Therapeutic Effects of Saponins for the Prevention and Treatment of Cancer by Ameliorating Inflammation and Angiogenesis and Inducing Antioxidant and Apoptotic Effects in Human Cells
Abstract
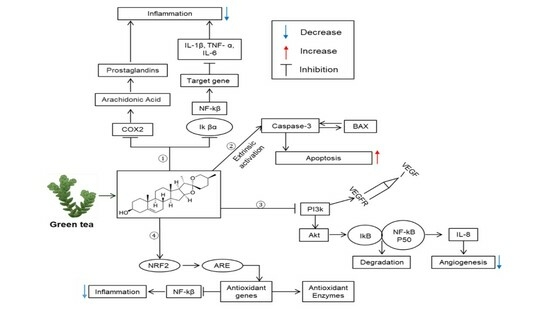
:1. Introduction
2. Results
2.1. Identification, Structure Elucidation, and Quantification of the Isolated Compounds
2.2. Cytotoxic Effects of Saponins
2.3. Anti-Angiogenesis Effect of Saponins
2.4. Free Radical-Scavenging Effects of Saponins
2.5. Saponins Effect on Intercellular ROS Levels
2.6. Pro-Apoptotic Effects of Saponins
2.7. Effects of Saponins on Proinflammatory Cytokines
3. Discussion
4. Materials and Methods
4.1. Cell Lines and Conditions
4.2. MTT Assay
4.3. Cell Viability Assay
4.4. HUVECs Tubes Formation Assay
4.5. Angiogenesis-Associated mRNA Expression
4.6. DPPH Radical Scavenging Activities
4.7. ABTS Radical Scavenging Activities
4.8. Reactive Oxygen Species (ROS) Assay
4.9. mRNA Expression Level of the NRF2 and GCL Genes
4.10. Caspase-3 Activity Assay
4.11. RT-PCR for Apoptosis-Related and Pro-Inflammatory Genes
4.12. ELISA Quantification of Proinflammatory Cytokines
4.13. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef] [PubMed]
- Gordon, M.; Cragg, D.; Kingston, G.I.; David, M.N. Anticancer Agents from Natural Products, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2012; p. 767. [Google Scholar]
- Harvey, A.L.; Edrada-Ebel, R.; Quinn, R.J. The re-emergence of natural products for drug discovery in the genomics era. Nat. Rev. Drug. Discov. 2015, 14, 111–129. [Google Scholar] [CrossRef] [PubMed]
- Eissa, S.; Labib, R.; Khalifa, A.; Swelam, N.; Khalil, F.; El-Shenawy, A.M. Regulators of apoptosis in human breast cancer. Clin. Biochem. 1999, 32, 321–326. [Google Scholar] [CrossRef]
- Won, K.H.; Barve, A.; Yu, S.; Huang, M.-T.; Kong, A.-N.T. Cancer chemoprevention by phytochemicals: Potential molecular targets, biomarkers and animal models. Acta. Pharmacol. Sin. 2007, 28, 1409–1421. [Google Scholar]
- Magkouta, S.; Stathopoulos, G.T.; Psallidas, I.; Kolisis, F.N.; Roussos, C.; Loutrari, H.; Magkouta, S.; Stathopoulos, G.T.; Psallidas, I. Carcinoma Protective Effects of Mastic Oil from Pistacia Lentiscus Variation Chia Against Experimental Growth of Lewis Lung. Nutr. Cancer 2015, 61, 37–41. [Google Scholar]
- Fathi, P.; Fouladdel, S.; Hassani, S.; Yousefbeyk, F.; Mahmood, S.; Amin, G.; Azizi, E. Induction of apoptosis and cell cycle arrest by pericarp polyphenol-rich extract of Baneh in human colon carcinoma HT29 cells. Food Chem. Toxicol. 2012, 50, 1054–1059. [Google Scholar]
- Germain, M.; D’Amours, D.; Dixit, V. Cleavage of automodified poly (ADP-ribose) polymerase during apoptosis. J. Biol. Chem. 1999, 274, 28379–28384. [Google Scholar] [CrossRef]
- Funahashi, H.; Imai, T.; Tanaka, Y.; Tsukamura, K.; Hayakawa, Y.; Kikumori, T.; Mase, T.; Itoh, T.; Nishikawa, M.; Hayashi, H.; et al. Wakame seaweed suppresses the proliferation of 7,12-dimethylbenz(a)-anthracene-induced mammary tumors in rats. Jpn. J. Cancer Res. 1999, 90, 922–927. [Google Scholar] [CrossRef]
- Nathan, C. Points of control in inflammation. Nature 2002, 420, 846–852. [Google Scholar] [CrossRef]
