Effect of Mono- and Multichlorinated Organic Compounds—Chlorocyclohexane and Hexachloro-p-xylene—On the Catalytic Properties of Titanium–Magnesium Catalysts in the Homo- and Copolymerization of Ethylene with 1-Hexene
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effect of the Nature of Chlorinated Organic Compounds on Catalytic Activity of TMC
2.1.1. Ethylene Homopolymerization in the Presence of CHC and HCPX
2.1.2. Ethylene/1-Hexene Copolymerization in the Presence of CHC and HCPX
2.2. The Effect of TMC Modification with Chlorinated Organic Compounds on Polyethylene Properties
2.2.1. Molecular Characteristics and the MFI of the Polymers
2.2.2. Thermal Properties of Nascent PE Powders
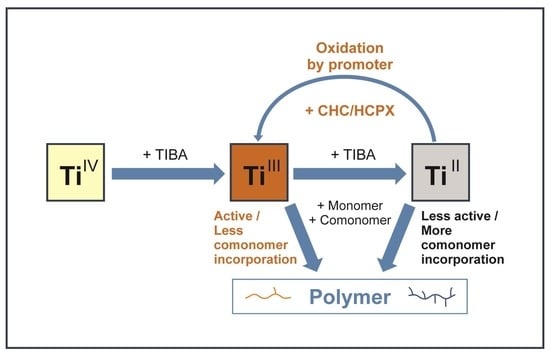
2.3. The Mechanistic Aspects of TMC Modification with Chlorinated Organic Discussion Compounds and Variation in Its Copolymerization Ability
- The lower reducing ability of organoaluminum compounds as alkyl groups are substituted with chlorine atoms in the reactions between AlR3 and RCl, as well as the lower rates of reductive processes in the catalytic system [8]; it can change the Ti2+/Ti3+ ratio in active sites.
- Halogenated hydrocarbons can be weakly coordinated to the titanium active site, thus changing its reactivity [14].
3. Materials and Methods
3.1. Reagents and Catalysts
3.2. Chemical Analysis of the Catalysts
3.3. Determining Particle Size Distribution and the Average Catalyst Particle Size
3.4. Slurry-Phase Polymerization
3.5. Determining the Copolymerization Constants
3.6. Analyzing Properties of the Polymers
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Spalding, M.A.; Chatterjee, A. (Eds.) Handbook of Industrial Polyethylene and Technology: Definitive Guide to Manufacturing, Properties, Processing, Applications and Markets Set; Scrivener Publishing LLC: Beverly, MA, USA, 2017. [Google Scholar] [CrossRef]
- Albunia, A.R.; Prades, F.; Jeremic, D. (Eds.) Multimodal Polymers with Supported Catalysts; Springer: Cham, Switzerland, 2019; pp. 243–265. [Google Scholar] [CrossRef]
- Stürzel, M.; Mihan, S.; Mülhaupt, R. From Multisite Polymerization Catalysis to Sustainable Materials and All-Polyolefin Composites. Chem. Rev. 2016, 116, 1398–1433. [Google Scholar] [CrossRef]
- Bahri-Laleh, N.; Arabi, H.; Mehdipor-Ataei, S.; Nekoomanesh-Haghighi, M.; Zohuri, G.; Seifali, M.; Akbari, Z. Activation of Ziegler–Natta catalysts by organohalide promoters: A combined experimental and density functional theory study. J. Appl. Polym. Sci. 2012, 123, 2526–2533. [Google Scholar] [CrossRef]
- Abedi, S.; Majdabadi-Farahani, N.; Daftari-Besheli, M.; Ghasempour, H.; Azadi, F. Promoting ethylene polymerization through cocatalyst modification. Polym. Bull. 2015, 72, 2377–2388. [Google Scholar] [CrossRef]
