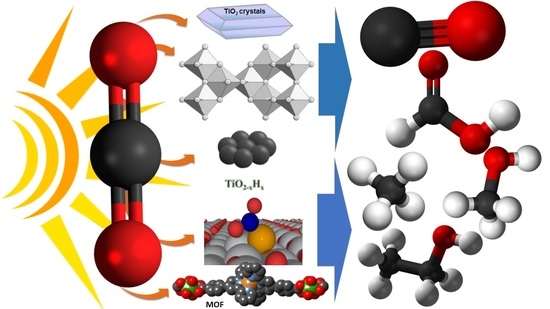
Photocatalytic Carbon Dioxide Conversion by Structurally and Materially Modified Titanium Dioxide Nanostructures
Abstract
:1. Introduction
2. Strategy I: Crystal Facet Engineering of TiO2 Photocatalyst
3. Strategy II: Nanostructured TiO2
3.1. Zero-Dimensional Nanostructured TiO2
3.2. One-Dimensional Nanostructured TiO2
3.3. Two-Dimensional Nanostructured TiO2
- First and foremost, 2D materials possess larger surface-to-volume ratio over their bulk counterparts [94]. Hence, 2D materials have more active sites on their surface that can enhance their photocatalytic performance significantly.
- Third, the high fraction of coordinated unsaturated centers can work as active centers and interact with the substrate intimately [96].
4. Strategy III: Formation of the Junction with TiO2
4.1. A Semiconductor-Semiconductor Heterojunction
4.2. Semiconductor-Metal Heterojunction
4.3. Semiconductor-Carbon Heterojunction
5. Strategy IV: Modified TiO2 Nanostructures by Hydrogenation
6. Strategy V: Single Atom Photocatalysts
7. Strategy VI: Metal Organic Framework
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Mardani, A.; Streimikiene, D.; Cavallaro, F.; Loganathan, N.; Khoshnoudi, M. Carbon dioxide (CO2) emissions and economic growth: A systematic review of two decades of research from 1995 to 2017. Sci. Total Environ. 2017, 649, 31–49. [Google Scholar] [CrossRef] [PubMed]
- Pipes, R.; Bhargav, A.; Manthiram, A. Phenyl Disulfide Additive for Solution-Mediated Carbon Dioxide Utilization in Li−CO2 Batteries. Adv. Energy Mater. 2019, 9, 1900453. [Google Scholar] [CrossRef]
- Song, C.; Liu, Q.; Deng, S.; Li, H.; Kitamura, Y. Cryogenicbased CO2 capture technologies: State-of-the-art developments and current challenges. Renew. Sustain. Energy Rev. 2019, 101, 265–278. [Google Scholar] [CrossRef]
- Duan, Y.-X.; Meng, F.-L.; Liu, K.-H.; Yi, S.-S.; Li, S.-J.; Yan, J.-M.; Jiang, Q. Amorphizing of Cu nanoparticles toward highly efficient and robust electrocatalyst for CO2 reduction to liquid fuels with high faradaic efficiencies. Adv. Mater. 2018, 30, 1706194. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Ma, T.; Tao, H.; Fan, Q.; Han, B. Fundamentals and challenges of electrochemical CO2 reduction using two-dimensional materials. Chem 2017, 3, 560–587. [Google Scholar] [CrossRef] [Green Version]
- Zhang, N.; Long, R.; Gao, C.; Xiong, Y. Recent progress on advanced design for photoelectrochemical reduction of CO2 to fuels. Sci. China Mater. 2018, 61, 771–805. [Google Scholar] [CrossRef] [Green Version]
- Yang, D.; Yu, H.; He, T.; Zuo, S.; Liu, X.; Yang, H.; Ni, B.; Li, H.; Gu, L.; Wang, D.; et al. Visible-light-switched electron transfer over single porphyrin-metal atom center for highly selective electroreduction of carbon dioxide. Nat. Commun. 2019, 10, 3844. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Kang, F.; Hu, C.; Wang, L.; Xu, Z.; Zheng, D.; Gong, W.; Lu, Y.; Ma, Y.; Wang, J. A genetically encoded photosensitizer protein facilitates the rational design of a miniature photocatalytic CO2-reducing enzyme. Nat. Chem. 2018, 10, 1201. [Google Scholar] [CrossRef]
- Meng, A.; Zhang, L.; Cheng, B.; Yu, J. TiO2−MnOx−Pt Hybrid Multiheterojunction Film Photocatalyst with Enhanced Photocatalytic CO2-Reduction Activity. ACS Appl. Mater. Interfaces 2019, 11, 5581–5589. [Google Scholar] [CrossRef]
- Lisovskaya, A.; Bartels, D.M. Reduction of CO2 by hydrated electrons in high temperature water. Radiat. Phys. Chem. 2019, 158, 61–63. [Google Scholar] [CrossRef]
- Ferrah, D.; Haines, A.R.; Galhenage, R.P.; Bruce, J.P.; Babore, A.D.; Hunt, A.; Waluyo, I.; Hemminger, J.C. Wet Chemical Growth and Thermocatalytic Activity of Cu-Based Nanoparticles Supported on TiO2 Nanoparticles/HOPG: In Situ Ambient Pressure XPS Study of the CO2 Hydrogenation Reaction. ACS Catal. 2019, 9, 6783–6802. [Google Scholar] [CrossRef]
- Huang, J.; Buonsanti, R. Colloidal nanocrystals as heterogeneous catalysts for electrochemical CO2 conversion. Chem. Mater. 2019, 31, 13–25. [Google Scholar] [CrossRef] [Green Version]
- Kang, M.J.; Kim, C.W.; Pawar, A.U.; Cha, H.G.; Ji, S.; Cai, W.-B.; Kang, Y.S. Selective Alcohol on Dark Cathode by Photoelectrochemical CO2 Valorization and Their in-situ Characterization. ACS Energy Lett. 2019, 4, 1549–1555. [Google Scholar] [CrossRef]
- Ji, Y.; Luo, Y. Direct Donation of Protons from H2O to CO2 in Artificial Photosynthesis on the Anatase TiO2 (101) Surface. J. Phys. Chem. Commun. 2019, 123, 3019–3023. [Google Scholar] [CrossRef]
- Xu, S.; Carter, E.A. Theoretical insights into heterogeneous (Photo) electrochemical CO2 reduction. Chem. Rev. 2019, 119, 6631–6669. [Google Scholar] [CrossRef]
- Raizada, P.; Soni, V.; Kumar, A.; Singh, P.; Khan, A.A.P.; Asiri, A.M.; Thakur, V.K.; Nguyen, V.-H. Surface defect engineering of metal oxides photocatalyst for energy application and water treatment. J. Mater. 2021, 7, 388–418. [Google Scholar] [CrossRef]
- Wang, P.; Wang, S.; Wang, H.; Wu, Z.; Wang, L. Recent progress on photo-electrocatalytic reduction of carbon dioxide. Part. Part. Syst. Charact. 2018, 35, 1700371. [Google Scholar] [CrossRef]
- Wang, L.; Chen, W.; Zhang, D.; Du, Y.; Amal, R.; Qiao, S.; Wu, J.; Yin, Z. Surface strategies for catalytic CO2 reduction: From two-dimensional materials to nanoclusters to single atoms. Chem. Soc. Rev. 2019, 48, 5310–5349. [Google Scholar] [CrossRef]
- Kong, L.; Qiao, J.; Ruan, Q.; Wang, H.; Xi, X.; Zha, W.; Zhou, Z.; He, W.; Zhang, W.; Sun, Z. A very low charge potential for zinc-air battery promoted by photochemical effect of triazine-based conjugated polymer nanolayer coated TiO2. J. Power Sources 2022, 536, 231507. [Google Scholar] [CrossRef]
- Pawar, A.U.; Kim, C.W.; Nguyen-Le, M.-T.; Kang, Y.S. General review on the components and parameters of photoelectrochemical system for CO2 reduction with in situ analysis. ACS Sustain. Chem. Eng. 2019, 7, 7431–7455. [Google Scholar] [CrossRef]
- Inoue, T.; Fujishima, A.; Konishi, S.; Honda, K. Photoelectrocatalytic reduction of carbon dioxide in aqueous suspensions of semiconductor powders. Nature 1979, 277, 637–638. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Tamgadge, R.M.; Shukla, A. Fluorine-doped anatase for improved supercapacitor electrode. Electrochim. Acta 2018, 289, 342–353. [Google Scholar] [CrossRef]
