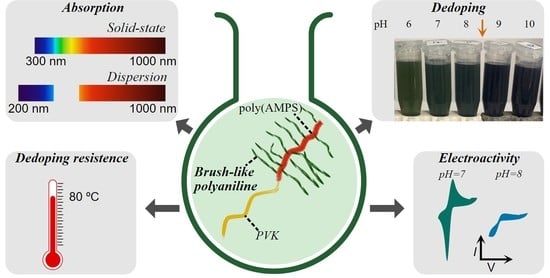
Brush-like Polyaniline with Optical and Electroactive Properties at Neutral pH and High Temperature
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structural Characterization of the Polymerization Sequence
2.1.1. Macro-RAFT
2.1.2. Block Copolymer
2.1.3. Poly(2-acrylamide-2-methyl-1-propanesulfonate-graft-polyaniline)-Block-Poly(N-vinylcarbazole), BL PAni
2.2. UV–Vis Study
2.2.1. Optical Properties of Macro-RAFT, PAAMP-b-PVK, and BL PAni
2.2.2. Dedoping Point of Poly(2-acrylamide-2-methyl-1-propanesulfonate-graft-polyaniline)-Block-Poly(N-vinylcarbazole)
2.2.3. Optical Properties in Correlation with Temperature
2.3. Electroactive Properties of BL PAni
2.4. Thermal Stability
2.4.1. Thermogravimetric Analysis
2.4.2. Differential Scanning Calorimetry
3. Materials and Methods
3.1. Materials
3.2. Poly(anilinium 2-acrylamide-2-methyl-1-propanesulfonate), Macro-RAFT
3.3. Poly(anilinium 2-acrylamide-2-methyl-1-propanesulfonate)-Block-Poly(N-vinylcarbazole), PAAMP-b-PVK
3.4. Poly(2-acrylamide-2-methyl-1-propanesulfonate)-Block-Poly(N-vinylcarbazole)/Graft-Polyaniline, BL PAni
3.5. Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Menzel, V.C.; Yi, X.; Bößl, F.; Kirk, C.; Robertson, N.; Tudela, I. Additive manufacturing of polyaniline electrodes for electrochemical applications. Addit. Manuf. 2022, 54, 102710. [Google Scholar] [CrossRef]
- Bhadra, J.; Alkareem, A.; Al-Thani, N. A review of advances in the preparation and application of polyaniline based thermoset blends and composites. J. Polym. Res. 2020, 27, 122. [Google Scholar] [CrossRef] [Green Version]
- Biabangard, F.; Nazari, H.; Arefinia, R. Effect of pH on the electrochemical properties of polyaniline nanoparticle suspension in strongly acidic solution: An experimental and theoretical study. J. Solid State Electrochem. 2021, 25, 881–893. [Google Scholar] [CrossRef]
- Masdarolomoor, F.; Hajizadeh, S.; Arab Chamjangali, M.; Innis, P.C. Novel approach to the synthesis of polyaniline possessing electroactivity at neutral pH. Synth. Met. 2019, 250, 121–130. [Google Scholar] [CrossRef]
- Bharati, A.; Hejmady, P.; van der Donck, T.; Seo, J.W.; Cardinaels, R.; Moldenaers, P. Developing conductive immiscible polystyrene/polypropylene blends with a percolated conducting polyaniline/polyamide filler by tuning its specific interactions with the styrene-based triblock compatibilizer grafted with maleic anhydride. J. Appl. Polym. Sci. 2020, 137, 48433. [Google Scholar] [CrossRef]
- Massoumi, B.; Sorkhishams, N.; Sarvari, R.; Agbolaghi, S. Synthesis and characterization of electroactive bottlebrush nano-copolymers based on polystyrene and polyaniline as side chains and poly(3-(2-hydroxyethyl)thiophene) as backbone. Polym. Bull. 2020, 77, 3707–3724. [Google Scholar] [CrossRef]
- Mažeikienė, R.; Niaura, G.; Malinauskas, A. Poly(N-methylaniline) vs. polyaniline: An extended pH range of polaron stability as revealed by Raman spectroelectrochemistry. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 262, 120140. [Google Scholar] [CrossRef]
- Park, Y.R.; Kim, H.J.; Im, S.; Seo, S.; Shin, K.; Choi, W.K.; Hong, Y.J. Tailoring the highest occupied molecular orbital level of poly(N-vinylcarbazole) hole transport layers in organic multilayer heterojunctions. Appl. Phys. Lett. 2016, 108, 023301. [Google Scholar] [CrossRef]
