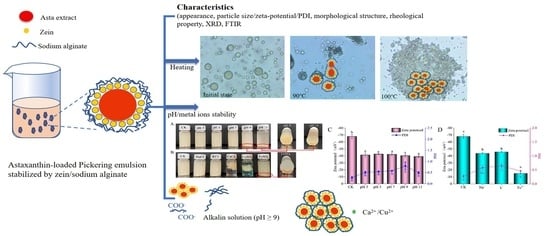
Property and Stability of Astaxanthin Emulsion Based on Pickering Emulsion Templating with Zein and Sodium Alginate as Stabilizer
Abstract
:1. Introduction
2. Results and Discussion
2.1. Appearance, Particle Size and Morphological Property
2.2. Molecular Interaction
2.3. Rheological Property
2.4. Thermal Stability
2.5. Effects of pH and Metal Ions on APEs Stability
2.5.1. Visual Appearance
2.5.2. Zeta-Potential and PDI
2.5.3. Asta Retention Rate
2.5.4. Asta Degradation Kinetics
3. Materials and Methods
3.1. Materials
3.2. Preparation of APEs
3.3. Asta Encapsulation Efficiency
3.4. Asta Retention
3.5. Particle Size, Particle Charge and PDI Measurements
3.6. Morphological Characterization
3.6.1. Appearance and Optical Characteristics
3.6.2. SEM and TEM Observation
3.6.3. CLSM Observation
3.7. Rheological Property
3.8. Molecular Interaction
3.8.1. XRD Analysis
3.8.2. FTIR Assay
3.9. Thermal Stability
3.10. Antioxidant Activity
3.11. pH and Metal Ions Stability
3.12. Asta Degradation Kinetics
3.13. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rousseau, S.; Kyomugasho, C.; Celus, M.; Hendrickx, M.E.G.; Grauwet, T. Barriers Impairing Mineral Bioaccessibility and Bioavailability in Plant-Based Foods and the Perspectives for Food Processing. Crit. Rev. Food Sci. 2020, 60, 826–843. [Google Scholar] [CrossRef] [PubMed]
- Higuera-Ciapara, I.; Felix-Valenzuela, L.; Goycoolea, F.M. Astaxanthin: A Review of Its Chemistry and Applications. Crit. Rev. Food Sci. 2006, 46, 185–196. [Google Scholar] [CrossRef]
- Rao, A.R.; Phang, S.M.; Ravi, S.; Ravishankar, G.A. Astaxanthin: Sources, Extraction, Stability, Biological Activities and Its Commercial Applications-a review. Mar. Drugs 2014, 12, 128–152. [Google Scholar]
- Demirel, P.B.; Tuna, B.G. Chapter 21-Anticancer Properties of Astaxanthin: A Molecule of Great Promise. In Global Perspectives on Astaxanthin; Ravishankar, G.A., Rao, A.R., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 427–445. [Google Scholar]
- Faraone, I.; Sinisgalli, C.; Ostuni, A.; Armentano, M.F.; Carmosino, M.; Milella, L.; Russo, D.; Labanca, F.; Khan, H. Astaxanthin Anticancer Effects are Mediated Through Multiple Molecular Mechanisms: A Systematic Review. Pharmacol. Res. 2020, 155, 104689. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.X.; Yang, L.; Xu, J.; Qiao, X.; Li, Z.J.; Wang, Y.M.; Xue, C.H. Evaluation of the Physicochemical Stability and Digestibility of Microencapsulated Esterified Astaxanthins Using In vitro and In vivo Models. Food Chem. 2018, 260, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Mcclements, D.J. Recent Advances in the Production and Application of Nano-Enabled Bioactive Food Ingredients. Curr. Opin. Food Sci. 2020, 33, 85–90. [Google Scholar] [CrossRef]
- Dickinson, E. Emulsion Gels: The Structuring of Soft Solids with Protein-Stabilized Oil Droplets. Food Hydrocoll. 2012, 28, 224–241. [Google Scholar] [CrossRef]
- Ren, Z.; Li, Z.; Chen, Z.; Zhang, Y.; Lin, X.; Weng, W.; Yang, H.; Li, B. Characteristics and Application of Fish Oil-in-Water Pickering Emulsions Structured with Tea Water-Insoluble Proteins/κ-Carrageenan Complexes. Food Hydrocoll. 2021, 114, 106562. [Google Scholar] [CrossRef]
