] [CrossRef]
- Khan, I.; Bahuguna, A.; Kumar, P.; Bajpai, V.K.; Kang, S.C. In vitro and in vivo antitumor potential of carvacrol nanoemulsion against human lung adenocarcinoma A549 cells via mitochondrial mediated apoptosis. Sci. Rep. 2018, 8, 144–158. [Google Scholar] [CrossRef] [Green Version]
- Bouhtit, F.; Najar, M.; Moussa Agha, D.; Melki, R.; Najimi, M.; Sadki, K.; Boukhatem, N.; Bron, D.; Meuleman, N.; Hamal, A.; et al. New anti-leukemic effect of carvacrol and thymol combination through synergistic induction of different cell death pathways. Molecules 2021, 26, 410. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Li, X.; Nishida, Y. Synthesis and bioactivity of novel carvacrol and thymol derivatives containing 5-phenyl-2-furan. Lett. Drug Des. Discov. 2014, 11, 877–885. [Google Scholar] [CrossRef]
- Rajput, J.D.; Bagul, S.D.; Bendre, R.S. Design, synthesis, biological screenings and docking simulations of novel carvacrol and thymol derivatives containing acetohydrazone linkage. Res. Chem. Intermed. 2017, 43, 4893–4906. [Google Scholar] [CrossRef]
- Rajput, J.D.; Bagul, S.D.; Hosamani, A.A.; Patil, M.M.; Bendre, R.S. Synthesis, characterizations, biological activities and docking studies of novel dihydroxy derivatives of natural phenolic monoterpenoids containing azomethine linkage. Res. Chem. Intermed. 2017, 43, 5377–5393. [Google Scholar] [CrossRef]
- Oertling, H.; Reckziegel, A.; Surburg, H.; Bertram, H.J. Application of menthol synthetic chemistry. Chem. Rev. 2007, 107, 2136–2164. [Google Scholar] [CrossRef] [PubMed]
- Kamatou, G.P.P.; Vermaak, I.; Viljoen, A.M.; Lawrence, B.M. Menthol: A simple monoterpene with remarkable biological properties. Phytochemistry 2013, 96, 15–25. [Google Scholar] [CrossRef]
- Kim, S.H.; Lee, S.; Piccolo, S.R.; Allen-Brady, K.; Park, E.J.; Chun, J.N.; Kim, T.W.; Cho, N.H.; Kim, I.G.; So, I.; et al. Menthol induces cell-cycle arrest in PC-3 cells by down-regulating G2/M genes, including polo-like kinase 1. Biochem. Biophys. Res. Commun. 2012, 422, 436–441. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Yang, Z.; Zhu, G.; Chen, D.; Meng, Z. Menthol inhibits the proliferation and motility of prostate cancer DU145 cells. Pathol. Oncol. Res. 2012, 18, 903–910. [Google Scholar] [CrossRef]
- Okamoto, Y.; Ohkubo, T.; Ikebe, T.; Yamazaki, J. Blockade of TRPM8 activity reduces the invasion potential of oral squamous carcinoma cell lines. Int. J. Oncol. 2012, 40, 1431–1440. [Google Scholar] [CrossRef] [Green Version]
- Yamamura, H.; Ugawa, S.; Ueda, T.; Morita, A.; Shimada, S. TRPM8 activation suppresses cellular viability in human melanoma. Am. J. Physiol. Cell Physiol. 2008, 295, C296–C301. [Google Scholar] [CrossRef] [Green Version]
- Kudryavtsev, K.V.; Ivantcova, P.M.; Muhle-Goll, C.; Churakov, A.V.; Sokolov, M.N.; Dyuba, A.V.; Arutyunyan, A.M.; Howard, J.A.K.; Yu, C.C.; Guh, J.H.; et al. Menthols as chiral auxiliaries for asymmetric cycloadditive oligomerization: Syntheses and studies of β-proline hexamers. Org. Lett. 2015, 17, 6178–6181. [Google Scholar] [CrossRef] [PubMed]
- Effenberger, K.; Breyer, S.; Schobert, R. Modulation of doxorubicin activity in cancer cells by conjugation with fatty acyl and terpenyl hydrazones. Eur. J. Med. Chem. 2010, 45, 1947–1954. [Google Scholar] [CrossRef] [PubMed]