- Fink, J.K. Carbazole Polymers. In High Performance Polymers; Elsevier: Oxford, UK, 2014; pp. 1–42. ISBN 9780323312226. [Google Scholar]
- Yu, J.-W.; Kim, J.K.; Cho, H.N.; Kim, D.Y.; Kim, C.Y.; Song, N.W.; Kim, D. Long-Range Energy Migration in Photoexcited Polymers. Macromolecules 2000, 33, 5443–5447. [Google Scholar] [CrossRef]
- Miyasaka, H.; Khan, S.R.; Itaya, A. Photoinduced Electron Transfer Dynamics in Aromatic Vinyl Polymers and Related Systems: Time-Resolved Detection of Primary Events. J. Photochem. Photobiol. C Photochem. Rev. 2003, 4, 195–214. [Google Scholar] [CrossRef]
- Borsenberger, P.M.; Ateya, A.I. Hole photogeneration in poly(N-vinylcarbazole). J. Appl. Phys. 1978, 49, 4035–4040. [Google Scholar] [CrossRef]
- Xie, J.; Piao, J.; Liu, L.; Chen, D.; Liu, Y.; Wang, W.; Cao, K.; Shen, W.; Chen, S. Ink formulation of in-situ crosslinkable hole-transporting composite for multilayer inkjet-printed organic light-emitting diodes. Org. Electron. 2021, 99, 106337. [Google Scholar] [CrossRef]
- Huang, B.-Y.; Huang, S.; Chuang, C.; Kuo, C. Electrically-Tunable Blue Phase Liquid Crystal Microlens Array Based on a Photoconductive Film. Polymers 2020, 12, 65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, F.; Li, X.; Duan, H.; Wang, H.; Fan, L.; Sun, Y.; Sui, Y.; Yang, J.; Yang, L. Toward efficient, moisture-resistant and lead-leakproofness perovskite solar cells: Coordination-driven reconstructing homogeneous amorphous perovskitoid/crystalline perovskite photoabsorber. Chem. Eng. J. 2022, 428, 132528. [Google Scholar] [CrossRef]
- Piravadili Mucur, S. Chromaticity tunable realizable solution process single layer white organic light-emitting diode. Color Res. Appl. 2021, 46, 1245–1254. [Google Scholar] [CrossRef]
- Claire, P.D. Sainte Molecular Simulation of Excimer Fluorescence in Polystyrene and Poly(vinylcarbazole ). J. Phys. Chem. B 2006, 110, 7334–7343. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.A.N.; Dong, S.; Li, P.; Li, Z.; Ye, C.; Qin, J. New PVK-Based Nonlinear Optical Polymers: Enhanced Nonlinearity and Improved Transparency. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 2983–2993. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, W.E.I.; Zhang, Z.; Cheng, Z.; Tu, Y.; Qiu, Y.; Zhu, X. Thermo-Responsive Fluorescent Micelles from Amphiphilic A 3 B Miktoarm Star Copolymers Prepared via a Combination of SET-LRP and RAFT Polymerization. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 4268–4278. [Google Scholar] [CrossRef]
- Xie, L.; Deng, X.; Chen, L.I.N.; Chen, S.; Liu, R.; Hou, X.; Wong, K.; Ling, Q.; Huang, W.E.I. A pi -Stacked and Conjugated Hybrid Based on Poly(N-vinylcarbazole) Postfunctionalized with Terfluorene for Stable Deep-Blue Hole-Transporting Materials. Polym. Chem. 2009, 47, 5221–5229. [Google Scholar] [CrossRef]
- Cadenas, J.L.; Hu, H. Chemically stable conducting polyaniline composite coatings. Sol. Energy Mater. Sol. Cells 1998, 55, 105–112. [Google Scholar] [CrossRef]
- Basavaraja, C.; Kim, N.R.; Jo, E.A.; Huh, D.S. Structure and DC conductivity studies in Poly-N-vinylcarbazole-polyaniline films. J. Polym. Res. 2010, 17, 861–867. [Google Scholar] [CrossRef]
- Park, Y.R.; Doh, J.H.; Shin, K.; Seo, Y.S.; Kim, Y.S.; Kim, S.Y.; Choi, W.K.; Hong, Y.J. Solution-processed quantum dot light-emitting diodes with PANI:PSS hole-transport interlayers. Org. Electron. 2015, 19, 131–139. [Google Scholar] [CrossRef]
