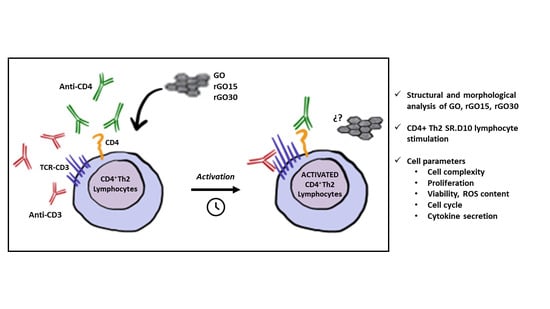
Effects of Graphene Oxide and Reduced Graphene Oxide Nanostructures on CD4+ Th2 Lymphocytes
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structural and Morphological Analysis of GO, rGO15 and rGO30 Nanostructures
2.2. Effects of GO, rGO15 and rGO30 on the Intracellular Complexity of CD4+ Th2 SR.D10 Lymphocytes in Basal and Stimulated Conditions
2.3. Proliferation of CD4+ Th2 SR.D10 Lymphocytes after GO, rGO15 and rGO30 Treatment in Basal and Stimulated Conditions
2.4. Effects of GO, rGO15 and rGO30 on Cell-Cycle Phases of CD4+ Th2 SR.D10 Lymphocytes in Basal and Stimulated Conditions
2.5. Effects of GO, rGO15 and rGO30 on Cell viability and Intracellular Reactive Oxygen Species (ROS) Content of CD4+ Th2 SR.D10 Lymphocytes in Basal and Stimulated Conditions
2.6. Effects of GO, rGO15 and rGO30 on Interleukin-4 (IL-4) secretion by CD4+ Th2 SR.D10 Lymphocytes in Basal and Stimulated Conditions
3. Materials and Methods
3.1. GO, rGO15 and rGO30 Nanostructures Preparation
3.2. Structural and Morphological Characterization of GO, rGO15 and rGO30 Nanostructures
3.3. Culture of CD4+ Th2 Lymphocyte Cell Line SR.D10 for Treatment with GO, rGO15 and rGO30
3.4. Effects of GO, rGO15 and rGO30 on the Intracellular Complexity of CD4+ Th2 SR.D10 Lymphocytes Evaluated by Flow Cytometry
3.5. Cell Viability Studies
3.6. Intracellular Reactive Oxygen Species (ROS) Content of CD4+ Th2 SR.D10 Lymphocytes Analyzed by Flow Cytometry
3.7. Cell-Cycle Phases of CD4+ Th2 SR.D10 Lymphocytes Analyzed by Flow Cytometry
3.8. Detection of Interleukin IL-4
3.9. Statistics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Girão, A.F.; Sousa, J.; Domínguez-Bajo, A.; González-Mayorga, A.; Bdikin, I.; Pujades-Otero, E.; Casañ-Pastor, N.; Hortigüela, M.J.; Otero-Irurueta, G.; Completo, A.; et al. 3D Reduced Graphene Oxide Scaffolds with a Combinatorial Fibrous-Porous Architecture for Neural Tissue Engineering. ACS Appl. Mater. Interfaces 2020, 12, 38962–38975. [Google Scholar] [CrossRef]
- Goncalves, G.; Marques, P.A.A.P.; Granadeiro, C.M.; Nogueira, H.I.S.; Singh, M.K.; Grácio, J. Surface Modification of Graphene Nanosheets with Gold Nanoparticles: The Role of Oxygen Moieties at Graphene Surface on Gold Nucleation and Growth. Chem. Mater. 2009, 21, 4796–4802. [Google Scholar] [CrossRef]
- Bramini, M.; Alberini, G.; Colombo, E.; Chiacchiaretta, M.; DiFrancesco, M.L.; Maya-Vetencourt, J.F.; Maragliano, L.; Benfenati, F.; Cesca, F. Interfacing Graphene-Based Materials with Neural Cells. Front. Syst. Neurosci. 2018, 12, 12. [Google Scholar] [CrossRef] [PubMed]
- Magaz, A.; Li, X.; Gough, J.E.; Blaker, J.J. Graphene oxide and electroactive reduced graphene oxide-based composite fibrous scaffolds for engineering excitable nerve tissue. Mater. Sci. Eng. C 2021, 119, 111632. [Google Scholar] [CrossRef] [PubMed]