- Bernhardt, G.; Biersack, B.; Bollwein, S.; Schobert, R.; Zoldakova, M. Terpene conjugates of diaminedichloridoplatinum(II) complexes: Antiproliferative effects in HL-60 leukemia, 518A2 melanoma, and HT-29 colon cancer cells. Chem. Biodivers. 2008, 5, 1645–1659. [Google Scholar] [CrossRef] [PubMed]
- Suchodolski, J.; Feder-Kubis, J.; Krasowska, A. Antifungal activity of ionic liquids based on (–)-menthol: A mechanism study. Microbiol. Res. 2017, 197, 56–64. [Google Scholar] [CrossRef]
- Feder-Kubis, J.; Wnętrzak, A.; Chachaj-Brekiesz, A. Terpene-based ionic liquids from natural renewable sources as selective agents in antifungal therapy. ACS Biomater. Sci. Eng. 2020, 6, 3832–3842. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Liang, Z.; Mi, Q.; Guo, Y. Limonene terpenoid obstructs human bladder cancer cell (T24 cell line) growth by inducing cellular apoptosis, caspase activation, G2/M phase cell cycle arrest and stops cancer metastasis. JBUON 2020, 25, 280–285. [Google Scholar] [PubMed]
- Yu, X.; Lin, H.; Wang, Y.; Lv, W.; Zhang, S.; Qian, Y.; Deng, X.; Feng, N.; Yu, H.; Qian, B. D-limonene exhibits antitumor activity by inducing autophagy and apoptosis in lung cancer. OncoTargets Ther. 2018, 11, 1833–1847. [Google Scholar] [CrossRef] [Green Version]
- Schobert, R.; Biersack, B.; Dietrich, A.; Grotemeier, A.; Müller, T.; Kalinowski, B.; Knauer, S.; Voigt, W.; Paschke, R. Monoterpenes as drug shuttles: Cytotoxic (6-aminomethylnicotinate)dichloridoplatinum(II) complexes with potential to overcome cisplatin resistance. J. Med. Chem. 2007, 50, 1288–1293. [Google Scholar] [CrossRef]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Tambyrajah, V. Novel solvent properties of choline chloride/urea mixtures. Chem. Commun. 2003, 70–71. [Google Scholar] [CrossRef] [Green Version]
- Paiva, A.; Matias, A.A.; Duarte, A.R.C. How do we drive deep eutectic systems towards an industrial reality? Curr. Opin. Green Sustain. Chem. 2018, 11, 81–85. [Google Scholar] [CrossRef] [Green Version]
- Silva, J.M.; Pereira, C.V.; Mano, F.; Silva, E.; Castro, V.I.B.; Sá-Nogueira, I.; Reis, R.L.; Paiva, A.; Matias, A.A.; Duarte, A.R.C. Therapeutic role of deep eutectic solvents based on menthol and saturated fatty acids on wound healing. ACS Appl. Bio Mater. 2019, 2, 4346–4355. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.S.; Roy, R.; Jadhav, B.; Hossain, M.N.; Halim, M.A.; Raynie, D.E. Formulation, structure, and applications of therapeutic and amino acid-based deep eutectic solvents: An overview. J. Mol. Liq. 2021, 321, 114745. [Google Scholar] [CrossRef]
- Rodrigues, L.A.; Pereira, C.V.; Leonardo, I.C.; Fernández, N.; Gaspar, F.B.; Silva, J.M.; Reis, R.L.; Duarte, A.R.C.; Paiva, A.; Matias, A.A. Terpene-based natural deep eutectic systems as efficient solvents to recover astaxanthin from brown crab shell residues. ACS Sustain. Chem. Eng. 2020, 8, 2246–2259. [Google Scholar] [CrossRef]
- Silva, E.; Oliveira, F.; Silva, J.M.; Matias, A.; Reis, R.L.; Duarte, A.R.C. Optimal design of THEDES based on perillyl alcohol and ibuprofen. Pharmaceutics 2020, 12, 1121. [Google Scholar] [CrossRef] [PubMed]
- Pereira, C.V.; Silva, J.M.; Rodrigues, L.; Reis, R.L.; Paiva, A.; Duarte, A.R.C.; Matias, A. Unveil the anticancer potential of limomene based therapeutic deep eutectic solvents. Sci. Rep. 2019, 9, 14926. [Google Scholar] [CrossRef] [PubMed] [Green Version]






