- Bouriche, O.; Bouzerafa, B.; Kouadri, H. Electrochemical, optical and morphological properties of poly(N-vinylcarbazole/TiO2) and (N-vinylcarbazole/aniline)/TiO2 copolymer prepared by electrochemical polymerization. e-Polymers 2018, 18, 111–122. [Google Scholar] [CrossRef]
- Pratap Khare, K.; Kathal, R.; Shukla, N.; Srivastava, R.; Srivastava, A. Copolymerization of aniline and 9 vinyl carbazole: A DFT study. Mater. Today Proc. 2022, 48, 602–604. [Google Scholar] [CrossRef]
- Bongiovanni Abel, S.; Riberi, K.; Rivarola, C.R.; Molina, M.; Barbero, C.A. Synthesis of a Smart Conductive Block Copolymer Responsive to Heat and Near Infrared Light. Polymers 2019, 11, 1744. [Google Scholar] [CrossRef] [Green Version]
- Massoumi, B.; Davtalab, S.; Jaymand, M.; Entezami, A.A. AB2 Y-shaped miktoarm star conductive polyaniline-modified poly(ethylene glycol) and its electrospun nanofiber blend with poly(ε-caprolactone). RSC Adv. 2015, 5, 36715–36726. [Google Scholar] [CrossRef]
- Maity, N.; Dawn, A. Conducting Polymer Grafting: Recent and Key Developments. Polymers 2020, 12, 709. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Guo, R.; Shen, Y.; Shao, Y.; Fei, G.; Zhu, K. Waterborne polyaniline-graft-alkyd for anticorrosion coating and comparison study with physical blend. Prog. Org. Coat. 2019, 126, 187–195. [Google Scholar] [CrossRef]
- Jung, J.W.; Lee, J.U.; Jo, W.H. High-Efficiency Polymer Solar Cells with Water-Soluble and Self-Doped Conducting Polyaniline Graft Copolymer as Hole Transport Layer. J. Phys. Chem. C 2010, 114, 633–637. [Google Scholar] [CrossRef]
- Tiwari, A. Gum Arabic-Graft-Polyaniline: An Electrically Active Redox Biomaterial for Sensor Applications. J. Macromol. Sci. Part A 2007, 44, 735–745. [Google Scholar] [CrossRef]
- Chen, F.; Dai, D.; Yang, J.; Fei, Z.; Zhong, M. Controlled Synthesis of Polyelectrolytes by 4-Cyanopentanoic Acid Dithiobenzoate Mediated RAFT Polymerization. J. Macromol. Sci. Part A 2013, 50, 1002–1006. [Google Scholar] [CrossRef]
- Zhou, J.; Yao, H.; Ma, J. Recent advances in RAFT-mediated surfactant-free emulsion polymerization. Polym. Chem. 2018, 9, 2532–2561. [Google Scholar] [CrossRef]
- Morales-Moctezuma, M.D.; Spain, S.G. The effects of cononsolvents on the synthesis of responsive particles via polymerisation-induced thermal self-assembly. Polym. Chem. 2021, 12, 4696–4706. [Google Scholar] [CrossRef]
- Xu, Y.; Li, Y.; Cao, X.; Chen, Q.; An, Z. Versatile RAFT dispersion polymerization in cononsolvents for the synthesis of thermoresponsive nanogels with controlled composition, functionality and architecture. Polym. Chem. 2014, 5, 6244–6255. [Google Scholar] [CrossRef]
- Fang, F.F.; Dong, Y.-Z.; Choi, H.J. Effect of oxidants on morphology of interfacial polymerized polyaniline nanofibers and their electrorheological response. Polymer 2018, 158, 176–182. [Google Scholar] [CrossRef]
- Conejo-Dávila, A.S.; Moya-Quevedo, M.A.; Chávez-Flores, D.; Vega-Rios, A.; Zaragoza-Contreras, E.A. Role of the Anilinium Ion on the Selective Polymerization of Anilinium 2-Acrylamide-2-methyl-1-propanesulfonate. Polymers 2021, 13, 2349. [Google Scholar] [CrossRef]