- Ray, P.; Haideri, N.; Haque, I.; Mohammed, O.; Chakraborty, S.; Banerjee, S.; Quadir, M.; Brinker, A.E.; Banerjee, S.K. The Impact of Nanoparticles on the Immune System: A Gray Zone of Nanomedicine. J. Immunol. Sci. 2021, 5, 19–33. [Google Scholar] [CrossRef]
- Franz, S.; Rammel, S.; Scharnweber, D.; Simon, J.C. Immune Responses to Implants—A Review of the Implications for the Design of Immunomodulatory Biomaterials. Biomaterials 2011, 32, 6692–6709. [Google Scholar] [CrossRef]
- Wang, J.; Chen, H.J.; Hang, T.; Yu, Y.; Liu, G.; He, G.; Xiao, S.; Yang, B.R.; Yang, C.; Liu, F.; et al. Physical activation of innate immunity by spiky particles. Nat. Nanotechnol. 2018, 13, 1078–1086. [Google Scholar] [CrossRef]
- Boraschi, D.; Italiani, P.; Palomba, R.; Decuzzi, P.; Duschl, A.; Fadeel, B.; Moghimi, S.M. Nanoparticles and innate immunity: New perspectives on host defence. Semin. Immunol. 2017, 34, 33–51. [Google Scholar] [CrossRef]
- Swartzwelter, B.J.; Fux, A.C.; Johnson, L.; Swart, E.; Hofer, S.; Hofstätter, N.; Geppert, M.; Italiani, P.; Boraschi, D.; Duschl, A.; et al. The Impact of Nanoparticles on Innate Immune Activation by Live Bacteria. Int. J. Mol. Sci. 2020, 21, 9695. [Google Scholar] [CrossRef]
- Ernst, L.M.; Casals, E.; Italiani, P.; Boraschi, D.; Puntes, V. The Interactions between Nanoparticles and the Innate Immune System from a Nanotechnologist Perspective. Nanomaterials 2021, 11, 2991. [Google Scholar] [CrossRef]
- Dowling, J.K.; Mansell, A. Toll-like receptors: The swiss army knife of immunity and vaccine development. Clin. Transl. Immunol. 2016, 5, e85. [Google Scholar] [CrossRef] [PubMed]
- Kapsenberg, M.L. Dendritic-cell control of pathogen-driven T-cell polarization. Nat. Rev. Immunol. 2003, 3, 984–993. [Google Scholar] [CrossRef] [PubMed]
- Cantor, H.; Boyse, E.A. Lymphocytes as Models for the Study of Mammalian Cellular Differentiation. Immunol. Rev. 1977, 33, 105–124. [Google Scholar] [CrossRef] [PubMed]
- Shiku, H.; Kisielow, P.; Bean, M.A.; Takahashi, T.; Boyse, E.A.; Oettgen, H.F.; Old, L.J. Expression of T-cell differentiation antigens on effector cells in cell-mediated cytotoxicity in vitro. Evidence for functional heterogeneity related to the surface phenotype of T cells. J. Exp. Med. 1975, 141, 227–241. [Google Scholar] [CrossRef]
- Janeway, C.A., Jr.; Carding, S.; Jones, B.; Murray, J.; Portoles, P.; Rasmussen, R.; Rojo, J.M.; Saizawa, K.; West, J.; Bottomly, J. CD4+ T Cells: Specificity and Function. Immunol. Rev. 1992, 101, 39–80. [Google Scholar] [CrossRef]
- Parnes, J.R. Molecular Biology and Function of CD4 and CD8. Adv. Immunol. 1989, 44, 265–311. [Google Scholar]
- Na, H.; Cho, M.; Chung, Y. Regulation of Th2 cell immunity by dendritic cells. Immune Netw. 2016, 16, 1–12. [Google Scholar] [CrossRef]
- Mϕrch, A.M.; Bálint, S.; Santos, A.M.; Davis, S.J.; Dustin, M.L. Coreceptors and TCR Signaling—the Strong and the Weak of It. Front. Cell Dev. Biol. 2020, 8, 597627. [Google Scholar] [CrossRef]