- Hussain, I.; Chowdhury, A.R.; Jaksik, J.; Grissom, G.; Touhami, A.; Ibrahim, E.E.; Schauer, M.; Okoli, O.; Uddin, M.J. Conductive glass free carbon nanotube micro yarn based perovskite solar cells. Appl. Surf. Sci. 2019, 478, 327–333. [Google Scholar] [CrossRef]
- Mino, L.; Ferrari, A.M.; Lacivita, V.; Spoto, G.; Bordiga, S.; Zecchina, A. CO adsorption on anatase nanocrystals: A combined experimental and periodic DFT study. J. Phys. Chem. C 2011, 115, 7694–7700. [Google Scholar] [CrossRef]
- Das, S.; Dhara, S. Chemical Solution Synthesis for Materials Design and Thin Film Device Applications; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Mino, L.; Zecchina, A.; Martra, G.; Rossi, A.M.; Spoto, G. A surface science approach to TiO2 P25 photocatalysis: An in situ FTIR study of phenol photodegradation at controlled water coverages from sub-monolayer to multilayer. Appl. Catal. B Environ. 2016, 196, 135–141. [Google Scholar] [CrossRef]
- Li, G.; Fang, K.; Ou, Y.; Yuan, W.; Yang, H.; Zhang, Z.; Wang, Y. Surface study of the reconstructed anatase TiO2 (001) surface. Prog. Nat. Sci. Mater. Int. 2021, 31, 1–13. [Google Scholar] [CrossRef]
- Liu, Y.; Du, Y.E.; Bai, Y.; An, J.; Li, J.; Yang, X.; Feng, Q. Facile Synthesis of {101},{010} and [111]-Faceted Anatase-TiO2 Nanocrystals Derived from Porous Metatitanic Acid H2TiO3 for Enhanced Photocatalytic Performance. ChemistrySelect 2018, 3, 2867–2876. [Google Scholar] [CrossRef]
- Mollavali, M.; Rohani, S.; Elahifard, M.; Behjatmanesh-Ardakani, R.; Nourany, M. Band gap reduction of (Mo+ N) co-doped TiO2 nanotube arrays with a significant enhancement in visible light photo-conversion: A combination of experimental and theoretical study. Int. J. Hydrog. Energy 2021, 46, 21475–21498. [Google Scholar] [CrossRef]
- Grissom, G.; Jaksik, J.; McEntee, M.; Durke, E.M.; Aishee, S.T.; Cua, M.; Okoli, O.; Touhami, A.; Moore, H.J.; Uddin, M.J. Three-dimensional carbon nanotube yarn based solid state solar cells with multiple sensitizers exhibit high energy conversion efficiency. Solar Energy 2018, 171, 16–22. [Google Scholar] [CrossRef]
- Likodimos, V. Photonic crystal-assisted visible light activated TiO2 photocatalysis. Appl. Catal. B Environ. 2018, 230, 269–303. [Google Scholar] [CrossRef]
- Cravanzola, S.; Cesano, F.; Gaziano, F.; Scarano, D. Sulfur-doped TiO2: Structure and surface properties. Catalysts 2017, 7, 214. [Google Scholar] [CrossRef] [Green Version]
- Humayun, M.; Raziq, F.; Khan, A.; Luo, W. Modification strategies of TiO2 for potential applications in photocatalysis: A critical review. Green Chem. Lett. Rev. 2018, 11, 86–102. [Google Scholar] [CrossRef] [Green Version]
- Uddin, M.J.; Daramola, D.E.; Velasquez, E.; Dickens, T.J.; Yan, J.; Hammel, E.; Cesano, F.; Okoli, O.I. A high efficiency 3D photovoltaic microwire with carbon nanotubes (CNT)-quantum dot (QD) hybrid interface. Phys. Status Solidi (RRL)–Rapid Res. Lett. 2014, 8, 898–903. [Google Scholar] [CrossRef]
- Jia, S.; Li, X.; Zhang, B.; Yang, J.; Zhang, S.; Li, S.; Zhang, Z. TiO2/CuS heterostructure nanowire array photoanodes toward water oxidation: The role of CuS. Appl. Surf. Sci. 2019, 463, 829–837. [Google Scholar] [CrossRef]
- Uddin, M.J.; Cesano, F.; Chowdhury, A.R.; Trad, T.; Cravanzola, S.; Martra, G.; Mino, L.; Zecchina, A.; Scarano, D. Surface structure and phase composition of TiO2 P25 particles after thermal treatments and HF etching. Front. Mater. 2020, 7, 192. [Google Scholar] [CrossRef]
- Peng, Y.-K.; Chou, H.-L.; Tsang, S.C.E. Differentiating surface titanium chemical states of anatase TiO2 functionalized with various groups. Chem. Sci. 2018, 9, 2493–2500. [Google Scholar] [CrossRef] [Green Version]
- Zou, Y.; Gao, G.; Wang, Z.; Shi, J.-W.; Wang, H.; Ma, D.; Fan, Z.; Chen, X.; Wang, Z.; Niu, C. Formation mechanism of rectangular-ambulatory-plane TiO2 plates: An insight into the role of hydrofluoric acid. Chem. Commun. 2018, 54, 7191–7194. [Google Scholar] [CrossRef]
- Ma, M.J.; Li, W.; Dambournet, D. Solution-Based Synthesis of Nano-Sized TiO2 Anatase in Fluorinating Media. In Modern Synthesis Processes and Reactivity of Fluorinated Compounds; Elsevier: Amsterdam, The Netherlands, 2017; pp. 651–669. [Google Scholar]
- Wang, A.; Wu, S.; Dong, J.; Wang, R.; Wang, J.; Zhang, J.; Zhong, S.; Bai, S. Interfacial facet engineering on the Schottky barrier between plasmonic Au and TiO2 in boosting the photocatalytic CO2 reduction under ultraviolet and visible light irradiation. Chem. Eng. J. 2021, 404, 127145. [Google Scholar] [CrossRef]
- Dong, J.; Wang, Z.; Cao, H.; Xue, J.; Liu, C.; Sun, S.; Gao, C.; Zhu, X.; Bao, J. Independent Cr2O3 functions as efficient cocatalyst on the crystal facets engineered TiO2 for photocatalytic CO2 reduction. Appl. Surf. Sci. 2021, 554, 149634. [Google Scholar] [CrossRef]
- Butburee, T.; Kotchasarn, P.; Hirunsit, P.; Sun, Z.; Tang, Q.; Khemthong, P.; Sangkhun, W.; Thongsuwan, W.; Kumnorkaew, P.; Wang, H. New understanding of crystal control and facet selectivity of titanium dioxide ruling photocatalytic performance. J. Mater. Chem. A 2019, 7, 8156–8166. [Google Scholar] [CrossRef]
- Li, X. Synthesis and Metal-Insulator Transition Properties of Vanadium Dioxide Nanostructures; The Pennsylvania State University: State College, PA, USA, 2019. [Google Scholar]
- Du, Y.-E.; Niu, X.; He, X.; Hou, K.; Liu, H.; Zhang, C. Synthesis and photocatalytic activity of TiO2/CdS nanocomposites with co-exposed anatase highly reactive facets. Molecules 2021, 26, 6031. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.-E.; Niu, X.; He, J.; Liu, L.; Liu, Y.; Chen, C.; Yang, X.; Feng, Q. Hollow square rodlike microtubes composed of anatase nanocuboids with coexposed {100},{010}, and {001} facets for improved photocatalytic performance. ACS Omega 2020, 5, 14147–14156. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.Z.; Zhou, J.Z.; Jiang, H.B.; Hu, Q.H.; Qiao, S.Z.; Yang, H.G. Synthesis of micro-sized titanium dioxide nanosheets wholly exposed with high-energy {001} and {100} facets. Chem. Commun. 2011, 47, 4400–4402. [Google Scholar] [CrossRef] [Green Version]
- Li, W. Sol-Gel Synthesis of TiO2 Anatase in a Fluorinated Medium and Its Applications as Negative Electrode for Li+ and Na+ Batteries. Ph.D. Thesis, Paris 6, Doctoral School of Physical Chemistry and Analytical Chemistry of Paris Center, Paris, France, 2015. [Google Scholar]
- Bokare, A.; Erogbogbo, F. Photocatalysis and Li-Ion Battery Applications of {001} Faceted Anatase TiO2-Based Composites. J 2021, 4, 500–530. [Google Scholar] [CrossRef]
