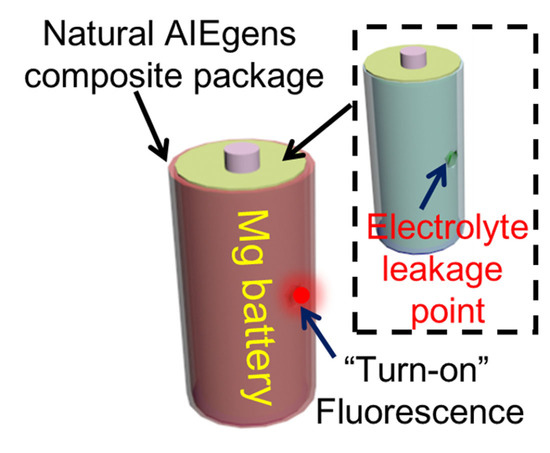
Sensing Leakage of Electrolytes from Magnesium Batteries Enabled by Natural AIEgens
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Characterization
3.3. Preparation of L-AIEgen
3.4. Preparation of L-AIE-F
3.5. Sensing Electrolyte Leakage
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shterenberg, I.; Salama, M.; Gofer, Y.; Levi, E.; Aurbach, D. The challenge of developing rechargeable magnesium batteries. MRS Bull. 2014, 39, 453–460. [Google Scholar] [CrossRef]
- Tutusaus, O.; Mohtadi, R.; Arthur, T.S.; Mizuno, F.; Nelson, E.G.; Sevryugina, Y.V. An efficient halogen-free electrolyte for use in rechargeable magnesium batteries. Angew. Chem. Int. Ed. 2015, 54, 7900–7904. [Google Scholar] [CrossRef]
- Barile, C.J.; Barile, E.C.; Zavadil, K.R.; Nuzzo, R.G.; Gewirth, A.A. Electrolytic conditioning of a magnesium aluminum chloride complex for reversible magnesium deposition. J. Phys. Chem. C 2014, 118, 27623–27630. [Google Scholar] [CrossRef]
- Tian, H.; Gao, T.; Li, X.; Wang, X.; Luo, C.; Fan, X.; Yang, C.; Suo, L.; Ma, Z.; Han, W. High power rechargeable magnesium/iodine battery chemistry. Nat. Commun. 2017, 8, 14083. [Google Scholar] [CrossRef] [PubMed]
- Canepa, P.; Jayaraman, S.; Cheng, L.; Rajput, N.N.; Richards, W.D.; Gautam, G.S.; Curtiss, L.A.; Persson, K.A.; Ceder, G. Elucidating the structure of the magnesium aluminum chloride complex electrolyte for magnesium-ion batteries. Energy Environ. Sci. 2015, 8, 3718–3730. [Google Scholar] [CrossRef]
- Lu, D.; Liu, H.; Huang, T.; Xu, Z.; Ma, L.; Yang, P.; Qiang, P.; Zhang, F.; Wu, D. Magnesium ion based organic secondary batteries. J. Mater. Chem. A 2018, 6, 17297–17302. [Google Scholar] [CrossRef]
- Song, M.; Niu, J.; Gao, H.; Kou, T.; Wang, Z.; Zhang, Z. Phase engineering in lead-bismuth system for advanced magnesium ion batteries. J. Mater. Chem. A 2020, 8, 13572–13584. [Google Scholar] [CrossRef]
- Tarascon, J.M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef]
- Kim, H.S.; Arthur, T.S.; Allred, G.D.; Zajicek, J.; Newman, J.G.; Rodnyansky, A.E.; Oliver, A.G.; Boggess, W.C.; Muldoon, J. Structure and compatibility of a magnesium electrolyte with a sulphur cathode. Nat. Commun. 2011, 2, 427. [Google Scholar] [CrossRef]
- Crowther, O.; West, A.C. Effect of electrolyte composition on lithium dendrite growth. J. Electrochem. Soc. 2008, 155, A806. [Google Scholar] [CrossRef]
- Xu, K. Nonaqueous liquid electrolytes for lithium-based rechargeable batteries. Chem. Rev. 2004, 104, 4303–4418. [Google Scholar] [CrossRef] [PubMed]
- Aurbach, D.; Cohen, Y.; Moshkovich, M. The study of reversible magnesium deposition by in situ scanning tunneling microscopy. Electrochem. Solid-State Lett. 2001, 4, A113. [Google Scholar] [CrossRef]
- Attias, R.; Salama, M.; Hirsch, B.; Goffer, Y.; Aurbach, D. Anode-electrolyte interfaces in secondary magnesium batteries. Joule 2018, 3, 27–52. [Google Scholar] [CrossRef]