- Talaat, R.M.; Mohamed, S.F.; Bassyouni, I.H.; Raouf, A.A. Th1/Th2/Th17/Treg cytokine imbalance in systemic lupus erythematosus (SLE) patients: Correlation with disease activity. Cytokine 2015, 72, 146–153. [Google Scholar] [CrossRef]
- Yu, S.L.; Kuan, W.P.; Wong, C.K.; Li, E.K.; Tam, L.S. Immunopathological roles of cytokines, chemokines, signaling molecules, and pattern-recognition receptors in systemic lupus erythematosus. Clin. Dev. Immunol. 2012, 2012, 715190. [Google Scholar] [CrossRef]
- McHeyzer-Williams, L.J.; Pelletier, N.; Mark, L.; Fazilleau, N.; McHeyzer-Williams, M.G. Follicular helper T cells as cognate regulators of B cell immunity. Curr. Opin. Immunol. 2009, 2, 266–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gong, N.; Sheppard, N.C.; Billingsley, M.M.; June, C.H.; Mitchell, M.J. Nanomaterials for T-cell cancer immunotherapy. Nat. Nanotechnol. 2021, 16, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Cicuéndez, M.; Casarrubios, L.; Barroca, N.; Silva, D.; Feito, M.J.; Diez-Orejas, R.; Marques, P.A.A.P.; Portolés, M.T. Benefits in the Macrophage Response Due to Graphene Oxide Reduction by Thermal Treatment. Int. J. Mol. Sci. 2021, 22, 6701. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Li, D.; Fu, O.; Pan, C. Influence of graphene microstructures on electrochemical performance for supercapacitors. Prog. Nat. Sci. 2015, 25, 379–385. [Google Scholar] [CrossRef]
- Kuila, A.; Maity, N.; Layek, R.K.; Nandi, A.K. On the pH sensitive optoelectronic properties of amphiphilic reduced graphene oxide via grafting of poly(dimethylaminoethyl methacrylate): A signature of p- and n-type doping. J. Mater. Chem. A 2014, 2, 16039–16050. [Google Scholar] [CrossRef]
- Guo, S.; Raya, J.; Ji, D.; Nishina, Y.; Ménard-Moyon, C.; Bianco, A. Is carboxylation an efficient method for graphene oxide functionalization? Nanoscale Adv. 2020, 2, 4085–4092. [Google Scholar] [CrossRef]
- Jasim, D.A.; Lozano, N.; Kostarelos, K. Synthesis of few-layered, high-purity graphene oxide sheets from different graphite sources for biology. 2D Mater. 2016, 3, 014006. [Google Scholar] [CrossRef]
- Bazán, A.; Akashi, L.; Quintana, M.; Champi, A. Morphological and structural properties in graphene oxides and reduced graphene oxides. Revista de Investigación de Física 2019, 22, 9–16. [Google Scholar] [CrossRef]
- López-Díaz, D.; López Holgado, M.; García-Fierro, J.L.; Velázquez, M.M. Evolution of the Raman Spectrum with the Chemical Composition of Graphene Oxide. J. Phys. Chem. C 2017, 121, 20489–20497. [Google Scholar] [CrossRef]
- Ma, B.; Rodriguez, R.D.; Ruban, A.; Pavlov, S.; Shereme, E. The correlation between electrical conductivity and second-order Raman modes of laser-reduced graphene oxide. Phys. Chem. Chem. Phys. 2019, 21, 10125–10134. [Google Scholar] [CrossRef]