- Du, D.-J.; Du, Y.-E.; Yue, W.-B.; Yang, X.-J. Lithium storage performance of {010}-faceted and [111]-faceted anatase TiO2 nanocrystals. J. Cent. South Univ. 2019, 26, 1530–1539. [Google Scholar] [CrossRef]
- Maitani, M.M.; Tateyama, A.; Boix, P.P.; Han, G.; Nitta, A.; Ohtani, B.; Mathews, N.; Wada, Y. Effects of energetics with {001} facet-dominant anatase TiO2 scaffold on electron transport in CH3NH3PbI3 perovskite solar cells. Electrochim. Acta 2019, 300, 445–454. [Google Scholar] [CrossRef]
- Liu, X.; Du, G.; Li, M. True photoreactivity origin of Ti3+-doped anatase TiO2 crystals with respectively dominated exposed {001},{101}, and {100} facets. ACS Omega 2019, 4, 14902–14912. [Google Scholar] [CrossRef] [Green Version]
- Liu, G.; Wang, T.; Zhou, W.; Meng, X.; Zhang, H.; Liu, H.; Kako, T.; Ye, J. Crystal-facet-dependent hot-electron transfer in plasmonic-Au/semiconductor heterostructures for efficient solar photocatalysis. J. Mater. Chem. C 2015, 3, 7538–7542. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Liu, Z.; Liu, A.; Gu, X.; Ge, C.; Gao, F.; Dong, L. Engineering the TiO2–graphene interface to enhance photocatalytic H2 production. ChemSusChem 2014, 7, 618–626. [Google Scholar] [CrossRef]
- Pathakoti, K.; Manubolu, M.; Hwang, H.-M. Nanotechnology applications for environmental industry. In Handbook of Nanomaterials for Industrial Applications; Elsevier: Amsterdam, The Netherlands, 2018; pp. 894–907. [Google Scholar]
- Kozak, M.; Mazierski, P.; Żebrowska, J.; Kobylański, M.; Klimczuk, T.; Lisowski, W.; Trykowski, G.; Nowaczyk, G.; Zaleska-Medynska, A. Electrochemically obtained TiO2/CuxOy nanotube arrays presenting a photocatalytic response in processes of pollutants degradation and bacteria inactivation in aqueous phase. Catalysts 2018, 8, 237. [Google Scholar] [CrossRef] [Green Version]
- Qu, J.; Sha, L.; Wu, C.; Zhang, Q. Applications of mechanochemically prepared layered double hydroxides as adsorbents and catalysts: A mini-review. Nanomaterials 2019, 9, 80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xue, Y.; Wu, Z.; He, X.; Yang, X.; Chen, X.; Gao, Z. Constructing a Z-scheme heterojunction of egg-like core@ shell CdS@ TiO2 photocatalyst via a facile reflux method for enhanced photocatalytic performance. Nanomaterials 2019, 9, 222. [Google Scholar] [CrossRef] [Green Version]
- Shahvaranfard, F. Modification of Low Dimensional Nanostructured TiO2 for Energy Application. Ph.D. Thesis, Friedrich-Alexander-Universität Erlangen-Nürnberg (FAU), Erlangen, Germany, 2022. [Google Scholar]
- Lee, K.; Mazare, A.; Schmuki, P. One-dimensional titanium dioxide nanomaterials: Nanotubes. Chem. Rev. 2014, 114, 9385–9454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haider, A.J.; Jameel, Z.N.; Al-Hussaini, I.H. Review on: Titanium dioxide applications. Energy Procedia 2019, 157, 17–29. [Google Scholar] [CrossRef]
- Ranjan, S.; Ramalingam, C. Titanium dioxide nanoparticles induce bacterial membrane rupture by reactive oxygen species generation. Environ. Chem. Lett. 2016, 14, 487–494. [Google Scholar] [CrossRef]
- Liu, Q.; Huang, J.; Tang, H.; Yu, X.; Shen, J. Construction 0D TiO2 nanoparticles/2D CoP nanosheets heterojunctions for enhanced photocatalytic H2 evolution activity. J. Mater. Sci. Technol. 2020, 56, 196–205. [Google Scholar] [CrossRef]
- Tahir, M.; Amin, N.S. Indium-doped TiO2 nanoparticles for photocatalytic CO2 reduction with H2O vapors to CH4. Appl. Catal. B Environ. 2015, 162, 98–109. [Google Scholar] [CrossRef]
- Wada, K.; Ranasinghe, C.S.K.; Kuriki, R.; Yamakata, A.; Ishitani, O.; Maeda, K. Interfacial manipulation by rutile TiO2 nanoparticles to boost CO2 reduction into CO on a metal-complex/semiconductor hybrid photocatalyst. ACS Appl. Mater. Interfaces 2017, 9, 23869–23877. [Google Scholar] [CrossRef]
- Tseng, I.-H.; Chang, W.-C.; Wu, J.C. Photoreduction of CO2 using sol–gel derived titania and titania-supported copper catalysts. Appl. Catal. B Environ. 2002, 37, 37–48. [Google Scholar] [CrossRef]
- Wang, Y.; Lai, Q.; Zhang, F.; Shen, X.; Fan, M.; He, Y.; Ren, S. High efficiency photocatalytic conversion of CO2 with H2O over Pt/TiO2 nanoparticles. RSC Adv. 2014, 4, 44442–44451. [Google Scholar] [CrossRef]
- Liu, Y.; Lee, C.-C.; Horn, M.W.; Lee, H. Toward efficient photocatalysts for light-driven CO2 reduction: TiO2 nanostructures decorated with perovskite quantum dots. Nano Express 2021, 2, 020003. [Google Scholar] [CrossRef]
- Xu, Y.-F.; Yang, M.-Z.; Chen, B.-X.; Wang, X.-D.; Chen, H.-Y.; Kuang, D.-B.; Su, C.-Y. A CsPbBr3 perovskite quantum dot/graphene oxide composite for photocatalytic CO2 reduction. J. Am. Chem. Soc. 2017, 139, 5660–5663. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.; Yang, S.; Chen, R.; Zhu, X.; Liao, Q.; Huang, Y. Copper-decorated TiO2 nanorod thin films in optofluidic planar reactors for efficient photocatalytic reduction of CO2. Int. J. Hydrog. Energy 2017, 42, 9722–9732. [Google Scholar] [CrossRef]
- Devi, A.D.; Pushpavanam, S.; Singh, N.; Verma, J.; Kaur, M.P.; Roy, S.C. Enhanced methane yield by photoreduction of CO2 at moderate temperature and pressure using Pt coated, graphene oxide wrapped TiO2 nanotubes. Results Eng. 2022, 14, 100441. [Google Scholar] [CrossRef]
- Rambabu, Y.; Kumar, U.; Singhal, N.; Kaushal, M.; Jaiswal, M.; Jain, S.L.; Roy, S.C. Photocatalytic reduction of carbon dioxide using graphene oxide wrapped TiO2 nanotubes. Appl. Surf. Sci. 2019, 485, 48–55. [Google Scholar] [CrossRef]
- Ouyang, W.; Teng, F.; He, J.H.; Fang, X. Enhancing the photoelectric performance of photodetectors based on metal oxide semiconductors by charge-carrier engineering. Adv. Funct. Mater. 2019, 29, 1807672. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Z.; Jiang, X.; Tao, J.; Gong, Z.; Cheng, Y.; Zhang, M.; Yang, L.; Lv, J.; He, G. Silver-decorated TiO2 nanorod array films with enhanced photoelectrochemical and photocatalytic properties. J. Electrochem. Soc. 2016, 163, H943. [Google Scholar] [CrossRef]
- Qu, J.; Lai, C. One-dimensional nanostructures as photoanodes for dye-sensitized solar cells. J. Nanomater. 2013, 2013, 2. [Google Scholar] [CrossRef]
- Subramanian, A.; Pan, Z.; Li, H.; Zhou, L.; Li, W.; Qiu, Y.; Xu, Y.; Hou, Y.; Muzi, C.; Zhang, Y. Synergistic promotion of photoelectrochemical water splitting efficiency of TiO2 nanorods using metal-semiconducting nanoparticles. Appl. Surf. Sci. 2017, 420, 631–637. [Google Scholar] [CrossRef]