- Mikenas, T.B.; Zakharov, V.A.; Nikitin, V.E.; Echevskaya, L.G.; Matsko, M.A. The new generation of supported Ziegler-type catalysts for polyethylene production. Russ. J. Appl. Chem. 2010, 83, 2210–2219. [Google Scholar] [CrossRef]
- Mikenas, T.B.; Koshevoy, E.I.; Cherepanova, S.V.; Zakharov, V.A. Study of the composition and morphology of new modifications of titanium-magnesium catalysts with improved properties in ethylene polymerization. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 545–2558. [Google Scholar] [CrossRef]
- Chirkov, N.M.; Matkovsky, P.E.; Dyachkovsky, F.S. Polymerization with Complex Organometallic Compounds; Khimiya: Moscow, Russia, 1976. [Google Scholar]
- Adisson, E.; Deffieux, A.; Fontanille, M.; Bujadoux, K. Polymerization of ethylene at high temperature by vanadium-based heterogeneous Ziegler-Natta catalysts. II. Study of the activation by halocarbons. J. Polym. Sci. Part A 1994, 32, 1033–1041. [Google Scholar] [CrossRef]
- Rośario Ribeiro, M.; Deffieux, A.; Fontanille, M.; Portela, M.F. Homo and copolymerization of ethylene: Improvement of supported vanadium catalysts performance by halocarbons. Macromol. Chem. Phys. 1995, 196, 3833. [Google Scholar] [CrossRef]
- Spitz, R.; Pasquet, V.; Patin, M.; Guyot, A. The Activation of Supported Vanadium Catalysts in Ethylene Polymerization. In Ziegler Catalysts; Fink, G., Mülhaupt, R., Brintzinger, H.H., Eds.; Springer: Berlin/Heidelberg, Germany, 1995. [Google Scholar]
- Winslow, L.N. Vanadium-Containing Catalyst System. U.S. Patent 5534472, 9 July 1996. [Google Scholar]
- Reinking, M.K.; Bauer, P.D.; Seyler, J.W. A study of halocarbon promoter influence on catalyst reactivity and polymer Mn in vanadium-based ethylene polymerizations. J. Appl. Catal. A Gen. 1999, 189, 23–34. [Google Scholar] [CrossRef]
- Luo, H.K.; Tang, R.G.; Yang, H.; Zhao, Q.-F.; An, J.-Y. Studies on highly efficient promoters for titanium-based Ziegler–Natta catalyst for ethylene polymerization. Appl. Catal. A Gen. 2000, 203, 269–273. [Google Scholar] [CrossRef]
- Farrer, D.K. Process for the Polymerization of Ethylene and Interpolymers Thereof. U.S. Patent 6646073, 11 November 2003. [Google Scholar]
- Bahri-Laleh, N.; Abbas-Abadi, M.S.; Haghighi, M.N.; Akbari, Z.; Tavasoli, M.R.; Mirjahanmardi, S.H. Effect of halocarbon promoters on polyethylene properties using MgCl2(ethoxide type)/TiCl4/AlEt3/H2 catalyst system. J. Appl. Polym. Sci. 2010, 117, 1780–1786. [Google Scholar] [CrossRef]
- Abedi, S.; Azadi, F.; Daftari-Besheli, M.; Majdabadi-Farahani, N.; Safinejad, A. Butyl chloride as promoter in ethylene polymerization by magnesium ethoxide-supported catalyst. J. Appl. Polym. Sci. 2014, 131, 40189. [Google Scholar] [CrossRef]
- Abbas-Abadi, M.S. The production of high efficiency Ziegler–Natta catalyst with dual active sites nature using cyclohexyl chloride as promoter with super activity and produced superior polyethylene with controllable molecular weight distribution. Des. Monomers Polym. 2017, 20, 524–531. [Google Scholar] [CrossRef]
