Role of Endothelin-1 in Right Atrial Arrhythmogenesis in Rabbits with Monocrotaline-Induced Pulmonary Arterial Hypertension
Abstract
:1. Introduction
2. Results
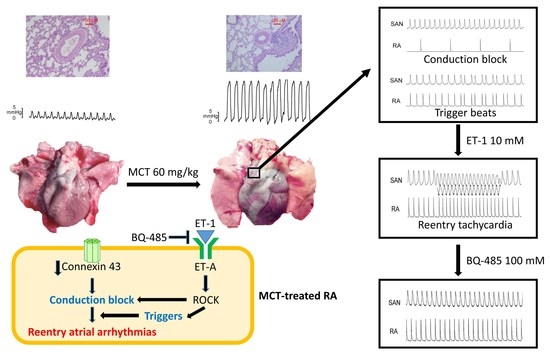
2.1. Pulmonary Arteriole and RV Remodeling in MCT-Induced PAH Rabbits
2.2. Action Potential (AP) Morphology of the RA and Electrical Activity in Sinoatrial Node (SAN)-RA Preparations
2.3. Effects of BQ 485 on Electrical Activity on ET-1-Treated SAN–RA Preparations from MCT-Treated Rabbits
2.4. Western Blotting Analysis
3. Discussion
4. Materials and Methods
4.1. Animal Model
4.2. Hemodynamic Analysis
4.3. Tissue Preparation Add Histological Analysis
4.4. Electropharmacological Experiments
4.5. Western Blotting Analysis
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Masaki, T. The discovery of endothelins. Cardiovasc. Res. 1998, 39, 530–533. [Google Scholar] [CrossRef]
- Russell, F.D.; Molenaar, P. The human heart endothelin system: ET-1 synthesis, storage, release and effect. Trends Pharmacol. Sci. 2000, 21, 353–359. [Google Scholar] [CrossRef]
- Macarthur, H.; Warner, T.D.; Wood, E.G.; Corder, R.; Vane, J.R. Endothelin-1 release from endothelial cells in culture is elevated both acutely and chronically by short periods of mechanical stretch. Biochem. Biophys. Res. Commun. 1994, 200, 395–400. [Google Scholar] [CrossRef]
- Hasdai, D.; Holmes, D.R., Jr.; Garratt, K.N.; Edwards, W.D.; Lerman, A. Mechanical pressure and stretch release endothelin-1 from human atherosclerotic coronary arteries in vivo. Circulation 1997, 95, 357–362. [Google Scholar] [CrossRef]
- Cheng, C.P.; Ukai, T.; Onishi, K.; Ohte, N.; Suzuki, M.; Zhang, Z.S.; Cheng, H.J.; Tachibana, H.; Igawa, A.; Little, W.C. The role of ANG II and endothelin-1 in exercise-induced diastolic dysfunction in heart failure. Am. J. Physiol. Heart Circ. Physiol. 2001, 280, H1853–H1860. [Google Scholar] [CrossRef]
- Burstein, B.; Libby, E.; Calderone, A.; Nattel, S. Differential behaviors of atrial versus ventricular fibroblasts: A potential role for platelet-derived growth factor in atrial-ventricular remodeling differences. Circulation 2008, 117, 1630–1641. [Google Scholar] [CrossRef]
- Burrell, K.M.; Molenaar, P.; Dawson, P.J.; Kaumann, A.J. Contractile and arrhythmic effects of endothelin receptor agonists in human heart in vitro: Blockade with SB 209670. J. Pharmacol. Exp. Ther. 2000, 292, 449–459. [Google Scholar]
- Udyavar, A.R.; Chen, Y.C.; Chen, Y.J.; Cheng, C.C.; Lin, C.I.; Chen, S.A. Endothelin-1 modulates the arrhythmogenic activity of pulmonary veins. J. Cardiovasc. Electrophysiol. 2008, 19, 285–292. [Google Scholar] [CrossRef]
- Li, X.; Zima, A.V.; Sheikh, F.; Blatter, L.A.; Chen, J. Endothelin-1-induced arrhythmogenic Ca2+ signaling is abolished in atrial myocytes of inositol-1,4,5-trisphosphate(IP3)-receptor type 2-deficient mice. Circ. Res. 2005, 96, 1274–1281. [Google Scholar] [CrossRef]