- Jiang, L.; He, J.; Yang, Y.; Mao, D.; Chen, D.; Wang, W.; Chen, Y.; Sharma, V.K.; Wang, J. Enhancing visible-light photocatalytic activity of hard-biotemplated TiO2: From macrostructural morphology replication to microstructural building units design. J. Alloys Compd. 2022, 898, 162886. [Google Scholar] [CrossRef]
- Ghosh, M.; Liu, J.; Chuang, S.S.; Jana, S.C. Fabrication of hierarchical V2O5 nanorods on TiO2 nanofibers and their enhanced photocatalytic activity under visible light. ChemCatChem 2018, 10, 3305–3318. [Google Scholar] [CrossRef]
- Attar, A.S.; Ghamsari, M.S.; Hajiesmaeilbaigi, F.; Mirdamadi, S.; Katagiri, K.; Koumoto, K. Sol–gel template synthesis and characterization of aligned anatase-TiO2 nanorod arrays with different diameter. Mater. Chem. Phys. 2009, 113, 856–860. [Google Scholar] [CrossRef]
- Wang, W.-N.; An, W.-J.; Ramalingam, B.; Mukherjee, S.; Niedzwiedzki, D.M.; Gangopadhyay, S.; Biswas, P. Size and structure matter: Enhanced CO2 photoreduction efficiency by size-resolved ultrafine Pt nanoparticles on TiO2 single crystals. J. Am. Chem. Soc. 2012, 134, 11276–11281. [Google Scholar] [CrossRef]
- Ping, G.; Wang, C.; Chen, D.; Liu, S.; Huang, X.; Qin, L.; Huang, Y.; Shu, K. Fabrication of self-organized TiO2 nanotube arrays for photocatalytic reduction of CO2. J. Solid State Electrochem. 2013, 17, 2503–2510. [Google Scholar] [CrossRef]
- Feng, X.; Sloppy, J.D.; LaTempa, T.J.; Paulose, M.; Komarneni, S.; Bao, N.; Grimes, C.A. Synthesis and deposition of ultrafine Pt nanoparticles within high aspect ratio TiO2 nanotube arrays: Application to the photocatalytic reduction of carbon dioxide. J. Mater. Chem. 2011, 21, 13429–13433. [Google Scholar] [CrossRef]
- Li, X.; Liu, H.; Luo, D.; Li, J.; Huang, Y.; Li, H.; Fang, Y.; Xu, Y.; Zhu, L. Adsorption of CO2 on heterostructure CdS (Bi2S3)/TiO2 nanotube photocatalysts and their photocatalytic activities in the reduction of CO2 to methanol under visible light irradiation. Chem. Eng. J. 2012, 180, 151–158. [Google Scholar] [CrossRef]
- Li, Y.; Wang, C.; Song, M.; Li, D.; Zhang, X.; Liu, Y. TiO2−x/CoOx photocatalyst sparkles in photothermocatalytic reduction of CO2 with H2O steam. Appl. Catal. B Environ. 2019, 243, 760–770. [Google Scholar] [CrossRef]
- Tahir, M.; Tahir, B.; Amin, N.A.S. Gold-nanoparticle-modified TiO2 nanowires for plasmon-enhanced photocatalytic CO2 reduction with H2 under visible light irradiation. Appl. Surf. Sci. 2015, 356, 1289–1299. [Google Scholar] [CrossRef]
- Tahir, M.; Tahir, B.; Amin, N.A.S.; Zakaria, Z.Y. Photo-induced reduction of CO2 to CO with hydrogen over plasmonic Ag-NPs/TiO2 NWs core/shell hetero-junction under UV and visible light. J. CO2 Util. 2017, 18, 250–260. [Google Scholar] [CrossRef]
- Low, J.; Qiu, S.; Xu, D.; Jiang, C.; Cheng, B. Direct evidence and enhancement of surface plasmon resonance effect on Ag-loaded TiO2 nanotube arrays for photocatalytic CO2 reduction. Appl. Surf. Sci. 2018, 434, 423–432. [Google Scholar] [CrossRef]
- Su, K.-Y.; Chen, C.-Y.; Wu, R.-J. Preparation of Pd/TiO2 nanowires for the photoreduction of CO2 into renewable hydrocarbon fuels. J. Taiwan Inst. Chem. Eng. 2019, 96, 409–418. [Google Scholar] [CrossRef]
- Song, G.; Xin, F.; Yin, X. Photocatalytic reduction of carbon dioxide over ZnFe2O4/TiO2 nanobelts heterostructure in cyclohexanol. J. Colloid Interface Sci. 2015, 442, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Razzaq, A.; Grimes, C.A.; In, S.-I. Facile fabrication of a noble metal-free photocatalyst: TiO2 nanotube arrays covered with reduced graphene oxide. Carbon 2016, 98, 537–544. [Google Scholar] [CrossRef]
- Zhang, H. Ultrathin two-dimensional nanomaterials. ACS Nano 2015, 9, 9451–9469. [Google Scholar] [CrossRef]
- Madkour, L.H. Carbon Nanomaterials and Two-Dimensional Transition Metal Dichalcogenides (2D TMDCs). In Nanoelectronic Materials; Springer: Berlin/Heidelberg, Germany, 2019; pp. 165–245. [Google Scholar]
- Tan, C.; Cao, X.; Wu, X.-J.; He, Q.; Yang, J.; Zhang, X.; Chen, J.; Zhao, W.; Han, S.; Nam, G.-H. Recent advances in ultrathin two-dimensional nanomaterials. Chem. Rev. 2017, 117, 6225–6331. [Google Scholar] [CrossRef]
- Late, D.J.; Bhat, A.; Rout, C.S. Fundamentals and properties of 2D materials in general and sensing applications. In Fundamentals and Sensing Applications of 2D Materials; Elsevier: Amsterdam, The Netherlands, 2019; pp. 5–24. [Google Scholar]
- Chen, F.; Ma, T.; Zhang, T.; Zhang, Y.; Huang, H. Atomic-level charge separation strategies in semiconductor-based photocatalysts. Adv. Mater. 2021, 33, 2005256. [Google Scholar] [CrossRef]
- Liu, Y.; Zou, J.; Guo, B.; Ren, Y.; Wang, Z.; Song, Y.; Yu, Y.; Wu, L. Selective photocatalytic oxidation of thioanisole on DUT-67 (Zr) mediated by surface coordination. Langmuir 2020, 36, 2199–2208. [Google Scholar] [CrossRef]
- Sadeghfar, F.; Zalipour, Z.; Taghizadeh, M.; Taghizadeh, A.; Ghaedi, M. Photodegradation processes. In Interface Science and Technology; Elsevier: Amsterdam, The Netherlands, 2021; Volume 32, pp. 55–124. [Google Scholar]
- Tu, W.; Zhou, Y.; Liu, Q.; Yan, S.; Bao, S.; Wang, X.; Xiao, M.; Zou, Z. An in situ simultaneous reduction-hydrolysis technique for fabrication of TiO2-graphene 2D sandwich-like hybrid nanosheets: Graphene-promoted selectivity of photocatalytic-driven hydrogenation and coupling of CO2 into methane and ethane. Adv. Funct. Mater. 2013, 23, 1743–1749. [Google Scholar] [CrossRef]
- Zhou, S.; Liu, Y.; Li, J.; Wang, Y.; Jiang, G.; Zhao, Z.; Wang, D.; Duan, A.; Liu, J.; Wei, Y. Facile in situ synthesis of graphitic carbon nitride (g-C3N4)-N-TiO2 heterojunction as an efficient photocatalyst for the selective photoreduction of CO2 to CO. Appl. Catal. B Environ. 2014, 158, 20–29. [Google Scholar] [CrossRef]
- Crake, A.; Christoforidis, K.C.; Godin, R.; Moss, B.; Kafizas, A.; Zafeiratos, S.; Durrant, J.R.; Petit, C. Titanium dioxide/carbon nitride nanosheet nanocomposites for gas phase CO2 photoreduction under UV-visible irradiation. Appl. Catal. B Environ. 2019, 242, 369–378. [Google Scholar] [CrossRef]
- Qamar, S.; Lei, F.; Liang, L.; Gao, S.; Liu, K.; Sun, Y.; Ni, W.; Xie, Y. Ultrathin TiO2 flakes optimizing solar light driven CO2 reduction. Nano Energy 2016, 26, 692–698. [Google Scholar] [CrossRef]