- Gholami, Y.; Abdouss, M.; Abedi, S.; Azadi, F.; Baniani, P.; Arsalanfar, M. An Investigation on Polymerization of Ethylene by Ziegler-Natta Catalyst in the Presence of a Promoter: Polymerization Behavior and Polymer Microstructure. Bull. Chem. React. Eng. Catal. 2018, 13, 412–419. [Google Scholar] [CrossRef]
- Abbas-Abadi, M.S.; Rashedi, R.; Sepahi, A.; Heydari, L.; Farhadi, A.; Biglarkhani, M. The effect of different process parameters on the TiCl4/internal donor/MgCl2/AlEt3 catalytic system using external donor and cyclohexylchloride. Iran. Polym. J. 2020, 29, 659–667. [Google Scholar] [CrossRef]
- Bazgir, H.; Abbas-Abadi, M.S.; Haghighi, M.N.; Darounkola, M.R.R.; Issaabadi, Z.; Rashedi, R. Synthesis of novel Ziegler Natta catalyst in the presence of internal promoter and electron donors for ethylene and ethylene/ 1-hexene polymerization. J. Polym. Res. 2021, 28, 301. [Google Scholar] [CrossRef]
- Malpass, D.B. Polyethylene with Broad Molecular Weight Distribution. U.S. Patent 4,657,998, 14 March 1987. [Google Scholar]
- Wu, Z.; Wu, Y. Titanium-Based Catalyst System for Olefin Polymerization. Patent CN 1189505A, 5 August 1998. [Google Scholar]
- Daire, E. Ethylene Polymerisation Process. U.S. Patent 5863995, 26 January 1999. [Google Scholar]
- Pietro, B.; Massimo, C.; Maria, D.D.; Lorella, M.; Antonio, M. Process for the polymerization of olefins. Patent WO 2018083161 A1, 4 November 2018. [Google Scholar]
- Meng, W.; Li, H.; Li, J.; Chen, B. The Effect of Comonomer Type and Content on the Properties of Ziegler-Natta Bimodal High-Density Polyethylene. J. Korean Chem. Soc. 2011, 55, 673–679. [Google Scholar] [CrossRef]
- Gupta, P.; Wilkes, G.L.; Sukhadia, A.M.; Krishnaswamy, R.K.; Lamborn, M.J.; Wharry, S.M.; Tso, C.C.; DesLauriers, P.J.; Mansfield, T.; Beyer, F.L. Does the length of the short chain branch affect the mechanical properties of linear low density polyethylenes? An investigation based on films of copolymers of ethylene/1-butene, ethylene/1-hexene and ethylene/1-octene synthesized by a single site metallocene catalyst. Polymer 2005, 46, 8819–8837. [Google Scholar] [CrossRef]
- Salakhov, I.I.; Akhmetov, I.G.; Kozlov, V.G. Polymerization of butadiene during the action of the catalytic system neodymium versatate-diisobutylaluminum hydride-hexachloro-p-xylene. Polym. Sci. Ser. B 2011, 53, 385–390. [Google Scholar] [CrossRef]
- Salakhov, I.; Tkacheva, E.; Kozlov, V.; Galanin, D.; Sakhabutdinov, A. A highly chlorinated xylene promoter for ethylene-propylene copolymerisation over a vanadium catalyst. RSC Adv. 2021, 11, 20916–20925. [Google Scholar] [CrossRef] [PubMed]