- Laveti, D.; Kumar, M.; Hemalatha, R.; Sistla, R.; Naidu, V.G.; Talla, V.; Verma, V.; Kaur, N.; Nagpal, R. Anti-inflammatory treatments for chronic diseases: A review. Inflamm. Allergy Drug Targets 2013, 12, 349–361. [Google Scholar] [CrossRef]
- Caughey, G.H. Mast cell proteases as pharmacological targets. Eur. J. Pharmacol. 2016, 778, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Allavena, P.; Garlanda, C.; Borrello, M.G.; Sica, A.; Mantovani, A. Pathways connecting inflammation and cancer. Curr. Opin. Genet. Dev. 2008, 18, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Ajith, Y.; Dimri, U.; Dixit, S.K.; Singh, S.K.; Gopalakrishnan, A.; Madhesh, E.; Rajesh, J.B.; Sangeetha, S.G. Immunomodulatory basis of antioxidant therapy and its future prospects: An appraisal. Inflammopharmacology 2017, 25, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Sagin, F.G.; Sozmen, E.Y. Anti-inflammatory effects of dietary antioxidants. Curr. Med. Chem.-Anti-Inflamm. Anti-Allergy Agents 2004, 3, 19–30. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; International Natural Product Sciences Taskforce; Supuran, C.T. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef]
- Veeresham, C. Natural products derived from plants as a source of drugs. J. Adv. Pharm. Technol. Res. 2012, 3, 200–201. [Google Scholar] [CrossRef]
- Dias, D.A.; Urban, S.; Roessner, U. A historical overview of natural products in drug discovery. Metabolites 2012, 2, 303–336. [Google Scholar] [CrossRef]
- Welz, A.N.; Emberger-Klein, A.; Menrad, K. Why people use herbal medicine: Insights from a focus-group study in Germany. BMC Complement. Altern. Med. 2018, 18, 92. [Google Scholar] [CrossRef]
- George, B.P.; Abrahamse, H.; Parimelazhagan, T. Caspase dependent apoptotic activity of Rubus fairholmianus Gard. on MCF-7 human breast cancer cell lines. J. Appl. Biomed. 2016, 14, 211–219. [Google Scholar] [CrossRef]
- Harvey, A.A. Natural products in drug discovery. Drug Discov. Today 2008, 13, 894–901. [Google Scholar] [CrossRef]
- Elkady, A.I.; Abuzinadah, O.A.; Baeshen, N.A.; Rahmy, T.R. Differential control of growth, apoptotic activity, and gene expression in human breast cancer cells by extracts derived from medicinal herbs Zingiber officinale. J. Biomed. Biotechnol. 2012, 2012, 614356. [Google Scholar] [CrossRef]
- Sagesaka, Y.M.; Uemura, T.; Suzuki, Y.; Sugiura, T.; Yoshida, M.; Yamaguchi, K.; Kyuki, K. Antimicrobial and anti-inflammatory actions of tea-leaf saponin. J. Pharm. Soc. Jpn. 1996, 116, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Sagesaka, Y.M.; Sugiura, T.; Miwa, Y.; Yamaguchi, K.; Kyuki, K. Effect of tea-leaf saponin on blood pressure of spontaneously hypertensive rats. J. Pharm. Soc. Jpn. 1996, 116, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Akagi, M.; Fukuishi, N.; Kan, T.; Sagesaka, Y.M.; Akagi, R. Anti-allergic effect of tea-leaf saponin (TLS) from tea leaves (Camellia sinensis var. sinensis). Biol. Pharm. Bull. 1997, 20, 565–567. [Google Scholar] [CrossRef]
- Matsui, Y.; Kumagai, H.; Masuda, H. Antihypercholesterolemic Activity of Catechin-free Saponin-rich Extract from Green Tea Leaves. Food Sci. Technol. Res. 2006, 12, 50–54. [Google Scholar] [CrossRef]