- Liu, Y.; Miao, C.; Yang, P.; He, Y.; Feng, J.; Li, D. Synergetic promotional effect of oxygen vacancy-rich ultrathin TiO2 and photochemical induced highly dispersed Pt for photoreduction of CO2 with H2O. Appl. Catal. B Environ. 2019, 244, 919–930. [Google Scholar] [CrossRef]
- Low, J.; Zhang, L.; Tong, T.; Shen, B.; Yu, J. TiO2/MXene Ti3C2 composite with excellent photocatalytic CO2 reduction activity. J. Catal. 2018, 361, 255–266. [Google Scholar] [CrossRef]
- Yuan, L.; Lu, K.-Q.; Zhang, F.; Fu, X.; Xu, Y.-J. Unveiling the interplay between light-driven CO2 photocatalytic reduction and carbonaceous residues decomposition: A case study of Bi2WO6-TiO2 binanosheets. Appl. Catal. B Environ. 2018, 237, 424–431. [Google Scholar] [CrossRef]
- Reddy, K.M.; Manorama, S.V.; Reddy, A.R. Bandgap studies on anatase titanium dioxide nanoparticles. Mater. Chem. Phys. 2003, 78, 239–245. [Google Scholar] [CrossRef]
- Munir, S.; Shah, S.M.; Hussain, H. Effect of carrier concentration on the optical band gap of TiO2 nanoparticles. Mater. Des. 2016, 92, 64–72. [Google Scholar] [CrossRef]
- Wang, X.; Li, Z.; Shi, J.; Yu, Y. One-dimensional titanium dioxide nanomaterials: Nanowires, nanorods, and nanobelts. Chem. Rev. 2014, 114, 9346–9384. [Google Scholar] [CrossRef]
- Lee, M.; Seo, Y.; Shin, H.S.; Jo, C.; Ryoo, R. Anatase TiO2 nanosheets with surface acid sites for Friedel–Crafts alkylation. Microporous Mesoporous Mater. 2016, 222, 185–191. [Google Scholar] [CrossRef]
- Xu, C.; Zhang, Z.; Zhang, S.; Si, H.; Ma, S.; Fan, W.; Xiong, Z.; Liao, Q.; Sattar, A.; Kang, Z. Manipulation of perovskite crystallization kinetics via Lewis base additives. Adv. Funct. Mater. 2021, 31, 2009425. [Google Scholar] [CrossRef]
- Sheng, Y.; Wei, Z.; Miao, H.; Yao, W.; Li, H.; Zhu, Y. Enhanced organic pollutant photodegradation via adsorption/photocatalysis synergy using a 3D g-C3N4/TiO2 free-separation photocatalyst. Chem. Eng. J. 2019, 370, 287–294. [Google Scholar] [CrossRef]
- Yang, K.; Cheng, G.; Chen, R.; Zhao, K.; Liang, Y.; Li, W.; Han, C. Simply Coupling TiO2 Nanospheres with Cu2O Particles to Boost the Photocatalytic Hydrogen Evolution through p–n Heterojunction-Induced Charge Transfer. Energy Technol. 2022, 10, 2100259. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, X.; Yang, Y.; Li, J.; Liu, W.; Shen, Y.; Tian, X. Photocatalytic Hydrogen Evolution Using Ternary-Metal-Sulfide/TiO2 Heterojunction Photocatalysts. ChemCatChem 2022, 14, e202101439. [Google Scholar] [CrossRef]
- Guo, Q.; Huang, Y.; Xu, H.; Luo, D.; Huang, F.; Gu, L.; Wei, Y.; Zhao, H.; Fan, L.; Wu, J. The effects of solvent on photocatalytic properties of Bi2WO6/TiO2 heterojunction under visible light irradiation. Solid State Sci. 2018, 78, 95–106. [Google Scholar] [CrossRef]
- Shang, M.; Wang, W.; Zhang, L.; Sun, S.; Wang, L.; Zhou, L. 3D Bi2WO6/TiO2 hierarchical heterostructure: Controllable synthesis and enhanced visible photocatalytic degradation performances. J. Phys. Chem. C 2009, 113, 14727–14731. [Google Scholar] [CrossRef]
- Li, Q.; Zong, L.; Li, C.; Yang, J. Reprint of “Photocatalytic reduction of CO2 on MgO/TiO2 nanotube films”. Appl. Surf. Sci. 2014, 319, 16–20. [Google Scholar] [CrossRef]
- Ran, H.; Fan, J.; Zhang, X.; Mao, J.; Shao, G. Enhanced performances of dye-sensitized solar cells based on Au-TiO2 and Ag-TiO2 plasmonic hybrid nanocomposites. Appl. Surf. Sci. 2018, 430, 415–423. [Google Scholar] [CrossRef]
- Yao, G.-Y.; Zhao, Z.-Y.; Liu, Q.-L.; Dong, X.-D.; Zhao, Q.-M. Theoretical calculations for localized surface plasmon resonance effects of Cu/TiO2 nanosphere: Generation, modulation, and application in photocatalysis. Sol. Energy Mater. Sol. Cells 2020, 208, 110385. [Google Scholar] [CrossRef]
- Wang, Z.; Lee, H.; Chen, J.; Wu, M.; Leung, D.Y.; Grimes, C.A.; Lu, Z.; Xu, Z.; Feng, S.-P. Synergistic effects of Pd–Ag bimetals and g-C3N4 photocatalysts for selective and efficient conversion of gaseous CO2. J. Power Sources 2020, 466, 228306. [Google Scholar] [CrossRef]
- Taghizadeh, A.; Taghizadeh, M.; Sabzehmeidani, M.M.; Sadeghfar, F.; Ghaedi, M. Electronic structure: From basic principles to photocatalysis. In Interface Science and Technology; Elsevier: Amsterdam, The Netherlands, 2021; Volume 32, pp. 1–53. [Google Scholar]
- Wang, R.; Shen, J.; Sun, K.; Tang, H.; Liu, Q. Enhancement in photocatalytic activity of CO2 reduction to CH4 by 0D/2D Au/TiO2 plasmon heterojunction. Appl. Surf. Sci. 2019, 493, 1142–1149. [Google Scholar] [CrossRef]
- Saraev, A.A.; Kurenkova, A.Y.; Gerasimov, E.Y.; Kozlova, E.A. Broadening the Action Spectrum of TiO2-Based Photocatalysts to Visible Region by Substituting Platinum with Copper. Nanomaterials 2022, 12, 1584. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, Z.; Cao, S.-W.; Xue, C. Au/Pt nanoparticle-decorated TiO2 nanofibers with plasmon-enhanced photocatalytic activities for solar-to-fuel conversion. J. Phys. Chem. C 2013, 117, 25939–25947. [Google Scholar] [CrossRef]
- Mankidy, B.D.; Joseph, B.; Gupta, V.K. Photo-conversion of CO2 using titanium dioxide: Enhancements by plasmonic and co-catalytic nanoparticles. Nanotechnology 2013, 24, 405402. [Google Scholar] [CrossRef] [PubMed]
- Tayebi, M.; Kolaei, M.; Tayyebi, A.; Masoumi, Z.; Belbasi, Z.; Lee, B.-K. Reduced graphene oxide (RGO) on TiO2 for an improved photoelectrochemical (PEC) and photocatalytic activity. Sol. Energy 2019, 190, 185–194. [Google Scholar] [CrossRef]
- Mondal, A.; Prabhakaran, A.; Gupta, S.; Subramanian, V.R. Boosting photocatalytic activity using reduced graphene oxide (RGO)/semiconductor nanocomposites: Issues and future scope. ACS Omega 2021, 6, 8734–8743. [Google Scholar] [CrossRef]
- Shin, D.H.; Choi, S.-H. Graphene-based semiconductor heterostructures for photodetectors. Micromachines 2018, 9, 350. [Google Scholar] [CrossRef] [Green Version]
- Sun, P.; Zhou, S.; Yang, Y.; Liu, S.; Cao, Q.; Wang, Y.; Wågberg, T.; Hu, G. Artificial chloroplast-like phosphotungstic acid—Iron oxide microbox heterojunctions penetrated by carbon nanotubes for solar photocatalytic degradation of tetracycline antibiotics in wastewater. Adv. Compos. Hybrid Mater. 2022, 1–18. [Google Scholar] [CrossRef]
- Padmanabhan, N.T.; Thomas, N.; Louis, J.; Mathew, D.T.; Ganguly, P.; John, H.; Pillai, S.C. Graphene coupled TiO2 photocatalysts for environmental applications: A review. Chemosphere 2021, 271, 129506. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, J.; Tao, F. Interface Modification of TiO2 Nanotubes by Biomass-Derived Carbon Quantum Dots for Enhanced Photocatalytic Reduction of CO2. ACS Appl. Energy Mater. 2021, 4, 13120–13131. [Google Scholar] [CrossRef]