- Humbert, M.; Morrell, N.W.; Archer, S.L.; Stenmark, K.R.; MacLean, M.R.; Lang, I.M.; Christman, B.W.; Weir, E.K.; Eickelberg, O.; Voelkel, N.F.; et al. Cellular and molecular pathobiology of pulmonary arterial hypertension. J. Am. Coll. Cardiol. 2004, 43 (Suppl. S12), 13S–24S. [Google Scholar] [CrossRef]
- Runo, J.R.; Loyd, J.E. Primary pulmonary hypertension. Lancet 2003, 361, 1533–1544. [Google Scholar] [CrossRef] [Green Version]
- Grapsa, J.; Gibbs, J.S.; Cabrita, I.Z.; Watson, G.F.; Pavlopoulos, H.; Dawson, D.; Gin-Sing, W.; Howard, L.S.; Nihoyannopoulos, P. The association of clinical outcome with right atrial and ventricular remodelling in patients with pulmonary arterial hypertension: Study with real-time three-dimensional echocardiography. Eur. Heart J. Cardiovasc. Imaging 2012, 13, 666–672. [Google Scholar] [CrossRef]
- Mercurio, V.; Peloquin, G.; Bourji, K.I.; Diab, N.; Sato, T.; Enobun, B.; Housten-Harris, T.; Damico, R.; Kolb, T.M.; Mathai, S.C.; et al. Pulmonary arterial hypertension and atrial arrhythmias: Incidence, risk factors, and clinical impact. Pulm. Circ. 2018, 8, 2045894018769874. [Google Scholar] [CrossRef]
- Cannillo, M.; Grosso Marra, W.; Gili, S.; D’Ascenzo, F.; Morello, M.; Mercante, L.; Mistretta, E.; Salera, D.; Zema, D.; Bissolino, A.; et al. Supraventricular Arrhythmias in Patients With Pulmonary Arterial Hypertension. Am. J. Cardiol. 2015, 116, 1883–1889. [Google Scholar] [CrossRef]
- Tongers, J.; Schwerdtfeger, B.; Klein, G.; Kempf, T.; Schaefer, A.; Knapp, J.M.; Niehaus, M.; Korte, T.; Hoeper, M.M. Incidence and clinical relevance of supraventricular tachyarrhythmias in pulmonary hypertension. Am. Heart J. 2007, 153, 127–132. [Google Scholar] [CrossRef]
- Galie, N.; Manes, A.; Branzi, A. The endothelin system in pulmonary arterial hypertension. Cardiovasc. Res. 2004, 61, 227–237. [Google Scholar] [CrossRef]
- Ono, K.; Tsujimoto, G.; Sakamoto, A.; Eto, K.; Masaki, T.; Ozaki, Y.; Satake, M. Endothelin-A receptor mediates cardiac inhibition by regulating calcium and potassium currents. Nature 1994, 370, 301–304. [Google Scholar] [CrossRef]
- Stenmark, K.R.; Meyrick, B.; Galie, N.; Mooi, W.J.; McMurtry, I.F. Animal models of pulmonary arterial hypertension: The hope for etiological discovery and pharmacological cure. Am. J. Physiol. Lung Cell. Mol. Physiol. 2009, 297, L1013–L1032. [Google Scholar] [CrossRef]
- Rosenberg, H.C.; Rabinovitch, M. Endothelial injury and vascular reactivity in monocrotaline pulmonary hypertension. Am. J. Physiol. 1988, 255, H1484–H1491. [Google Scholar] [CrossRef]
- Hiram, R.; Naud, P.; Xiong, F.; Al-U’datt, D.; Algalarrondo, V.; Sirois, M.G.; Tanguay, J.F.; Tardif, J.C.; Nattel, S. Right Atrial Mechanisms of Atrial Fibrillation in a Rat Model of Right Heart Disease. J. Am. Coll. Cardiol. 2019, 74, 1332–1347. [Google Scholar] [CrossRef]