- Pang, B.; Liu, H.; Zhang, K. Recent Progress on Pickering Emulsions Stabilized by Polysaccharides-Based Micro/nanoparticles. Adv. Colloid Interface Sci. 2021, 296, 102522. [Google Scholar] [CrossRef]
- Zhang, T.; Xu, J.M.; Chen, J.H.; Wang, Z.Q.; Wang, X.C.; Zhong, J. Protein Nanoparticles for Pickering Emulsions: A Comprehensive Review on Their Shapes, Preparation Methods, and Modification Methods. Trends Food Sci. Technol. 2021, 113, 26–41. [Google Scholar] [CrossRef]
- Patel, A.; Hu, Y.; Tiwari, J.K.; Velikov, K.P. Synthesis and Characterisation of Zein-Curcumin Colloidal Particles. Soft Matter. 2010, 6, 6192–6199. [Google Scholar] [CrossRef]
- Lawton, J.W. Zein: A History of Processing and Use. Cereal Chem. 2002, 79, 1–18. [Google Scholar] [CrossRef]
- Wang, L.; Hu, Y.; Yin, S.; Yang, X.; Lai, F.; Wang, S. Fabrication and Characterization of Antioxidant Pickering Emulsions Stabilized by Zein/chitosan Complex Particles (ZCPs). J. Agric. Food Chem. 2015, 63, 2514–2524. [Google Scholar] [CrossRef]
- Meng, R.; Wu, Z.; Xie, Q.T.; Zhang, B.; Li, P.J. Zein/carboxymethyl Dextrin Nanoparticles Stabilized Pickering Emulsions as Delivery Vehicles: Effect of Interfacial Composition on Lipid Oxidation and In Vitro Digestion. Food Hydrocoll. 2020, 108, 106020. [Google Scholar] [CrossRef]
- Yan, J.; Liang, X.P.; Ma, C.C.; McClements, D.J.; Liu, X.B.; Liu, F.G. Design and Characterization of Double-Cross-Linked Emulsion Gels Using Mixed Biopolymers: Zein and Sodium Alginate. Food Hydrocoll. 2021, 113, 106473. [Google Scholar] [CrossRef]
- Barresi, A.A.; Pisano, R.; Fissore, D.; Rasetto, V.; Velardi, S.A.; Vallan, A.; Galan, M. Monitoring of the Primary Drying of a Lyophilization Process in Vials. Chem. Eng. Process. 2009, 48, 408–423. [Google Scholar] [CrossRef] [Green Version]
- Sun, C.; Dai, L.; Gao, Y. Interaction and Formation Mechanism of Binary Complex Between Zein and Propylene Glycol Alginate. Carbohydr. Polym. 2017, 157, 1638–1649. [Google Scholar] [CrossRef]
- Liang, X.; Ma, C.; Yan, X.; Zeng, H.; Mcclements, D.J.; Liu, X.; Liu, F. Structure, Rheology and Functionality of Whey Protein Emulsion Gels: Effects of Double Cross-Linking with Transglutaminase and Calcium Ions. Food Hydrocoll. 2020, 102, 105569. [Google Scholar] [CrossRef]
- Hong, H.L.; Suo, Q.L.; Han, L.M.; Li, C.P. Study on Precipitation of Astaxanthin in Supercritical Fluid. Powder Technol. 2009, 191, 294–298. [Google Scholar] [CrossRef]
- Sun, C.; Chen, S.; Dai, L.; Gao, Y. Structural Characterization and Formation Mechanism of Zein-Propylene Glycol Alginate Binary Complex Induced by Calcium Ions. Food Res. Int. 2017, 100, 57–68. [Google Scholar] [CrossRef]
- Yoksan, R.; Jirawutthiwongchai, J.; Arpo, K. Encapsulation of Ascorbyl Palmitate in Chitosan Nanoparticles by Oil-in-Water Emulsion and Ionic Gelation Processes. Colloids Surf. B 2010, 76, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Jing, Y.; Han, C.; Zhang, H.; Tian, Y. Encapsulation of Curcumin in Zein/caseinate/sodium Alginate Nanoparticles with Improved Physicochemical and Controlled Release Properties. Food Hydrocoll. 2019, 93, 432–442. [Google Scholar] [CrossRef]
- Sow, L.C.; Toh, N.Z.Y.; Wong, C.W.; Yang, H. Combination of Sodium Alginate with Tilapia Fish Gelatin for Improved Texture Properties and Nanostructure Modification. Food Hydrocoll. 2019, 94, 459–467. [Google Scholar] [CrossRef]