- Iqbal, J.; Abbasi, B.A.; Mahmood, T.; Kanwal, S.; Ali, B.; Shah, S.A.; Khalil, A.T. Plant-derived anticancer agents: A green anticancer approach. Asian Pac. J. Trop. Biomed. 2017, 12, 1129–1150. [Google Scholar] [CrossRef]
- Lu, Z.; Zhang, Q.; Chen, R.; Yu, D. Alkaloids and anthraquinones from branches and leaves of Uvaria kurzii. China J. Chin. Mater. Med. 2011, 36, 1190–1192. [Google Scholar]
- Nakano, D.; Ishitsuka, K.; Kamikawa, M.; Matsuda, M.; Tsuchihashi, R.; Okawa, M.; Okabe, H.; Tamura, K.; Kinjo, J. Screening of promising chemotherapeutic candidates from plants against human adult T-cell leukemia/lymphoma (III). J. Nat. Med. 2013, 67, 894–903. [Google Scholar] [CrossRef]
- Woo, S.H.; Sun, N.-J.; Cassady, J.M.; Snapka, R.M. Topoisomerase II inhibition by aporphine alkaloids. Biochem. Pharmacol. 1999, 57, 1141–1145. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Chen, S.-Y.; Chen, C.-H. Liriodenine induces G1/S cell cycle arrest in human colon cancer cells via nitric oxide- and p53-mediated pathway. Process Biochem. 2012, 47, 1460–1468. [Google Scholar] [CrossRef]
- Nordin, N.; Majid, N.A.; Hashim, N.M.; Rahman, M.A.; Hassan, Z.; Ali, H.M. Liriodenine, an aporphine alkaloid from Enicosanthellum pulchrum, inhibits proliferation of human ovarian cancer cells through induction of apoptosis via the mitochondrial signaling pathway and blocking cell cycle progression. Drug Des. Devel. Ther. 2015, 9, 1437–1448. [Google Scholar] [PubMed]
- Cain, K.; Brown, D.G.; Langlais, C.; Cohen, G.M. Caspase activation involves the formation of the aposome, a large (approximately 700 kDa) caspase activating complex. J. Biol. Chem. 1999, 274, 22686–22692. [Google Scholar] [CrossRef] [PubMed]
- Zou, H.; Li, Y.; Liu, X.; Wang, X. An APAF-1. cytochrome c multimeric complex is a functional apoptosome that activates procaspase-9. J. Biol. Chem. 1999, 274, 11549–11556. [Google Scholar] [CrossRef] [PubMed]
- Tóthová, Z.; Šemeláková, M.; Solárová, Z.; Tomc, J.; Debeljak, N.; Solár, P. The Role of PI3K/AKT and MAPK Signaling Pathways in Erythropoietin Signalization. Int. J. Mol. Sci. 2021, 22, 7682. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q. Role of nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef]
- Krejsa, C.M.; Franklin, C.C.; White, C.C.; Ledbetter, J.A.; Schieven, G.L.; Kavanagh, T.J. Rapid activation of glutamate cysteine ligase following oxidative stress. J. Biol. Chem. 2010, 285, 16116–16124. [Google Scholar] [CrossRef]
- Khan, M.I.; Khan, M.Z.; Shin, J.H.; Shin, T.S.; Lee, Y.B.; Kim, M.Y.; Kim, J.D. Neuroprotective Effects of Green Tea Seed Isolated Saponin Due to the Amelioration of Tauopathy and Alleviation of Neuroinflammation: A Therapeutic Approach to Alzheimer’s Disease. Molecules 2022, 27, 2079. [Google Scholar] [CrossRef]
- Fan, X.G.; Pei, S.Y.; Zhou, D.; Zhou, P.C.; Huang, Y.; Hu, X.W.; Li, T.; Wang, Y.; Huang, Z.B.; Li, N. Melittin ameliorates inflammation in mouse acute liver failure via inhibition of PKM2-mediated Warburg effect. Acta. Pharm. Sin. 2021, 42, 1256–1266. [Google Scholar] [CrossRef]