- Wu, J.B.; Lin, M.L.; Cong, X.; Liu, H.N.; Tan, P.H. Raman spectroscopy of graphene-based materials and its applications in related devices. Chem. Soc. Rev. 2018, 47, 1822–1873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Claramunt, S.; Varea, A.; López-Díaz, D.; Velázquez, M.M.; Cornet, A.; Cirera, A. The Importance of Interbands on the Interpretation of the Raman Spectrum of Graphene Oxide. J. Phys. Chem. C 2015, 119, 10123–10129. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, L.; Wei, S.; He, Y.; Xia, H.; Chen, Q.; Sun, H.B.; Xiao, F.S. Direct imprinting of microcircuits on graphene oxides film by femtosecond laser reduction. Nano Today 2010, 5, 15–20. [Google Scholar] [CrossRef]
- Ojeda, G.; Ronda, M.; Ballester, S.; Díez-Orejas, R.; Feito, M.J.; Garcia-Albert, L.; Rojo, J.M.; Portolés, P.; Díez-Orejas, R. A Hyperreactive Variant of a CD4+ T Cell Line Is Activated by Syngeneic Antigen Presenting Cells in the Absence of Antigen. Cell. Immunol. 1995, 164, 265–278. [Google Scholar] [CrossRef] [PubMed]
- Kaye, J.; Porcelli, S.; Tite, J.; Jones, B.; Janeway, C.A., Jr. Both a monoclonal antibody and antisera specific for determinants unique to individual cloned helper T cell lines can substitute for antigen and antigen-presenting cells in the activation of T cells. J. Exp. Med. 1983, 158, 836–856. [Google Scholar] [CrossRef] [PubMed]
- Udall, J.N.; Moscicki, R.A.; Preffer, F.I.; Ariniello, P.D.; Carter, E.A.; Bhan, A.K.; Bloch, K.J. Flow cytometry: A new approach to the isolation and characterization of Kupffer cells. In Recent Advances in Mucosal Immunology; Advances in Experimental Medicine and Biology; Mestecky, J., McGhee, J.R., Bienenstock, J., Ogra, P.L., Eds.; Springer: Boston, MA, USA, 1987; Volume 216A, pp. 821–827. [Google Scholar]
- Ribeiro, A.; Laranjeira, P.; Mendes, S.; Velada, I.; Leite, C.; Andrade, P.; Santos, F.; Henriques, A.; Grãos, M.; Cardoso, C.M.P.; et al. Mesenchymal stem cells from umbilical cord matrix, adipose tissue and bone marrow exhibit different capability to suppress peripheral blood B, natural killer and T cells. Stem Cell Res. Ther. 2013, 4, 125. [Google Scholar] [CrossRef]
- Greulich, C.; Diendorf, J.; Simon, T.; Eggeler, G.; Epple, M. Uptake and intracellular distribution of silver nanoparticles in human mesenchymal stem cells. Acta Biomater. 2011, 7, 347–354. [Google Scholar] [CrossRef]
- Suzuki, H.; Toyooka, T.; Ibuki, Y. Simple and easy method to evaluate uptake potential of nanoparticles in mammalian cells using a flow cytometric light scatter analysis. Environ. Sci. Technol. 2007, 41, 3018–3024. [Google Scholar] [CrossRef]
- Casarrubios, L.; Gómez-Cerezo, N.; Feito, M.J.; Vallet-Regí, M.; Arcos, D.; Portolés, M.T. Incorporation and effects of mesoporous SiO2-CaO nanospheres loaded with ipriflavone on osteoblast/osteoclast cocultures. Eur. J. Pharm. Biopharm. 2018, 133, 258–268. [Google Scholar] [CrossRef]