- Morawski, A.W.; Ćmielewska, K.; Witkowski, K.; Kusiak-Nejman, E.; Pełech, I.; Staciwa, P.; Ekiert, E.; Sibera, D.; Wanag, A.; Gano, M. CO2 Reduction to Valuable Chemicals on TiO2-Carbon Photocatalysts Deposited on Silica Cloth. Catalysts 2021, 12, 31. [Google Scholar] [CrossRef]
- Rajaraman, T.S.; Parikh, S.P.; Gandhi, V.G. Black TiO2: A review of its properties and conflicting trends. Chem. Eng. 2020, 389, 123918. [Google Scholar] [CrossRef]
- Mezzat, F.; Zaari, H.; El Kenz, A.; Benyoussef, A. Effect of metal and non metal doping of TiO2 on photocatalytic activities: Ab initio calculations. Opt. Quantum Electron. 2021, 53, 1–14. [Google Scholar] [CrossRef]
- Jeon, J.P.; Kweon, D.H.; Jang, B.J.; Ju, M.J.; Baek, J.B. Enhancing the photocatalytic activity of TiO2 catalysts. Adv. Sustain. Syst. 2020, 4, 2000197. [Google Scholar] [CrossRef]
- Xu, T.; Wang, M.; Wang, T. Effects of N doping on the microstructures and optical properties of TiO2. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2019, 34, 55–63. [Google Scholar] [CrossRef]
- Yamazaki, Y.; Toyonaga, T.; Doshita, N.; Mori, K.; Kuwahara, Y.; Yamazaki, S.; Yamashita, H. Crystal Facet Engineering and Hydrogen Spillover-Assisted Synthesis of Defective Pt/TiO2–x Nanorods with Enhanced Visible Light-Driven Photocatalytic Activity. ACS Appl. Mater. Interfaces 2021, 14, 2291–2300. [Google Scholar] [CrossRef]
- Chen, X.; Peng, X.; Jiang, L.; Yuan, X.; Fei, J.; Zhang, W. Photocatalytic removal of antibiotics by MOF-derived Ti3+-and oxygen vacancy-doped anatase/rutile TiO2 distributed in a carbon matrix. Chem. Eng. J. 2022, 427, 130945. [Google Scholar] [CrossRef]
- Sorcar, S.; Hwang, Y.; Grimes, C.A.; In, S.-I. Highly enhanced and stable activity of defect-induced titania nanoparticles for solar light-driven CO2 reduction into CH4. Mater. Today 2017, 20, 507–515. [Google Scholar] [CrossRef]
- Nas, M.S.; Calimli, M.H. Recent Development of Nanoparticle by Green-Conventional Methods and Applications for Corrosion and Fuel Cells. Curr. Nanosci. 2021, 17, 525–539. [Google Scholar] [CrossRef]
- Behera, A. Nanomaterials. In Advanced Materials; Springer: Berlin/Heidelberg, Germany, 2022; pp. 77–125. [Google Scholar]
- Gupta, T.; Cho, J.; Prakash, J. Hydrothermal synthesis of TiO2 nanorods: Formation chemistry, growth mechanism, and tailoring of surface properties for photocatalytic activities. Mater. Today Chem. 2021, 20, 100428. [Google Scholar] [CrossRef]
- Wang, Y.-H.; Rahman, K.H.; Wu, C.-C.; Chen, K.-C. A review on the pathways of the improved structural characteristics and photocatalytic performance of titanium dioxide (TiO2) thin films fabricated by the magnetron-sputtering technique. Catalysts 2020, 10, 598. [Google Scholar] [CrossRef]
- Li, Z.; Wang, S.; Wu, J.; Zhou, W. Recent progress in defective TiO2 photocatalysts for energy and environmental applications. Renew. Sustain. Energy Rev. 2022, 156, 111980. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, W.; Zhang, K.; Wang, J.; Sun, B.; Li, H.; Qiao, P.; Wang, L.; Zhou, W. Ti3+ self-doped black TiO2 nanotubes with mesoporous nanosheet architecture as efficient solar-driven hydrogen evolution photocatalysts. ACS Sustain. Chem. Eng. 2017, 5, 901. [Google Scholar] [CrossRef]
- Wu, J.; Qiao, P.; Li, H.; Xu, Y.; Yang, W.; Yang, F.; Lin, K.; Pan, K.; Zhou, W. Engineering surface defects on two-dimensional ultrathin mesoporous anatase TiO2 nanosheets for efficient charge separation and exceptional solar-driven photocatalytic hydrogen evolution. Mater. Chem. 2020, 8, 82. [Google Scholar] [CrossRef]
- Li, Z.; Wang, S.; Xie, Y.; Yang, W.; Tao, B.; Lu, J.; Wu, J.; Qu, Y.; Zhou, W. Surface defects induced charge imbalance for boosting charge separation and solar-driven photocatalytic hydrogen evolution. Colloid Interface Sci. 2021, 596, 21. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.T.; Li, M.; Pan, K.; Guo, L.P.; Wu, J.X.; Li, Z.J.; Yang, F.; Lin, K.; Zhou, W. Surface engineering of mesoporous anatase titanium dioxide nanotubes for rapid spatial charge separation on horizontal-Vertical dimensions and efficient solar-driven photocatalytic hydrogen evolution. Colloid Interface Sci. 2021, 586, 83. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhu, G.; Wang, X.; Yuan, X.; Lin, T.; Huang, F. Progress in black titania: A new material for advanced photocatalysis. Adv. Energy Mater. 2016, 6, 1600452. [Google Scholar] [CrossRef]
- Shoneye, A.; Sen Chang, J.; Chong, M.N.; Tang, J. Recent progress in photocatalytic degradation of chlorinated phenols and reduction of heavy metal ions in water by TiO2-based catalysts. Int. Mater. Rev. 2022, 67, 47–64. [Google Scholar] [CrossRef]
- Janczarek, M.; Kowalska, E. Defective dopant-free TiO2 as an efficient visible light-active photocatalyst. Catalysts 2021, 11, 978. [Google Scholar] [CrossRef]
- Dobrosielska, M.; Zieliński, M.; Frydrych, M.; Pietrowski, M.; Marciniak, P.; Martyła, A.; Sztorch, B.; Przekop, R.E. Sol–Gel Approach for Design of Pt/Al2O3-TiO2 System—Synthesis and Catalytic Tests. Ceramics 2021, 4, 667–680. [Google Scholar] [CrossRef]
- Lluna-Galán, C.; Izquierdo-Aranda, L.; Adam, R.; Cabrero-Antonino, J.R. Catalytic Reductive Alcohol Etherifications with Carbonyl-Based Compounds or CO2 and Related Transformations for the Synthesis of Ether Derivatives. ChemSusChem 2021, 14, 3744–3784. [Google Scholar] [CrossRef]
- Tian, J.; Hu, X.; Yang, H.; Zhou, Y.; Cui, H.; Liu, H. High yield production of reduced TiO2 with enhanced photocatalytic activity. Appl. Surf. Sci. 2016, 360, 43. [Google Scholar] [CrossRef]
- Fang, W.; Xing, M.; Zhang, J. A new approach to prepare Ti3+ self-doped TiO2 via NaBH4 reduction and hydrochloric acid treatment. Appl. Catal. B Environ. 2014, 240, 160–161. [Google Scholar] [CrossRef]
- Tan, H.; Zhao, Z.; Niu, M.; Mao, C.; Cao, D.; Cheng, D.; Feng, P.; Sun, Z. A facile and versatile method for preparation of colored TiO2 with enhanced solar-driven photocatalytic activity. Nanoscale 2014, 6, 23. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, C.; Lin, T.; Yin, H.; Chen, P.; Wan, D.; Xu, F.; Huang, F.; Lin, J.; Xie, X.; et al. H-doped black titania with very high solar absorption and excellent photocatalysis enhanced by localized surface plasmon resonance. Adv. Funct Mater. 2013, 23, 50. [Google Scholar] [CrossRef]