- Rodriguez, J.; Chenoy, L.; Roobroeck, A.; Godet, S.; Olivier, M.G. Effect of the electrolyte pH on the corrosion mechanisms of Zn-Mg coated steel. Corros. Sci. 2016, 108, 47–59. [Google Scholar] [CrossRef]
- Zalyhina, V.; Cheprasova, V.; Romanovski, V. Pigments from spent ammonium chloride zinc plating electrolytes. J. Chem. Technol. Biotechnol. 2021, 96, 2767–2774. [Google Scholar] [CrossRef]
- Henriksen, M.; Vaagsaether, K.; Lundberg, J.; Forseth, S.; Bjerketvedt, D. Explosion characteristics for Li-ion battery electrolytes at elevated temperatures. J. Hazard. Mater. 2019, 371, 1–7. [Google Scholar] [CrossRef]
- Shi, L.; Gao, X.; Yuan, W.; Xu, L.; Deng, H.; Wu, C.; Yang, J.; Jin, X.; Zhang, C.; Zhu, X. Endoplasmic Reticulum-Targeted Fluorescent Nanodot with Large Stokes Shift for Vesicular Transport Monitoring and Long-Term Bioimaging. Small 2018, 14, 1800223. [Google Scholar] [CrossRef]
- Chen, X.; Liu, X.; Lei, J.; Xu, L.; Zhao, Z.; Kausar, F.; Xie, X.; Zhu, X.; Zhang, Y.; Yuan, W.Z. Synthesis, clustering-triggered emission, explosive detection and cell imaging of nonaromatic polyurethanes. Mol. Syst. Des. Eng. 2018, 3, 364–375. [Google Scholar] [CrossRef]
- Yuan, W.Z.; Wang, Q.; Dou, X.; Chen, X.; Zhao, Z.; Wang, S.; Wang, Y.; Sui, K.; Tan, Y.; Gong, Y. Reevaluating the Protein Emission: Remarkable Visible Luminescence and Emissive Mechanism. Angew. Chem. Int. Ed. 2019, 58, 12667–12673. [Google Scholar]
- Dong, S.; Xu, J.; Jia, T.; Xu, M.; Zhong, C.; Yang, G.; Li, J.; Yang, D.; He, F.; Gai, S. Upconversion-mediated ZnFe2O4 nanoplatform for NIR-enhanced chemodynamic and photodynamic therapy. Chem. Sci. 2019, 10, 4259–4271. [Google Scholar] [CrossRef]
- Feng, L.; He, F.; Dai, Y.; Liu, B.; Yang, G.; Gai, S.; Niu, N.; Lv, R.; Li, C.; Yang, P. A versatile near infrared light triggered dual-photosensitizer for synchronous bioimaging and photodynamic therapy. ACS Appl. Mater. Interfaces 2017, 9, 12993–13008. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Feng, L.; Yang, P.; Liu, B.; Gai, S.; Yang, G.; Dai, Y.; Lin, J. Enhanced up/down-conversion luminescence and heat: Simultaneously achieving in one single core-shell structure for multimodal imaging guided therapy. Biomaterials 2016, 105, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Li, S.; Häupler, B.; Liu, J.; Jin, S.; Steffen, W.; Schubert, U.S.; Butt, H.J.; Liang, X.J.; Wu, S. An amphiphilic ruthenium polymetallodrug for combined photodynamic therapy and photochemotherapy in vivo. Adv. Mater. 2017, 29, 1603702. [Google Scholar] [CrossRef]
- Sun, W.; Parowatkin, M.; Steffen, W.; Butt, H.J.; Mailänder, V.; Wu, S.J. Ruthenium-Containing Block Copolymer Assemblies: Red-Light-Responsive Metallopolymers with Tunable Nanostructures for Enhanced Cellular Uptake and Anticancer Phototherapy. Adv. Healthc. Mater. 2016, 5, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Saengsrichan, A.; Saikate, C.; Silasana, P.; Khemthong, P.; Wanmolee, W.; Phanthasri, J.; Youngjan, S.; Posoknistakul, P.; Ratchahat, S.; Laosiripojana, N.; et al. The Role of N and S Doping on Photoluminescent Characteristics of Carbon Dots from Palm Bunches for Fluorimetric Sensing of Fe3+ Ion. Int. J. Mol. Sci. 2022, 23, 5001. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Yan, D. White-light emission and tunable room temperature phosphorescence of dibenzothiophene. Sci. China Chem. 2018, 61, 397–401. [Google Scholar] [CrossRef]