| Counterpart A | Counterpart B | Molar Ratio | Abbreviation |
|---|---|---|---|
| Perillyl alcohol | Camphor | 1:1 | PA:CA (1:1) |
| Menthol | Perillyl alcohol | 1:1 | ME:PA (1:1) |
| Menthol | Camphor | 1:1 | ME:CAM (1:1) |
| Menthol | Eucalyptol | 1:1 | ME:EU (1:1) |
| Menthol | Myristic acid | 8:1 | ME:MA (8:1) |
| Samples | Cytotoxicity (Caco-2 Cells) | Antiproliferative Effect (HT-29 Cells) |
|---|---|---|
| NADESs | ||
| PA:CA (1:1) | 0.89 ± 0.20 | 0.75 ± 0.36 |
| ME:PA (1:1) | 0.91 ± 0.08 | 0.57 ± 0.02 |
| ME:CAM (1:1) | 1.26 ± 0.02 | 1.54 ± 0.24 |
| ME:EU (1:1) | 1.58 ± 0.08 | 1.21 ± 0.07 |
| ME:MA (8:1) | 3.67 ± 0.34 | 0.84 ± 0.18 |
| Individual components | ||
| PA | 0.74 ± 0.24 | 0.36 ± 0.03 |
| CAM | >5.00 | >5.00 |
| ME | 1.68 ± 0.50 | 2.67 ± 1.28 |
| EU | >5.00 | 3.09 ± 0.24 |
| MA | >1.50 | >1.50 |
| Physical mixtures (PM) | ||
| PM—PA:CA (1:1) | 2.45 ± 0.29 | 3.82 ± 0.37 |
| PM—ME:PA (1:1) | 1.34 ± 0.11 | 1.61 ± 0.38 |
| PM—ME:CAM (1:1) | 3.63 ± 0.21 | 3.08 |
| PM—ME:EU (1:1) | 3.31 ± 0.34 | 2.95 ± 1.31 |
| PM—ME:MA (8:1) | 0.72 | 5.42 ± 1.58 |
| Counterpart A | Counterpart B | Molar Ratio | Abbreviation |
|---|---|---|---|
| Myristic acid | Limonene | 1:1 | MA:LIM |
| Myristic acid | Limonene | 1:2 | MA:LIM |
| Myristic acid | Limonene | 2:1 | MA:LIM |
| Capric acid | Limonene | 1:1 | CAP:LIM |
| Capric acid | Limonene | 1:2 | CAP:LIM |
| Capric acid | Limonene | 2:1 | CAP:LIM |
| Menthol | Limonene | 1:1 | ME:LIM |
| Menthol | Limonene | 1:2 | ME:LIM |
| Menthol | Limonene | 2:1 | ME:LIM |
| Ibuprofen | Limonene | 1:1 | IBU:LIM |
| Ibuprofen | Limonene | 1:2 | IBU:LIM |
| Ibuprofen | Limonene | 2:1 | IBU:LIM |
| Ibuprofen | Limonene | 1:4 | IBU:LIM |
| Ibuprofen | Limonene | 1:8 | IBU:LIM |
| Samples | Cytotoxicity (Caco-2 Cells) | Antiproliferative Effect (HT-29 Cells) |
|---|---|---|
| THEDESs | ||
| CAP:LIM (1:1) | 0.918 ± 0.042 | 0.6901 ± 0.105 |
| ME:LIM (1:1) | 2.314 ± 0.421 | 0.8023 ± 0.016 |
| IBU:LIM (1:4) | 10.50 ± 0.883 | 2.390 ± 2.919 |
| IBU:LIM (1:8) | 3.323 ± 0.228 | 1.137 ± 0.055 |
| Individual components | ||
| IBU | 2.893 ± 0.059 | 2.346 ± 0.088 |
| CAP | 1.334 ± 0.223 | 0.341 ± 0.081 |
| LIM | 2.638 ± 0.108 | 0.661 ± 0.025 |
| ME | 8.078 ± 0.810 | 4.730 ± 16.14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zielińska-Błajet, M.; Pietrusiak, P.; Feder-Kubis, J. Selected Monocyclic Monoterpenes and Their Derivatives as Effective Anticancer Therapeutic Agents. Int. J. Mol. Sci. 2021, 22, 4763. https://doi.org/10.3390/ijms22094763
Zielińska-Błajet M, Pietrusiak P, Feder-Kubis J. Selected Monocyclic Monoterpenes and Their Derivatives as Effective Anticancer Therapeutic Agents. International Journal of Molecular Sciences. 2021; 22(9):4763. https://doi.org/10.3390/ijms22094763
Chicago/Turabian StyleZielińska-Błajet, Mariola, Przemysław Pietrusiak, and Joanna Feder-Kubis. 2021. "Selected Monocyclic Monoterpenes and Their Derivatives as Effective Anticancer Therapeutic Agents" International Journal of Molecular Sciences 22, no. 9: 4763. https://doi.org/10.3390/ijms22094763
APA StyleZielińska-Błajet, M., Pietrusiak, P., & Feder-Kubis, J. (2021). Selected Monocyclic Monoterpenes and Their Derivatives as Effective Anticancer Therapeutic Agents. International Journal of Molecular Sciences, 22(9), 4763. https://doi.org/10.3390/ijms22094763