- Teng, F.; Li, M.; Gao, C.; Zhang, G.; Zhang, P.; Wang, Y.; Chen, L.; Xie, E. Preparation of black TiO2 by hydrogen plasma assisted chemical vapor deposition and its photocatalytic activity. Appl. Catal. B Environ. 2014, 339, 148–149. [Google Scholar] [CrossRef]
- Khan, M.M.; Ansari, S.A.; Pradhan, D.; Ansari, M.O.; Han, D.H.; Lee, J.; Cho, M.H. Band gap engineered TiO2 nanoparticles for visible light induced photoelectrochemical and photocatalytic studies. Mater. Chem. 2014, 2, 44. [Google Scholar]
- Liu, N.; Haublein, V.; Zhou, X.M.; Venkatesan, U.; Hartmann, M.; Mackovic, M.; Nakajima, T.; Spiecker, E.; Osvet, A.; Frey, L.; et al. “Black” TiO2 nanotubes formed by high-energy proton implantation show noble-metal-co-catalyst free photocatalytic H2-evolution. Nano Lett. 2015, 15, 20. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Yang, C.; Lin, T.; Yin, H.; Chen, P.; Wan, D.; Xu, F.; Huang, F.; Lin, J.; Xie, X.; et al. Visible-light photocatalytic, solar thermal and photoelectrochemical properties of aluminium-reduced black titania. Energy Environ. Sci. 2013, 6, 14. [Google Scholar] [CrossRef]
- Zhu, G.; Lin, T.; Lü, X.; Zhao, W.; Yang, C.; Wang, Z.; Yin, H.; Liu, Z.; Huang, F.; Lin, J. Black brookite titania with high solar absorption and excellent photocatalytic performance. Mater. Chem. 2013, 1, 3. [Google Scholar] [CrossRef]
- Yin, H.; Lin, T.; Yang, C.; Wang, Z.; Zhu, G.; Xu, T.; Xie, X.; Huang, F.; Jiang, M. Gray TiO2 nanowires synthesized by aluminum-mediated reduction and their excellent photocatalytic activity for water cleaning. Chem. Eur. J. 2013, 19, 6. [Google Scholar] [CrossRef]
- Yang, C.; Wang, Z.; Lin, T.; Yin, H.; Lü, X.; Wan, D.; Xu, T.; Zheng, C.; Lin, J.; Huang, F.; et al. Core-shell nanostructured “black” rutile titania as excellent catalyst for hydrogen production enhanced by sulfur doping. Am. Chem. Soc. 2013, 135, 8. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.; Yang, C.; Wang, Z.; Yin, H.; Lü, X.; Huang, F.; Lin, J.; Xie, X.; Jiang, M. Effective nonmetal incorporation in black titania with enhanced solar energy utilization. Energy Environ. Sci. 2014, 7, 72. [Google Scholar] [CrossRef]
- Zhao, Z.; Tan, H.; Zhao, H.; Lv, Y.; Zhou, L.-J.; Song, Y.; Sun, Z. Reduced TiO2 rutile nanorods with well-defined facets and their visible-light photocatalytic activity. Chem. Commun. 2014, 50, 7. [Google Scholar]
- Sinhamahapatra, A.; Jeon, J.-P.; Yu, J.-S. A new approach to prepare highly active and stable black titania for visible light-assisted hydrogen production. Energy Env. Sci. 2015, 8, 44. [Google Scholar] [CrossRef] [Green Version]
- Zou, X.; Liu, J.; Su, J.; Zuo, F.; Chen, J.; Feng, P. Facile synthesis of thermal- and photostable titania with paramagnetic oxygen vacancies for visible-light photocatalysis. Chem. Eur. J. 2013, 19, 73. [Google Scholar] [CrossRef]
- Zuo, F.; Wang, L.; Wu, T.; Zhang, Z.Y.; Borchardt, D.; Feng, P.Y. Self-doped Ti3+ enhanced photocatalyst for hydrogen production under visible light. Am. Chem. Soc. 2010, 132, 7. [Google Scholar] [CrossRef]
- Chen, X.; Liu, L.; Huang, F. Black titanium dioxide (TiO2) nanomaterials. Chem. Soc. Rev. 2015, 44, 85. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Chen, Z.; Tsang, C.K.; Li, Z.; Ran, X.; Lee, C.; Nie, B.; Zheng, L.; Hung, T.; Lu, J.; et al. Electrochemical doping of anatase TiO2 in organic electrolytes for high-performance supercapacitors and photocatalyst. Mater. Chem. 2014, 2, 36. [Google Scholar]
- Liu, X.; Gao, S.; Xu, H.; Lou, Z.; Wang, W.; Huang, B.; Dai, Y. Green synthetic approach for Ti3+ self-doped TiO2−x nanoparticles with efficient visible light photocatalytic activity. Nanoscale 2013, 5, 5. [Google Scholar] [CrossRef]
- Grabstanowicz, L.R.; Gao, S.; Li, T.; Rickard, R.M.; Rajh, T.; Liu, D.J.; Xu, T. Facile oxidative conversion of TiH2 to high-concentration Ti3+-self-doped rutile TiO2 with visible-light photoactivity. Inorg. Chem. 2013, 52, 90. [Google Scholar] [CrossRef]
- Liu, X.; Xu, H.; Grabstanowicz, L.R.; Gao, S.; Lou, Z.; Wang, W.; Huang, B.; Dai, Y.; Xu, T. Ti3+ self-doped TiO2−x anatase nanoparticles via oxidation of TiH2 in H2O2. Catal. Today 2014, 225, 9. [Google Scholar] [CrossRef]
- Li, X.; Yang, X.; Huang, Y.; Zhang, T.; Liu, B. Supported noble-metal single atoms for heterogeneous catalysis. Adv. Mater. 2019, 31, 1902031. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Huang, J.; Pantovich, S.A.; Carl, A.D.; Fenton, T.G.; Caputo, C.A.; Grimm, R.L.; Frenkel, A.I.; Li, G. Selective CO2 reduction catalyzed by single cobalt sites on carbon nitride under visible-light irradiation. J. Am. Chem. Soc. 2018, 140, 16042–16047. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Zhan, X.; Xu, L.; Fu, X.; Zheng, T.; Yang, X.; Xu, Q.; Wang, D.; Qi, D.; Sun, T. Mass production of a single-atom cobalt photocatalyst for high-performance visible-light photocatalytic CO2 reduction. J. Mater. Chem. A 2021, 9, 26286–26297. [Google Scholar] [CrossRef]
- Xiong, X.; Mao, C.; Yang, Z.; Zhang, Q.; Waterhouse, G.I.; Gu, L.; Zhang, T. Photocatalytic CO2 reduction to CO over Ni single atoms supported on defect-rich zirconia. Adv. Energy Mater. 2020, 10, 2002928. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Li, Z.; Garcia, H. Catalysis and photocatalysis by metal organic frameworks. Chem. Soc. Rev. 2018, 47, 8134–8172. [Google Scholar] [CrossRef]
- Chen, X.; Peng, X.; Jiang, L.; Yuan, X.; Yu, H.; Wang, H.; Zhang, J.; Xia, Q. Recent advances in titanium metal–organic frameworks and their derived materials: Features, fabrication, and photocatalytic applications. Chem. Eng. J. 2020, 395, 125080. [Google Scholar] [CrossRef]
- Yang, S.; Peng, L.; Bulut, S.; Queen, W.L. Recent Advances of MOFs and MOF-Derived Materials in Thermally Driven Organic Transformations. Chem. A Eur. J. 2019, 25, 2161–2178. [Google Scholar] [CrossRef]
- Zhu, J.; Li, P.-Z.; Guo, W.; Zhao, Y.; Zou, R. Titanium-based metal–organic frameworks for photocatalytic applications. Coord. Chem. Rev. 2018, 359, 80–101. [Google Scholar] [CrossRef]
- Khaletskaya, K.; Pougin, A.; Medishetty, R.; Rosler, C.; Wiktor, C.; Strunk, J.; Fischer, R.A. Fabrication of gold/titania photocatalyst for CO2 reduction based on pyrolytic conversion of the metal–organic framework NH2-MIL-125 (Ti) loaded with gold nanoparticles. Chem. Mater. 2015, 27, 7248–7257. [Google Scholar] [CrossRef]