- Li, X.; Baryshnikov, G.; Deng, C.; Bao, X.; Wu, B.; Zhou, Y.; Ågren, H.; Zhu, L. A three-dimensional ratiometric sensing strategy on unimolecular fluorescence-thermally activated delayed fluorescence dual emission. Nat. Commun. 2019, 10, 731. [Google Scholar] [CrossRef]
- Zhou, Y.; Baryshnikov, G.; Li, X.; Zhu, M.; Ågren, H.; Zhu, L. Anti-Kasha’s rule emissive switching induced by intermolecular H-bonding. Chem. Mater. 2018, 30, 8008–8016. [Google Scholar] [CrossRef]
- Wu, Y.; Xie, Y.; Zhang, Q.; Tian, H.; Zhu, W.; Li, A.D. Quantitative photoswitching in bis (dithiazole) ethene enables modulation of light for encoding optical signals. Angew. Chem. Int. Ed. 2014, 53, 2090–2094. [Google Scholar] [CrossRef]
- Li, D.; Hu, W.; Wang, J.; Zhang, Q.; Cao, X.-M.; Ma, X.; Tian, H. White-light emission from a single organic compound with unique self-folded conformation and multistimuli responsiveness. Chem. Sci. 2018, 9, 5709–5715. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, C.; Chen, M.; An, Y.; Zheng, Y.; Tian, H.; Shi, R.; He, X.; Lin, X. Solvent-Free Preparation of Tannic Acid Carbon Dots for Selective Detection of Ni2+ in the Environment. Int. J. Mol. Sci. 2022, 23, 6681. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Yan, Q.; Feng, L.; Deng, S.; Wang, C.; Cui, J.; Wang, C.; Zhang, Z.; James, T.D.; Ma, X. A far-red fluorescent probe for sensing laccase in fungi and its application in developing an effective biocatalyst for the biosynthesis of antituberculous dicoumarin. Chem. Commun. 2019, 55, 3951–3954. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Wang, Y.; James, T.D.; Jia, N.; Huang, C. A hemicyanine based ratiometric fluorescence probe for mapping lysosomal pH during heat stroke in living cells. Chem. Commun. 2018, 54, 5518–5521. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Shao, W.; Valiev, R.R.; Ohulchanskyy, T.Y.; He, G.S.; Ågren, H.; Prasad, P.N. Efficient Broadband Upconversion of Near-Infrared Light in Dye-Sensitized Core/Shell Nanocrystals. Adv. Opt. Mater. 2016, 4, 1760–1766. [Google Scholar] [CrossRef]
- Shao, W.; Chen, G.; Kuzmin, A.; Kutscher, H.L.; Pliss, A.; Ohulchanskyy, T.Y.; Prasad, P.N. Tunable narrow band emissions from dye-sensitized core/shell/shell nanocrystals in the second near-infrared biological window. J. Am. Chem. Soc. 2016, 138, 16192–16195. [Google Scholar] [CrossRef]
- Suzuki, Y.; Komatsu, H.; Ikeda, T.; Saito, N.; Araki, S.; Citterio, D.; Hisamoto, H.; Kitamura, Y.; Kubota, T.; Nakagawa, J.; et al. Design and synthesis of Mg2+-selective fluoroionophores based on a coumarin derivative and application for Mg2+ measurement in a living cell. Anal. Chem. 2002, 74, 1423–1428. [Google Scholar] [CrossRef]