- Salakhov, I.I.; Sakhabutdinov, A.G.; Batyrshin, A.Z.; Shajdullin, N.M.; Borodin, R.G.; Mikhajlov, V.N.; Nazarov, V.V.; Khakimova, T.N.; Volkov, V.L.; Suslova, T.N. Method for Continuous Promotion of a Titanium-Magnesium Ziegler-Natta Catalyst in the Processes of Olefin (co)polymerization. Patent RU 275972, 27 November 2021. [Google Scholar]
- Nikolaeva, M.I.; Matsko, M.A.; Mikenas, T.B.; Echevskaya, L.G.; Zakharov, V.A. Copolymerization of Ethylene with α-Olefins over Supported Titanium–Magnesium Catalysts. II. Comonomer as a Chain Transfer Agent. J. Appl. Polym. Sci. 2012, 125, 2042–2049. [Google Scholar] [CrossRef]
- Mikenas, T.B.; Tregubov, A.A.; Zakharov, V.A.; Echevskaya, L.G.; Matsko, M.A. Titanium-magnesium catalysts for olefin polymerization—Effect of titanium oxidation state on catalyst performance. Polimery 2008, 53, 353–357. [Google Scholar] [CrossRef]
- Bahri-Laleh, N.; Correa, A.; Mehdipour-Ataei, S.M.; Arabi, H.; Haghighi, M.N.; Zohuri, G.; Cavallo, L. Moving up and down the Titanium Oxidation State in Ziegler−Natta Catalysis. Macromolecules 2011, 44, 778–783. [Google Scholar] [CrossRef]
- Kryzhanovskii, A.V.; Pvanchev, S.S. Synthesis of linear polyethylene on supported Ziegler-Natta catalysts. Review. Polym. Sci. U.S.S.R. 1990, 32, 1312–1329. [Google Scholar] [CrossRef]
- Mikenas, T.B.; Nikitin, V.E.; Zakharov, V.A. Method of Producing Catalyst for Polymerisation of Ethylene and Copolymerisation of Ethylene with Alpha-Olefons. Patent RU 2570645 C1, 10 December 2015. [Google Scholar]
- Fineman, M.; Ross, S.D. Linear method for determining monomer reactivity ratios in copolymerization. J. Polym. Sci. 1950, 5, 259–262. [Google Scholar] [CrossRef]
- Bohm, L.L. Copolymerisation von Ethylen und α-Olefinen mit Ziegler-Katalysatoren. Macromol. Chem. 1981, 182, 3291–3310. [Google Scholar] [CrossRef]
- Echevskaya, L.G.; Zakharov, V.A.; Golovin, A.V.; Mikenas, T.B. Molecular structure of polyethylene produced with supported vanadium-magnesium catalyst. Macromol. Chem. Phys. 1999, 200, 1434–1438. [Google Scholar] [CrossRef]







| Exp. No. | Type of COC | COC/Ti Molar Ratio | Yield (WG), kg PE/g cat∙h | Activity (WTi), kg PE/g Ti∙h |
|---|---|---|---|---|
| 1 | Without promoter | - | 1.8 | 33.3 |
| 4 | CHC | 1:1 | 6.7 | 136.7 |
| 7 | HCPX | 1:1 | 3.8 | 122.6 |
| Exp. No. | Name of Promoter | COC/Ti (Molar Ratio) | [1-Hexene], M | [C6H12]/[C2H4] (mol/mol) | WG, kg PE/g cat∙h | WTi, kg PE/g Ti∙h |
|---|---|---|---|---|---|---|
| 1 | Without promoter | - | 0 | 0 | 1.8 | 33.3 |
| 2 | - | 0.16 | 0.57 | 4.3 | 79.6 | |
| 3 | - | 0.32 | 1.14 | 3.2 | 59.3 | |
| 4 | CHC | 1:1 | 0 | 0 | 6.7 | 136.7 |
| 5 | 1:1 | 0.16 | 0.57 | 7.0 | 142.9 | |
| 6 | 1:1 | 0.32 | 1.14 | 6.5 | 132.7 | |
| 7 | HCPX | 1:1 | 0 | 0 | 3.8 | 122.6 |
| 8 | 1:1 | 0.16 | 0.57 | 5.2 | 167.7 | |
| 9 | 1:1 | 0.32 | 1.14 | 4.0 | 129.0 |
| Exp. No. | Name of Promoter | [1-hexene], M | Initial [C6H12]/[C2H4] (mol/mol) | [C6H12] mol.%. Polymer | Density, g/cm3 |
|---|---|---|---|---|---|
| 1 | Without promoter | 0 | 0 | - | 0.949 |