- Jun, S.W.; Kim, M.S.; Kim, J.S.; Park, H.J.; Lee, S.; Woo, J.S.; Hwang, S.J. Preparation and Characterization of Simvastatin/hydroxypropyl-β-Cyclodextrin Inclusion Complex Using Supercritical Antisolvent (SAS) Process. Eur. J. Pharm. Biopharm. 2007, 66, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Sun, C.; Wang, D.; Gao, Y. The Interaction Between Zein and Lecithin in Ethanol-Water Solution and Characterization of Colloidal Nanoparticles. PLoS ONE 2016, 11, e0167172. [Google Scholar]
- Jridi, M.; Hajji, S.; Ayed, H.B.; Lassoued, I.; Mbarek, A.; Kammoun, M.; Nasri, M. Physical, Structural, Antioxidant and Antimicrobial Properties of Gelatin-Chitosan Composite Edible Films. Int. J. Biol. Macromol. 2014, 67, 373–379. [Google Scholar] [CrossRef]
- Cerqueira, M.A.; Souza, B.W.; Teixeira, J.A.; Vicente, A.A. Effect of Glycerol and Corn Oil on Physicochemical Properties of Polysaccharide Films-A Comparative Study. Food Hydrocoll. 2012, 27, 175–184. [Google Scholar] [CrossRef] [Green Version]
- Song, R.; Qi, Y.; Jia, Z.; Liu, X.; Wei, R. Astaxanthin-Loaded Zein/calcium Alginate Composite Microparticles: Characterization, Molecular Interaction and Release Kinetics in Fatty Food Simulant System. LWT-Food Sci. Technol. 2020, 134, 110146. [Google Scholar] [CrossRef]
- Huang, M.; Theng, A.H.P.; Yang, D.; Yang, H. Influence of κ-Carrageenan on the Rheological Behaviour of a Model Cake Flour System. LWT-Food Sci. Technol. 2021, 136, 110324. [Google Scholar] [CrossRef]
- Yang, W.G.; Zhang, M.; Wang, Q.Q.; Sun, J.Y.; Song, A.X. Pickering Emulsions Stabilized by Surfactant Particles with Smart Responses to pH and Metal-Ligands. J. Mol. Liq. 2021, 324, 114730. [Google Scholar] [CrossRef]
- Xiao, H.; Lin, Q.; Xia, X. Rheological Properties of Sweet Potato Starch Before and After Denaturalization. J. Cent. South Univ. Technol. 2008, 15, 500–505. [Google Scholar] [CrossRef]
- Ma, J.; Lin, Y.; Chen, X.; Zhao, B.; Zhang, J. Flow Behavior, Thixotropy and Dynamical Viscoelasticity of Sodium Alginate Aqueous Solutions. Food Hydrocoll. 2014, 38, 119–128. [Google Scholar] [CrossRef]
- Tunick, M.H. Small-Strain Dynamic Rheology of Food Protein Networks. J. Agric. Food Chem. 2011, 59, 1481–1486. [Google Scholar] [CrossRef] [PubMed]
- Mwangi, W.W.; Ho, K.W.; Tey, B.T.; Chan, E.S. Effects of Environmental Factors on the Physical Stability of Pickering-Emulsions Stabilized by Chitosan Particles. Food Hydrocoll. 2016, 60, 543–550. [Google Scholar] [CrossRef]
- Keerati-u-rai, M.; Corredig, M. Heat-Induced Changes in Oil-in-Water Emulsions Stabilized with Soy Protein Isolate. Food Hydrocoll. 2009, 23, 2141–2148. [Google Scholar] [CrossRef]
- Beliciu, C.M.; Moraru, C.I. Effect of Solvent and Temperature on the Size Distribution of Casein Micelles Measured by Dynamic Light Scattering. J. Dairy Sci. 2009, 92, 1829–1839. [Google Scholar] [CrossRef] [Green Version]
- Evans, M.; Ratcliffe, I.; Williams, P.A. Emulsion Stabilisation Using Polysaccharide-Protein Complexes. Curr. Opin. Colloid Interface Sci. 2013, 18, 272–282. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Elmer, C.; Low, N.H.; Nickerson, M.T. Effect of pH on the Functional Behaviour of Pea Protein Isolate-Gum Arabic Complexes. Food Res. Int. 2010, 43, 489–495. [Google Scholar] [CrossRef]