- Fermi, A.; Bergamini, G.; Roy, M.; Gingras, M.; Ceroni, P. Turn-on Phosphorescence by Metal Coordination to a Multivalent Terpyridine Ligand: A New Paradigm for Luminescent Sensors. J. Am. Chem. Soc. 2014, 136, 6395–6400. [Google Scholar] [CrossRef]
- Bian, Y.-J.; Wang, L.-Q.; Cao, F.-X.; Tang, L.-J. A Simple Fluorescence Probe Based on Aggregation-Induced Emission (AIE) Property for the Detection of Mg2+ Ions. J. Fluoresc. 2016, 26, 53–57. [Google Scholar] [CrossRef]
- Luo, J.; Xie, Z.; Lam, J.W.; Cheng, L.; Chen, H.; Qiu, C.; Kwok, H.S.; Zhan, X.; Liu, Y.; Zhu, D.; et al. Aggregation-induced emission of 1-methyl-1, 2, 3, 4, 5-pentaphenylsilole. Chem. Commun. 2001, 18, 1740–1741. [Google Scholar] [CrossRef]
- Chen, Y.; Lam, J.W.; Kwok, R.T.; Liu, B.; Tang, B.Z. Aggregation-induced emission: Fundamental understanding and future developments. Mater. Horiz. 2019, 6, 428–433. [Google Scholar] [CrossRef]
- Hong, Y.; Lam, J.W.Y.; Tang, B.Z. Aggregation-induced emission. Chem. Soc. Rev. 2011, 40, 5361–5388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mei, J.; Hong, Y.; Lam, J.W.; Qin, A.; Tang, Y.; Tang, B.Z. Aggregation-induced emission: The whole is more brilliant than the parts. Adv. Mater. 2014, 26, 5429–5479. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lin, X.; Shu, T.; Su, L.; Liang, F.; Zhang, X. Self-assembly of metal nanoclusters for aggregation-induced emission. Int. J. Mol. Sci. 2019, 20, 1891. [Google Scholar] [CrossRef] [PubMed]
- Feng, G.; Liu, B. Aggregation-induced emission (AIE) dots: Emerging theranostic nanolights. Acc. Chem. Res. 2018, 51, 1404–1414. [Google Scholar] [CrossRef] [PubMed]
- He, T.; Wang, H.; Chen, Z.; Liu, S.; Li, J.; Li, S. Natural Quercetin AIEgen Composite Film with Antibacterial and Antioxidant Properties for in Situ Sensing of Al3+ Residues in Food, Detecting Food Spoilage, and Extending Food Storage Times. ACS Appl. Bio Mater. 2018, 1, 636–642. [Google Scholar] [CrossRef]
- Ma, Z.; Liu, C.; Niu, N.; Chen, Z.; Li, S.; Liu, S.; Li, J. Seeking brightness from nature: J-aggregation-induced emission in cellulolytic enzyme lignin nanoparticles. ACS Sustain. Chem. Eng. 2018, 6, 3169–3175. [Google Scholar] [CrossRef]
- Wang, P.; Liu, C.; Tang, W.; Ren, S.; Chen, Z.; Guo, Y.; Rostamian, R.; Zhao, S.; Li, J.; Liu, S.; et al. Molecular Glue Strategy: Large-Scale Conversion of Clustering-Induced Emission Luminogen to Carbon Dots. ACS Appl. Mater. Interfaces 2019, 1, 19301–19307. [Google Scholar] [CrossRef]
- He, T.; Niu, N.; Chen, Z.; Li, S.; Liu, S.; Li, J. Novel quercetin aggregation-induced emission luminogen (AIEgen) with excited-state intramolecular proton transfer for in vivo bioimaging. Adv. Funct. Mater. 2018, 28, 1706196. [Google Scholar] [CrossRef]
- Case, D.R.; Zubieta, J.; Doyle, R.P. The Coordination Chemistry of Bio-Relevant Ligands and Their Magnesium Complexes. Molecules 2020, 25, 3172. [Google Scholar] [CrossRef]
- Chairat, M.; Rattanaphani, V.; Bremner, J.B.; Rattanaphani, S.; Perkins, D.F.J.D. An absorption spectroscopic investigation of the interaction of lac dyes with metal ions. Dyes Pigments 2004, 63, 141–150. [Google Scholar] [CrossRef]