- Yuan, J.; Huang, X.; Li, P.; Li, L.; Shen, J. Surface-initiated RAFT polymerization of sulfobetaine from cellulose membranes to improve hemocompatibility and antibiofouling property. Polym. Chem. 2013, 4, 5074. [Google Scholar] [CrossRef]
- Tammer, M.G. Sokrates: Infrared and Raman characteristic group frequencies: Tables and charts. Colloid Polym. Sci. 2004, 283, 235. [Google Scholar] [CrossRef]
- Watanabe, H.; Kanazawa, A.; Aoshima, S. Stereospecific Living Cationic Polymerization of N-Vinylcarbazole through the Design of ZnCl2-Derived Counteranions. ACS Macro Lett. 2017, 6, 463–467. [Google Scholar] [CrossRef]
- Liguori, R.; Botta, A.; Pragliola, S.; Rubino, A.; Venditto, V.; Velardo, A.; Aprano, S.; Maglione, M.G.; Prontera, C.T.; De Girolamo Del Mauro, A.; et al. Study of the electroluminescence of highly stereoregular poly(N-pentenyl-carbazole) for blue and white OLEDs. Semicond. Sci. Technol. 2017, 32, 065006. [Google Scholar] [CrossRef]
- Mbarek, M.; Almoneef, M.M.; Alimi, K. Elaboration and study of the new copolymer based on vinylcarbazole and Stilbene (VK-Stilbene): Correlation structure-proprieties. J. Mol. Struct. 2020, 1217, 128384. [Google Scholar] [CrossRef]
- Butoi, B.; Groza, A.; Dinca, P.; Balan, A.; Barna, V. Morphological and Structural Analysis of Polyaniline and Poly(o-anisidine) Layers Generated in a DC Glow Discharge Plasma by Using an Oblique Angle Electrode Deposition Configuration. Polymers 2017, 9, 732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daraeinejad, Z.; Shabani, I. Enhancing biocompatibility of polyaniline-based scaffolds by using a bioactive dopant. Synth. Met. 2021, 271, 116642. [Google Scholar] [CrossRef]
- Trchová, M.; Šeděnková, I.; Tobolková, E.; Stejskal, J. FTIR spectroscopic and conductivity study of the thermal degradation of polyaniline films. Polym. Degrad. Stab. 2004, 86, 179–185. [Google Scholar] [CrossRef]
- Skrabania, K.; Miasnikova, A.; Bivigou-Koumba, A.M.; Zehm, D.; Laschewsky, A. Examining the UV-vis absorption of RAFT chain transfer agents and their use for polymer analysis. Polym. Chem. 2011, 2, 2074. [Google Scholar] [CrossRef]
- Baker, B.; Cockram, C.J.; Dakein, S.; DeMaria, J.M.; George, D.M.; Malyk, K.R.; Sarno, M.J.F.; Cardenas, A.J.P. Synthesis and characterization of anilinium ionic liquids: Exploring effect of π-π ring stacking. J. Mol. Struct. 2021, 1225, 129122. [Google Scholar] [CrossRef]
- Abro, H.A.; Zhou, T.; Han, W.; Xue, T.; Wang, T. Carbazole-based compounds containing aldehyde and cyanoacetic acid: Optical properties and applications in photopolymerization. RSC Adv. 2017, 7, 55382–55388. [Google Scholar] [CrossRef] [Green Version]
- Cruz-Medina, R.; Vega-Rios, A.; Hernández-Escobar, C.A.; Estrada-Monje, A.; Rodríguez-Sánchez, I.; Zaragoza-Contreras, E.A. Polystyrene-polyaniline core-shell composite particles using a bifunctional selectively polymerizable monomer as the interfacial linkage. Synth. Met. 2020, 265, 116402. [Google Scholar] [CrossRef]
- Mombrú, D.; Romero, M.; Faccio, R.; Mombrú, A.W. Raman and Impedance Spectroscopy under Applied Dc Bias Insights on the Electrical Transport for Donor: Acceptor Nanocomposites Based on Poly(vinyl carbazole) and TiO2 Quantum Dots. J. Phys. Chem. C 2017, 121, 23383–23391. [Google Scholar] [CrossRef]
- Ali Mohsin, M.E.; Shrivastava, N.K.; Arsad, A.; Basar, N.; Hassan, A. The Effect of pH on the Preparation of Electrically Conductive and Physically Stable PANI/Sago Blend Film via in situ Polymerization. Front. Mater. 2020, 7, 1–11. [Google Scholar] [CrossRef]