- Can, S.; Tanriseve, T. A New Investigation with the Salting-Out Effect on Emulsifier-Free Emulsion Polymerization of Methyl Methacrylate. J. Appl. Polym. Sci. 2007, 103, 2494–2500. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, H. Effects of Calcium Ion on Gel Properties and Gelation of Tilapia (Oreochromis niloticus) Protein Isolates Processed with pH Shift Method. Food Chem. 2019, 277, 327–335. [Google Scholar] [CrossRef]
- Bokkhim, H.; Bansal, N.; Grondahl, L.; Bhandari, B. In-vitro Digestion of Different Forms of Bovine Lactoferrin Encapsulated in Alginate Micro-Gel Particles. Food Hydrocoll. 2016, 52, 231–242. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.; Chen, J.; Wang, R.; Xu, P.; Yang, G. Bio-Inspired Cu-Alginate to Smartly Enhance Safety Performance and the Thermal Decomposition of Ammonium Perchlorate. Appl. Surf. Sci. 2019, 470, 269–275. [Google Scholar] [CrossRef]
- Russo, R.; Malinconico, M.; Santagata, G. Effect of Cross-Linking with Calcium Ions on the Physical Properties of Alginate Films. Biomacromolecules 2007, 8, 3193. [Google Scholar] [CrossRef] [PubMed]
- Hernández, R.; Sacristán, J.; Mijangos, C. Sol/Gel Transition of Aqueous Alginate Solutions Induced by Fe2+ Cations. Macromol. Chem. Phys. 2010, 211, 1254–1260. [Google Scholar] [CrossRef]
- Criado-Gonzalez, M.; Corbella, L.; Senger, B.; Boulmedais, F.; Hernandez, R. Photoresponsive Nanometer-Scale Iron Alginate Hydrogels: A Study of Gel-Sol Transition Using a Quartz Crystal Microbalance. Langmuir 2019, 35, 11397–11405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shukla, R.; Cheryan, M. Zein: The Industrial Protein from Corn. Ind. Crops Prod. 2002, 13, 171–192. [Google Scholar] [CrossRef]
- Zhang, C.; Xu, W.; Jin, W.; Shah, B.R.; Li, Y.; Li, B. Influence of Anionic Alginate and Cationic Chitosan on Physicochemical Stability and Carotenoids Bioaccessibility of Soy Protein Isolate-Stabilized Emulsions. Food Res. Int. 2015, 77, 419–425. [Google Scholar] [CrossRef]
- Lovaglio, R.B.; dos Santos, F.J.; Jafelicci, M.; Contiero, J. Rhamnolipid Emulsifying Activity and Emulsion Stability: pH Rules. Colloids Surf. B 2011, 85, 301–305. [Google Scholar] [CrossRef]
- Anarjan, N.; Tan, C.P. Chemical Stability of Astaxanthin Nanodispersions in Orange Juice and Skimmed Milk as Model Food Systems. Food Chem. 2013, 139, 527–531. [Google Scholar] [CrossRef]
- Bustos-Garza, C.; Yáñez-Fernández, J.; Barragán-Huerta, B.E. Thermal and pH Stability of Spray-Dried Encapsulated Astaxanthin Oleoresin from Haematococcus Pluvialis Using Several Encapsulation Wall Materials. Food Res. Int. 2013, 54, 641–649. [Google Scholar] [CrossRef]
- Pu, J.; Bankston, J.D.; Sathivel, S. Production of Microencapsulated Crawfish (Procambarus Clarkii) Astaxanthin in Oil by Spray Drying Technology. Dry. Technol. 2011, 29, 1150–1160. [Google Scholar] [CrossRef]
- Niamnuy, C.; Devahastin, S.; Soponronnarit, S.; Raghavan, G.S.V. Kinetics of Astaxanthin Degradation and Color Changes of Dried Shrimp During Storage. J. Food Eng. 2008, 87, 591–600. [Google Scholar] [CrossRef]
- Meléndez, A.; Britton, G.; Vicario, I.; Heredia, F. Relationship Between the Color and the Chemical Structure of Carotenoid Pigments. Food Chem. 2006, 101, 1145–1150. [Google Scholar] [CrossRef]