- Xie, W.; Bao, Q.; Liu, Y.; Wen, H.; Wang, Q. Hydrogen Bond Association to Prepare Flame Retardant Polyvinyl Alcohol Film with High Performance. ACS Appl. Mater. Interfaces 2021, 13, 5508–5517. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Wang, H. High-Performance Polymeric Materials through Hydrogen-Bond Cross-Linking. Adv. Mater. 2020, 32, 1901244. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Sletten, E.M. Fluorescent cyanine dye J-Aggregates in the fluorous phase. J. Am. Chem. Soc. 2018, 140, 2727–2730. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Peng, H.J.; Li, B.Q.; Xie, J.; Kong, L.; Zhao, M.; Chen, X.; Huang, J.Q.; Zhang, Q. Conductive and Catalytic Triple-Phase Interfaces Enabling Uniform Nucleation in High-Rate Lithium-Sulfur Batteries. Adv. Energy Mater. 2019, 9, 1802768. [Google Scholar] [CrossRef]
- Tu, S.B.; Chen, X.; Zhao, X.X.; Cheng, M.R.; Xiong, P.X.; He, Y.W.; Zhang, Q.; Xu, Y. A Polysulfide-Immobilizing Polymer Retards the Shuttling of Polysulfide Intermediates in Lithium-Sulfur Batteries. Adv. Mater. 2018, 30, 1804581. [Google Scholar] [CrossRef]
- Yan, C.; Cheng, X.B.; Yao, Y.X.; Shen, X.; Li, B.Q.; Li, W.J.; Zhang, R.; Huang, J.Q.; Li, H.; Zhang, Q. An Armored Mixed Conductor Interphase on a Dendrite-Free Lithium-Metal Anode. Adv. Mater. 2018, 30, 1804461. [Google Scholar] [CrossRef] [PubMed]
- Li, B.Q.; Zhang, S.Y.; Kong, L.; Peng, H.J.; Zhang, Q. Porphyrin Organic Framework Hollow Spheres and Their Applications in Lithium-Sulfur Batteries. Adv. Mater. 2018, 30, 1707483. [Google Scholar] [CrossRef]




| Ethanol Fraction (%) | Solution A (mL) | Water (mL) | Ethanol (mL) |
|---|---|---|---|
| 0 | 0.1 | 9.9 | 0 |
| 20 | 0.1 | 7.9 | 2 |
| 40 | 0.1 | 5.9 | 4 |
| 50 | 0.1 | 4.9 | 5 |
| 60 | 0.1 | 3.9 | 6 |
| 70 | 0.1 | 2.9 | 7 |
| 80 | 0.1 | 1.9 | 8 |
| 90 | 0.1 | 0.9 | 9 |
| 99 | 0.1 | 0 | 9.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhai, Y.; Zhang, J.; Li, J.; Liu, S.; Chen, Z.; Li, S. Sensing Leakage of Electrolytes from Magnesium Batteries Enabled by Natural AIEgens. Int. J. Mol. Sci. 2022, 23, 10440. https://doi.org/10.3390/ijms231810440
Zhai Y, Zhang J, Li J, Liu S, Chen Z, Li S. Sensing Leakage of Electrolytes from Magnesium Batteries Enabled by Natural AIEgens. International Journal of Molecular Sciences. 2022; 23(18):10440. https://doi.org/10.3390/ijms231810440
Chicago/Turabian StyleZhai, Yingxiang, Jiguo Zhang, Jian Li, Shouxin Liu, Zhijun Chen, and Shujun Li. 2022. "Sensing Leakage of Electrolytes from Magnesium Batteries Enabled by Natural AIEgens" International Journal of Molecular Sciences 23, no. 18: 10440. https://doi.org/10.3390/ijms231810440
APA StyleZhai, Y., Zhang, J., Li, J., Liu, S., Chen, Z., & Li, S. (2022). Sensing Leakage of Electrolytes from Magnesium Batteries Enabled by Natural AIEgens. International Journal of Molecular Sciences, 23(18), 10440. https://doi.org/10.3390/ijms231810440