- El-Agamy, D.; Shebl, A.; Shaaban, A. Modulation of d-galactosamine/lipopolysacharride–induced fulminant hepatic failure by nilotinib. Hum. Exp. Toxicol. 2018, 37, 51–60. [Google Scholar] [CrossRef]
- Hu, H.B.; Liang, H.P.; Li, H.M.; Yuan, R.N.; Sun, J.; Zhang, L.L.; Han, M.H.; Wu, Y. Isolation, purification, characterization and antioxidant activity of polysaccharides from the stem barks of Acanthopanax leucorrhizus. Carbohydr. Polym. 2018, 196, 359–367. [Google Scholar] [CrossRef]
- Hwang, E.-S.; Thi, N.D. Effects of extraction and processing methods on antioxidant compound contents and radical scavenging activities of laver (Porphyra tenera). Prev. Nutr. Food Sci. 2014, 19, 40. [Google Scholar] [CrossRef] [PubMed]






| No. | Gene | Primer Sequence | Accession Number | Size [bp] |
|---|---|---|---|---|
| 1 | COX2 | CTTCCGATTGAAGCCCCCAT GGTCGTGTAGCGGTGAAAGT | KC753760 | 137 |
| 2 | iNOS | GAGCAGAGATCGTGCCACTC TTGGTGGAATGGCAGGTAGG | AF045477 | 182 |
| 3 | NF-kB | GGGCAGGAAGAGGAGGTTTC GCAGTGCCATCTGTGGTTGA | NM_001382627 | 600 |
| 4 | NRF2 | ATCTTCGAGGAGCTCACCCT TCAGTGTCTTGGGACTTGCC | AH010686 | 480 |
| 5 | Caspase-3 | TGTCCTGGGACACCGGTTAT TCTGTTGCCACCTTTCGGTT | AJ413269 | 646 |
| 6 | GCL | CAGTGGTTTGCTATGCTGCG CCGGGGAATTCGATTCACTAC | AF198534 | 843 |
| 7 | VEGFR2 | CGGTCAACAAAGTCGGGAGA CAGTGCACCACAAAGACACG | EU826563 | 123 |
| 8 | PI3K | TGGAGAGAGAGCAGTTCCAAT ATCTCTCGGCAGTCTTGTCG | NM_001256045 | 555 |
| 9 | AKT | GAAGACGGGAGCAGGCG AAGGTGCGTTCGATGACAGT | NM_001382431 | 694 |
| 10 | BCL-2 | TCTCATGCCAAGGGGGAAAC CAATCCTCCCCCAGTTCACC | KY098799 | 629 |
| 11 | BAX | CCAGAGGCGGGGGATGATTG GCAGGGTAGATGAATCGGGG | NM_001291430 | 461 |
| 12 | β-actin | GGCTCTTTTCCAGCCTTCCT AATGCCAGGGTACATGGTGG | HQ154074 | 151 |
| 13 | GAPDH | GCTCCCTCTTTCTTTGCAGC GTTGTCATGGATGACCTTGGC | JN613429 | 77 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, M.I.; Karima, G.; Khan, M.Z.; Shin, J.H.; Kim, J.D. Therapeutic Effects of Saponins for the Prevention and Treatment of Cancer by Ameliorating Inflammation and Angiogenesis and Inducing Antioxidant and Apoptotic Effects in Human Cells. Int. J. Mol. Sci. 2022, 23, 10665. https://doi.org/10.3390/ijms231810665
Khan MI, Karima G, Khan MZ, Shin JH, Kim JD. Therapeutic Effects of Saponins for the Prevention and Treatment of Cancer by Ameliorating Inflammation and Angiogenesis and Inducing Antioxidant and Apoptotic Effects in Human Cells. International Journal of Molecular Sciences. 2022; 23(18):10665. https://doi.org/10.3390/ijms231810665
Chicago/Turabian StyleKhan, Muhammad Imran, Gul Karima, Muhammad Zubair Khan, Jin Hyuk Shin, and Jong Deog Kim. 2022. "Therapeutic Effects of Saponins for the Prevention and Treatment of Cancer by Ameliorating Inflammation and Angiogenesis and Inducing Antioxidant and Apoptotic Effects in Human Cells" International Journal of Molecular Sciences 23, no. 18: 10665. https://doi.org/10.3390/ijms231810665
APA StyleKhan, M. I., Karima, G., Khan, M. Z., Shin, J. H., & Kim, J. D. (2022). Therapeutic Effects of Saponins for the Prevention and Treatment of Cancer by Ameliorating Inflammation and Angiogenesis and Inducing Antioxidant and Apoptotic Effects in Human Cells. International Journal of Molecular Sciences, 23(18), 10665. https://doi.org/10.3390/ijms231810665