- Casarrubios, L.; Gómez-Cerezo, N.; Feito, M.J.; Vallet-Regí, M.; Arcos, D.; Portolés, M.T. Ipriflavone-Loaded Mesoporous Nanospheres with Potential Applications for Periodontal Treatment. Nanomaterials 2020, 10, 2573. [Google Scholar] [CrossRef]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef] [PubMed]
- Matesanz, M.C.; Vila, M.; Feito, M.J.; Linares, J.; Gonçalves, G.; Vallet-Regí, M.; Marques, P.A.A.P.; Portolés, M.T. The effects of graphene oxide nanosheets localized on F-actin filaments on cell-cycle alterations. Biomaterials 2013, 34, 1562–1569. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T.A.; Vannella, K.M. Macrophages in Tissue Repair, Regeneration, and Fibrosis. Immunity 2016, 44, 450–462. [Google Scholar] [CrossRef] [PubMed]
- Criado, G.; Feito, M.J.; Rojo, J.M. CD4-dependent and -independent association of protein tyrosine kinases to the T cell receptor/CD3 complex of CD4+ mouse T lymphocytes. Eur. J. Immunol. 1996, 26, 1228–1234. [Google Scholar] [CrossRef] [PubMed]
- Diez-Orejas, R.; Ballester, S.; Feito, M.J.; Ojeda, G.; Criado, G.; Ronda, M.; Rojo, J.M. Genetic and immunochemical evidence for the CD4-dependent association of p56lck with the ab T-cell receptor (TCR): Regulation of TCR-induced activation. EMBO J. 1993, 13, 90. [Google Scholar] [CrossRef]
- Portolés, P.; Rojo, J.; Golby, A.; Bonneville, M.; Gromkowski, S.; Greenbaum, L.; Janeway, C.A., Jr.; Murphy, D.B.; Bottomly, K. Monoclonal antibodies to murine CD3e define distinct epitopes, one of which may interact with CD4 during T cell activation. J. Immunol. 1989, 142, 4169–4175. [Google Scholar]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feito, M.J.; Cicuéndez, M.; Casarrubios, L.; Diez-Orejas, R.; Fateixa, S.; Silva, D.; Barroca, N.; Marques, P.A.A.P.; Portolés, M.T. Effects of Graphene Oxide and Reduced Graphene Oxide Nanostructures on CD4+ Th2 Lymphocytes. Int. J. Mol. Sci. 2022, 23, 10625. https://doi.org/10.3390/ijms231810625
Feito MJ, Cicuéndez M, Casarrubios L, Diez-Orejas R, Fateixa S, Silva D, Barroca N, Marques PAAP, Portolés MT. Effects of Graphene Oxide and Reduced Graphene Oxide Nanostructures on CD4+ Th2 Lymphocytes. International Journal of Molecular Sciences. 2022; 23(18):10625. https://doi.org/10.3390/ijms231810625
Chicago/Turabian StyleFeito, María José, Mónica Cicuéndez, Laura Casarrubios, Rosalía Diez-Orejas, Sara Fateixa, Daniela Silva, Nathalie Barroca, Paula A. A. P. Marques, and María Teresa Portolés. 2022. "Effects of Graphene Oxide and Reduced Graphene Oxide Nanostructures on CD4+ Th2 Lymphocytes" International Journal of Molecular Sciences 23, no. 18: 10625. https://doi.org/10.3390/ijms231810625
APA StyleFeito, M. J., Cicuéndez, M., Casarrubios, L., Diez-Orejas, R., Fateixa, S., Silva, D., Barroca, N., Marques, P. A. A. P., & Portolés, M. T. (2022). Effects of Graphene Oxide and Reduced Graphene Oxide Nanostructures on CD4+ Th2 Lymphocytes. International Journal of Molecular Sciences, 23(18), 10625. https://doi.org/10.3390/ijms231810625