- Yue, J.; Wang, Z.H.; Cromack, K.R.; Epstein, A.J.; MacDiarmid, A.G. Effect of sulfonic acid group on polyaniline backbone. J. Am. Chem. Soc. 1991, 113, 2665–2671. [Google Scholar] [CrossRef]
- Ansari, R.; Price, W.E.; Wallace, G.G. Effect of thermal treatment on the electroactivity of polyaniline. Polymer 1996, 37, 917–923. [Google Scholar] [CrossRef]
- Desbene-Monvernay, A.; Dubois, J.-E.; Lacaze, P.-C. Oxidative electropolymerization of N-vinylcarbazole. J. Electroanal. Chem. Interfacial Electrochem. 1985, 189, 51–63. [Google Scholar] [CrossRef]
- Do, J.-S.; Chang, Y.-H.; Tsai, M.-L. Highly sensitive amperometric creatinine biosensor based on creatinine deiminase/Nafion®-nanostructured polyaniline composite sensing film prepared with cyclic voltammetry. Mater. Chem. Phys. 2018, 219, 1–12. [Google Scholar] [CrossRef]
- Frau, A.F.; Pernites, R.B.; Advincula, R.C. A Conjugated Polymer Network Approach to Anticorrosion Coatings: Poly(vinylcarbazole) Electrodeposition. Ind. Eng. Chem. Res. 2010, 49, 9789–9797. [Google Scholar] [CrossRef]
- Reyna-González, J.M.; Roquero, P.; Rivera, E. A Comparative Investigation Between Poly(N-vinylcarbazole) and Poly(3,6-N-vinylcarbazole): Spectroscopy, Conductivity, Thermal and Optical Properties. Des. Monomers Polym. 2009, 12, 233–245. [Google Scholar] [CrossRef]
- Moraes, S.R.; Huerta-Vilca, D.; Motheo, A.J. Characteristics of polyaniline synthesized in phosphate buffer solution. Eur. Polym. J. 2004, 40, 2033–2041. [Google Scholar] [CrossRef]
- Osuna, V.; Vega-Rios, A.; Zaragoza-Contreras, E.A.; Estrada-Moreno, I.A.; Dominguez, R.B. Progress of Polyaniline Glucose Sensors for Diabetes Mellitus Management Utilizing Enzymatic and Non-Enzymatic Detection. Biosensors 2022, 12, 137. [Google Scholar] [CrossRef]
- Bouacida, S.; Bouchene, R.; Berrah, F. Synthesis, crystal structure, hirshfeld surface analysis, DFT calculations and thermal properties of a new anilinium derivative chlorostannate(IV) hybrid compound. J. Mol. Struct. 2019, 1198, 126900. [Google Scholar] [CrossRef]
- Gong, C.; Pinatti, L.; Lavigne, G.; Shaw, M.T.; Scola, D.A. Thermal stability of end-capped and linear sulfonated polyimides, sulfonated polystyrene, and Nafion 117. J. Appl. Polym. Sci. 2018, 135, 45694. [Google Scholar] [CrossRef]
- Pal, S.; Mondal, R.; Guha, S.; Chatterjee, U.; Jewrajka, S.K. Homogeneous phase crosslinked poly(acrylonitrile-co-2-acrylamido-2-methyl-1-propanesulfonic acid) conetwork cation exchange membranes showing high electrochemical properties and electrodialysis performance. Polymer 2019, 180, 121680. [Google Scholar] [CrossRef]
- Basavaraja, C.; Jo, E.A.; Kim, B.S.; Kim, D.G.; Huh, D.S. Microwave Absorption of Poly-N-vinylcarbazole-Polyaniline Composites. Polym. Eng. Sci. 2011, 51, 54–61. [Google Scholar] [CrossRef]
- Malmonge, L.F.; Langiano, S.D.C.; Cordeiro, J.M.M.; Mattoso, L.H.C.; Malmonge, J.A. Thermal and mechanical properties of PVDF/PANI blends. Mater. Res. 2010, 13, 465–470. [Google Scholar] [CrossRef] [Green Version]
- Balitaan, J.N.I.; Martin, G.A.V.; Santiago, K.S. Revamping squid gladii to biodegradable composites: In situ grafting of polyaniline to β-chitin and their antibacterial activity. J. Bioact. Compat. Polym. 2021, 36, 13–28. [Google Scholar] [CrossRef]