- Villalobos-Castillejos, F.; Cerezal-Mezquita, P.; Hernández-De Jesús, M.L.; Barragán-Huerta, B.E. Production and Stability of Water-Dispersible Astaxanthin Oleoresin from Phaffia Rhodozyma. Int. J. Food Sci. Technol. 2013, 48, 1243–1251. [Google Scholar] [CrossRef]
- Jia, Z.; Xu, Y.; Wang, J.X.; Song, R. Antioxidant Activity and Degradation Kinetics of Astaxanthin Extracted from Penaeus Sinensis (Solenocera Crassicornis) Byproducts under Pasteurization Treatment. LWT-Food Sci. Technol. 2021, 152, 112336. [Google Scholar] [CrossRef]
- Song, R.; Jia, Z.; Xu, Y.; Zhang, X.X.; Wei, R.B.; Sun, J.P. Saponifification To Improve the Antioxidant Activity of Astaxanthin Extracts from Penaeus Sinensis (Solenocera Crassicornis) By-products and Intervention Effect on Paracetamol-Induced Acute Hepatic Injury in Rat. J. Funct. Foods 2020, 73, 104150. [Google Scholar] [CrossRef]
- Sun, Y.; Ma, G.; Ye, X.; Kakuda, Y.; Meng, R. Stability of All-Trans-β-Carotene Under Ultrasound Treatment in a Model System: Effects of Different Factors, Kinetics and Newly Formed Compounds. Ultrason. Sonochem. 2010, 17, 654–661. [Google Scholar] [CrossRef]







| Sample | α (s) | n | η0 (Pa·s) | η∞ (Pa·s) | R2 | Standard Deviation (SD) |
|---|---|---|---|---|---|---|
| PEs | 6.757 | 0.68 | 0.135 | 0.036 | 0.9897 | 0.00495 |
| APEs | 95.744 | 0.66 | 0.813 | 0.039 | 0.9974 | 0.00199 |
| Zero Order Kinetics | First Order Kinetics | Second Order Kinetics | t1/2▲ (day) | ||||
|---|---|---|---|---|---|---|---|
| k (%·day−1) | R2 | k (day−1) | R2 | k (%−1·day−1) | R2 | ||
| pH 3 | 16.841 (0.143) | 0.813 | 0.334 (0.009) | 0.994 | 0.009 (0.0003) | 0.962 | 2.070 (0.059) |
| pH 5 | 17.983 (0.689) | 0.943 | 0.419 (0.055) | 0.990 | 0.016 (0.0053) | 0.828 | 1.668 (0.216) |
| pH 7 | 19.969 (1.196) | 0.858 | 0.318 (0.043) | 0.991 | 0.005 (0.0004) | 0.923 | 2.200 (0.286) |
| pH 9 | 31.529 (1.282) | 0.918 | 0.605 (0.101) | 0.997 | 0.015 (0.0007) | 0.998 | 0.681 (0.033) |
| Na+ | 26.651 (0.697) | 0.950 | 0.633 (0.031) | 0.997 | 0.026 (0.0065) | 0.886 | 1.095 (0.053) |
| K+ | 33.042 (0.472) | 0.885 | 0.641 (0.020) | 0.994 | 0.016 (0.0003) | 0.988 | 1.080 (0.034) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.; Jia, Z.; Wang, J.; Sun, J.; Song, R. Property and Stability of Astaxanthin Emulsion Based on Pickering Emulsion Templating with Zein and Sodium Alginate as Stabilizer. Int. J. Mol. Sci. 2022, 23, 9386. https://doi.org/10.3390/ijms23169386
Xu Y, Jia Z, Wang J, Sun J, Song R. Property and Stability of Astaxanthin Emulsion Based on Pickering Emulsion Templating with Zein and Sodium Alginate as Stabilizer. International Journal of Molecular Sciences. 2022; 23(16):9386. https://doi.org/10.3390/ijms23169386
Chicago/Turabian StyleXu, Yan, Zhe Jia, Jiaxing Wang, Jipeng Sun, and Ru Song. 2022. "Property and Stability of Astaxanthin Emulsion Based on Pickering Emulsion Templating with Zein and Sodium Alginate as Stabilizer" International Journal of Molecular Sciences 23, no. 16: 9386. https://doi.org/10.3390/ijms23169386
APA StyleXu, Y., Jia, Z., Wang, J., Sun, J., & Song, R. (2022). Property and Stability of Astaxanthin Emulsion Based on Pickering Emulsion Templating with Zein and Sodium Alginate as Stabilizer. International Journal of Molecular Sciences, 23(16), 9386. https://doi.org/10.3390/ijms23169386