- Bueken, B.; Vermoortele, F.; Vanpoucke, D.E.; Reinsch, H.; Tsou, C.C.; Valvekens, P.; De Baerdemaeker, T.; Ameloot, R.; Kirschhock, C.E.; Van Speybroeck, V. A Flexible Photoactive Titanium Metal–Organic Framework Based on a [TiIV3 (μ3-O)(O) 2 (COO) 6] Cluster. Angew. Chem. Int. Ed. 2015, 54, 13912–13917. [Google Scholar] [CrossRef] [PubMed]
- Ao, D.; Zhang, J.; Liu, H. Visible-light-driven photocatalytic degradation of pollutants over Cu-doped NH2-MIL-125 (Ti). J. Photochem. Photobiol. A Chem. 2018, 364, 524–533. [Google Scholar] [CrossRef]
- Rahmani, A.; Emrooz, H.B.M.; Abedi, S.; Morsali, A. Synthesis and characterization of CdS/MIL-125 (Ti) as a photocatalyst for water splitting. Mater. Sci. Semicond. Processing 2018, 80, 44–51. [Google Scholar] [CrossRef]
- An, X.; Jimmy, C.Y.; Wang, F.; Li, C.; Li, Y. One-pot synthesis of In2S3 nanosheets/graphene composites with enhanced visible-light photocatalytic activity. Appl. Catal. B Environ. 2013, 129, 80–88. [Google Scholar] [CrossRef]





| Catalyst | Treatment | Note | H2 Evolution Rate/Removal Efficacy | Reference |
|---|---|---|---|---|
| Black TiO2 nanoparticles | Thermal plasma furnace | The absorption increases promptly and monotonously in visible spectrum, when the wavelength is >400 nm | Visible light: 83% | [154] |
| Black TiO2 nanotubes | Hydrogen plasma method | NaOH solution (10M, 50 mL), to be used in heating 2g of P25 for 12 h, then being washed with water and HCL. | 7 µmol h−1 cm−2 | [155] |
| Black TiO2 nanoparticles | Electron beam treatment | Electron-beam-assisted high energy electron used in changing the composition of TiO2. Electron beam maximum energy 0.7 MeV. Electron beam maximum power 28 kW | Visible light: 85% | [156] |
| TiO2 nanotubes with black appearance for the proton-implanted layer | Proton implantation | The top of the nanotubes is being modified via high energy proton ion-implantation strategy. Then implanting the substrate with Varian 350D ion implanter. The resulted nanotubes showed high performance in aqueous solution. | UV: 38% | [157] |
| Defective TiO2 | Metal reduction | Metals like Zn, Al, Mg are excellent reductants that for being cheap, safe and convenient in comparison with hydrogen. | Solar light: 95% | [142] |
| Black TiO2 and TiO2 nanotubes | Aluminum reduction | TiO2 and Al are being processed in a dual tube furnace below 0.5 Pa | 3.9 mmol g−1 h−1 | [158,159] |
| Gray TiO2 nanowires | Aluminum reduction | Titanate nanowires are being processed in double zone furnace in Al atmosphere for 4 h | Solar light: 95% | [160] |
| Black brookite TiO2 nanoparticles | Aluminum reduction | Brookite TiO2 and Al powder are being placed in dual vacuum furnace and heated for 4 h at 300–600 and 800 °C. This process promoted the absorption of visible spectrum and IR of brookite TiO2 | Solar light: 92% | [159] |
| Black rutile TiO2 Nanoparticles | Molten Aluminum | The sample is being heated at 550–800 °C at a pressure of 6 × 10−4 Pa in a vacuum-double-zone furnace. And the results showed enhanced absorption. | 932 µmol h−1 g−1 | [161] |
| Black TiO2−x nanoparticles | Al powder | Al powder and P25 (0.5 g) are being processed in a two-zone vacuum furnace. Then using thermal plasma furnace to apply hydrogen plasma for 5 h | 15 mmol h−1 g−1 | [162] |
| Black TiO2-N nanoparticles | The material is being heated in a gas stream of NH3-Ar | Solar light: 85% | [162] | |
| Rutile TiO2 nanoparticles | Zn reduction | Mixing aqueous TiCl2 (1 mL) and isopropanol (30 mL) at 180 °C in existence of Zn powder for 6 h. | 1.4 mmol h−1 g−1 | [163] |
| Black TiO2 photocatalyst | Mg reduction | Mixing TiO2 with Mg powder resulted black TiO2. But Mg and H2 resulted in highly stable and active reduced black TiO2. | 440 µmol h−1 g−1 | [164] |
| Porous amorphous Vo-TiO2 | Organic reduction | 300-Xe lamp has been used as a light source. The target is aqueous methanol solution (25 vol%, 120 mL) for 8 h in UV and visible light: 5.67 mmol h−1 g−1 For 14 h, in visible light radiation: 115 µmol h−1 g−1 | Visible light and UV: 5.67 mmol h−1 g−1 | [165] |
| Ti3+ doped TiO2 | Organic reduction | 300 W Xe lamp, aqueous methanol solution (25 vol%, 120 mL), for 4 h in visible light irradiation: 50 μmol h−1 g−1 | Visible light: 115 µmol h−1 g−1 | [166] |
| Defective TiO2−x | Organic reduction | Imidiazole and 2-ethylimidazole | - | [165,166,167] |
| Gray TiO2 | Organic reduction | A TiO2 precursor exposed to UV for one hour then annealed with hydrochloric acid, and imidazole (1 g) in a muffle furnace at 450 °C | 115 µmol h−1 g−1 | [165] |
| Black defective TiO2 nanotubes | Electrochemical reduction | TiO2 were synthesized via Ti foil anodization in (4 mA for 5000 s or 80 V for 7200 s). then calcined in air. | Visible light: 72% | [168] |
| Ti3+ self-doped TiO2−x nanoparticles | Chemical oxidation | The light source used is 300 W Xe lamp. Target and concentration are aqueous methanol solution (100 mL, 20%) MB (120 mL, 5 × 10−4 mol/L), for 4 h. | 250 µmol h−1 g−1 | [169] |
| Ti3+ self-doped rutile TiO2 | Chemical oxidation | Using solar simulator, MB (30 mL, 10−5 M), for 1 h | - | [170] |
| Ti3+ self-doped TiO2−x anatase nanoparticles | Chemical oxidation | Light source: 300 W Xe arc lamp MB (100 mL, 1.5 × 10−5 mol/L) aqueous methanol solution (20 vol%) for 30 min | 147 µmol h−1 g−1 | [171] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fawzi, T.; Rani, S.; Roy, S.C.; Lee, H. Photocatalytic Carbon Dioxide Conversion by Structurally and Materially Modified Titanium Dioxide Nanostructures. Int. J. Mol. Sci. 2022, 23, 8143. https://doi.org/10.3390/ijms23158143
Fawzi T, Rani S, Roy SC, Lee H. Photocatalytic Carbon Dioxide Conversion by Structurally and Materially Modified Titanium Dioxide Nanostructures. International Journal of Molecular Sciences. 2022; 23(15):8143. https://doi.org/10.3390/ijms23158143
Chicago/Turabian StyleFawzi, Tarek, Sanju Rani, Somnath C. Roy, and Hyeonseok Lee. 2022. "Photocatalytic Carbon Dioxide Conversion by Structurally and Materially Modified Titanium Dioxide Nanostructures" International Journal of Molecular Sciences 23, no. 15: 8143. https://doi.org/10.3390/ijms23158143
APA StyleFawzi, T., Rani, S., Roy, S. C., & Lee, H. (2022). Photocatalytic Carbon Dioxide Conversion by Structurally and Materially Modified Titanium Dioxide Nanostructures. International Journal of Molecular Sciences, 23(15), 8143. https://doi.org/10.3390/ijms23158143