| 2 | 0.16 | 0.57 | 0.96 | 0.936 | |
| 3 | 0.32 | 1.14 | 1.2 | 0.933 | |
| 4 | CHC | 0 | 0 | - | 0.949 |
| 5 | 0.16 | 0.57 | 0.58 | 0.938 | |
| 6 | 0.32 | 1.14 | 1.12 | 0.935 | |
| 7 | HCPX | 0 | 0 | - | 0.949 |
| 8 | 0.16 | 0.57 | 0.26 | 0.942 | |
| 9 | 0.32 | 1.14 | 0.56 | 0.938 |
| Exp. No. | Catalytic System | The Constants of Ethylene (Monomer 1)/ Hexene 1 (Monomer 2) Copolymerization | r1 × r2 | |
|---|---|---|---|---|
| r1 | r2 | |||
| 1 | TMC–TIBA | 75 | 0.013 | 1.0 |
| 2 | TMC–TIBA–CHC | 101 | 0.010 | 1.0 |
| 3 | TMC–TIBA–HCPX | 202 | 0.005 | 1.0 |
| Exp. No. | Name of Promoter | [1-Hexene], M | Mw, kg/mol | Mn, kg/mol | Mw/Mn | Tm, °C | Crystallinity, % |
|---|---|---|---|---|---|---|---|
| 1 | Without promoter | 0 | 170 | 39.5 | 4.3 | 137.9 | 62 |
| 2 | 0.16 | 145 | 37.0 | 3.9 | 130.3 | 55 | |
| 3 | 0.32 | 120 | 31.5 | 3.8 | 130.8 | 40 | |
| 4 | CHC | 0 | 180 | 46 | 3.9 | 137.5 | 64 |
| 5 | 0.16 | 140 | 37 | 3.8 | 133.7 | 54 | |
| 6 | 0.32 | 100 | 26 | 3.8 | 131.0 | 48 | |
| 7 | HCPX | 0 | 200 | 51 | 3.9 | 138.6 | 61 |
| 8 | 0.16 | 165 | 44 | 3.8 | 134.5 | 54 | |
| 9 | 0.32 | 160 | 42 | 3.8 | 132.3 | 51 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salakhov, I.I.; Mikenas, T.B.; Zakharov, V.A.; Kozlov, V.G.; Matsko, M.A.; Suslova, T.N. Effect of Mono- and Multichlorinated Organic Compounds—Chlorocyclohexane and Hexachloro-p-xylene—On the Catalytic Properties of Titanium–Magnesium Catalysts in the Homo- and Copolymerization of Ethylene with 1-Hexene. Int. J. Mol. Sci. 2022, 23, 10335. https://doi.org/10.3390/ijms231810335
Salakhov II, Mikenas TB, Zakharov VA, Kozlov VG, Matsko MA, Suslova TN. Effect of Mono- and Multichlorinated Organic Compounds—Chlorocyclohexane and Hexachloro-p-xylene—On the Catalytic Properties of Titanium–Magnesium Catalysts in the Homo- and Copolymerization of Ethylene with 1-Hexene. International Journal of Molecular Sciences. 2022; 23(18):10335. https://doi.org/10.3390/ijms231810335
Chicago/Turabian StyleSalakhov, Ildar I., Tatiana B. Mikenas, Vladimir A. Zakharov, Valeriy G. Kozlov, Mikhail A. Matsko, and Tatiana N. Suslova. 2022. "Effect of Mono- and Multichlorinated Organic Compounds—Chlorocyclohexane and Hexachloro-p-xylene—On the Catalytic Properties of Titanium–Magnesium Catalysts in the Homo- and Copolymerization of Ethylene with 1-Hexene" International Journal of Molecular Sciences 23, no. 18: 10335. https://doi.org/10.3390/ijms231810335
APA StyleSalakhov, I. I., Mikenas, T. B., Zakharov, V. A., Kozlov, V. G., Matsko, M. A., & Suslova, T. N. (2022). Effect of Mono- and Multichlorinated Organic Compounds—Chlorocyclohexane and Hexachloro-p-xylene—On the Catalytic Properties of Titanium–Magnesium Catalysts in the Homo- and Copolymerization of Ethylene with 1-Hexene. International Journal of Molecular Sciences, 23(18), 10335. https://doi.org/10.3390/ijms231810335