- Liang, C.; Hou, J.; Li, Y.; Liu, D.; Li, J.; Cui, X.; Duan, Q. Synthesis and self-assembly of brush-shaped block copolymer structure via ATRP and ROP. Opt. Mater. 2021, 111, 110590. [Google Scholar] [CrossRef]
- Shen, J.; Masaoka, H.; Tsuchiya, K.; Ogino, K. Synthesis and Properties of a Novel Brush-type Copolymers Bearing Thiophene Backbone and 3-(N-carbazolyl)propyl Acrylate Side Chains for Light-emitting Applications. Polym. J. 2008, 40, 421–427. [Google Scholar] [CrossRef] [Green Version]
- Crawford, C.B.; Quinn, B. Physiochemical properties and degradation. In Microplastic Pollutants; Elsevier: Amsterdam, The Netherlands, 2017; pp. 57–100. ISBN 9780128094068. [Google Scholar]










| Copolymers | NVK (g) | Macro-RAFT (mg) | Initiator * (mg) | Toluene (mL) | Water (mL) | Hexanol (mL) | Yield (%) |
|---|---|---|---|---|---|---|---|
| PAAMP-b-PVK(A) | 0.16 | 500 | 5 | 1 | 7 | 2 | 82 |
| PAAMP-b-PVK(B) | 0.32 | 500 | 5 | 1 | 7 | 2 | 75 |
| PAAMP-b-PVK(C) | 0.48 | 500 | 5 | 1 | 7 | 2 | 79 |
| Copolymers | Int. H1 | Poly(Ani-AMPS) DP | Int. Hc | PVK DP | PVK, Mn (g/mol) | Mn (g/mol) * |
|---|---|---|---|---|---|---|
| PAAMP-b-PVK(A) | 2 | 89 | 0.75 | 33 | 6449 | 32,358 |
| PAAMP-b-PVK(B) | 2 | 89 | 2.11 | 94 | 18,166 | 44,147 |
| PAAMP-b-PVK(C) | 2 | 89 | 3.25 | 145 | 28,021 | 54,002 |
| Copolymers | [NVK]:[Macro-RAFT]:[I] | Yield (%) | Mn (Theoretical) (g/mol) | Mn (NMR) (g/mol) |
|---|---|---|---|---|
| PAAMP-b-PVK(A) | 41.5:1:0.9 | 80 | 32,396 | 32,358 |
| PAAMP-b-PVK(B) | 83.0:1:0.9 | 75 | 38,010 | 44,147 |
| PAAMP-b-PVK(C) | 124.5:1:0.9 | 79 | 44,987 | 54,002 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Conejo-Dávila, A.S.; Casas-Soto, C.R.; Aparicio-Martínez, E.P.; Chávez-Flores, D.; Ramos-Sánchez, V.H.; Dominguez, R.B.; Osuna, V.C.; Estrada-Monje, A.; Vega-Rios, A.; Zaragoza-Contreras, E.A. Brush-like Polyaniline with Optical and Electroactive Properties at Neutral pH and High Temperature. Int. J. Mol. Sci. 2022, 23, 8085. https://doi.org/10.3390/ijms23158085
Conejo-Dávila AS, Casas-Soto CR, Aparicio-Martínez EP, Chávez-Flores D, Ramos-Sánchez VH, Dominguez RB, Osuna VC, Estrada-Monje A, Vega-Rios A, Zaragoza-Contreras EA. Brush-like Polyaniline with Optical and Electroactive Properties at Neutral pH and High Temperature. International Journal of Molecular Sciences. 2022; 23(15):8085. https://doi.org/10.3390/ijms23158085
Chicago/Turabian StyleConejo-Dávila, Alain Salvador, Carlos Rafael Casas-Soto, Eider Pedro Aparicio-Martínez, David Chávez-Flores, Víctor Hugo Ramos-Sánchez, Rocio Berenice Dominguez, Velia Carolina Osuna, Anayansi Estrada-Monje, Alejandro Vega-Rios, and Erasto Armando Zaragoza-Contreras. 2022. "Brush-like Polyaniline with Optical and Electroactive Properties at Neutral pH and High Temperature" International Journal of Molecular Sciences 23, no. 15: 8085. https://doi.org/10.3390/ijms23158085
APA StyleConejo-Dávila, A. S., Casas-Soto, C. R., Aparicio-Martínez, E. P., Chávez-Flores, D., Ramos-Sánchez, V. H., Dominguez, R. B., Osuna, V. C., Estrada-Monje, A., Vega-Rios, A., & Zaragoza-Contreras, E. A. (2022). Brush-like Polyaniline with Optical and Electroactive Properties at Neutral pH and High Temperature. International Journal of Molecular Sciences, 23(15), 8085. https://doi.org/10.3390/ijms23158085