- Campbell, S.E.; Korecky, B.; Rakusan, K. Remodeling of myocyte dimensions in hypertrophic and atrophic rat hearts. Circ. Res. 1991, 68, 984–996. [Google Scholar] [CrossRef] [Green Version]
- Yanucil, C.; Kentrup, D.; Li, X.; Grabner, A.; Schramm, K.; Martinez, E.C.; Li, J.; Campos, I.; Czaya, B.; Heitman, K.; et al. FGF21-FGFR4 signaling in cardiac myocytes promotes concentric cardiac hypertrophy in mouse models of diabetes. Sci. Rep. 2022, 12, 7326. [Google Scholar] [CrossRef]
- Kalaycioglu, E.; Cetin, M.; Cinier, G.; Ozyildiz, A.G.; Durmus, I.; Kiris, T.; Gokdeniz, T. Epicardial adipose tissue is associated with increased systolic pulmonary artery pressure in patients with chronic obstructive pulmonary disease. Clin. Respir. J. 2021, 15, 406–412. [Google Scholar] [CrossRef]
- Siontis, K.C.; Geske, J.B.; Gersh, B.J. Atrial fibrillation pathophysiology and prognosis: Insights from cardiovascular imaging. Circ. Cardiovasc. Imaging 2015, 8, e003020. [Google Scholar] [CrossRef]
- Kazbanov, I.V.; ten Tusscher, K.H.; Panfilov, A.V. Effects of Heterogeneous Diffuse Fibrosis on Arrhythmia Dynamics and Mechanism. Sci. Rep. 2016, 6, 20835. [Google Scholar] [CrossRef]
- Allessie, M.A.; Boyden, P.A.; Camm, A.J.; Kleber, A.G.; Lab, M.J.; Legato, M.J.; Rosen, M.R.; Schwartz, P.J.; Spooner, P.M.; Van Wagoner, D.R.; et al. Pathophysiology and prevention of atrial fibrillation. Circulation 2001, 103, 769–777. [Google Scholar] [CrossRef]
- Li, D.; Fareh, S.; Leung, T.K.; Nattel, S. Promotion of atrial fibrillation by heart failure in dogs: Atrial remodeling of a different sort. Circulation 1999, 100, 87–95. [Google Scholar] [CrossRef]
- Ozawa, K.; Funabashi, N.; Kataoka, A.; Tanabe, N.; Yanagawa, N.; Tatsumi, K.; Kobayashi, Y. Myocardial fibrosis in the right ventricle detected on ECG gated 320 slice CT showed a short term poor prognosis in subjects with pulmonary hypertension. Int. J. Cardiol. 2013, 168, 584–586. [Google Scholar] [CrossRef]
- Medi, C.; Kalman, J.M.; Ling, L.H.; Teh, A.W.; Lee, G.; Lee, G.; Spence, S.J.; Kaye, D.M.; Kistler, P.M. Atrial electrical and structural remodeling associated with longstanding pulmonary hypertension and right ventricular hypertrophy in humans. J. Cardiovasc. Electrophysiol. 2012, 23, 614–620. [Google Scholar] [CrossRef]
- Lowen, M.A.; Bergman, M.J.; Cutaia, M.V.; Porcelli, R.J. Age-dependent effects of chronic hypoxia on pulmonary vascular reactivity. J. Appl. Physiol. 1987, 63, 1122–1129. [Google Scholar] [CrossRef]
- Mohacsi, A.; Magyar, J.; Tamas, B.; Nanasi, P.P. Effects of endothelins on cardiac and vascular cells: New therapeutic target for the future? Curr. Vasc. Pharmacol. 2004, 2, 53–63. [Google Scholar] [CrossRef]
- Kistamas, K.; Veress, R.; Horvath, B.; Banyasz, T.; Nanasi, P.P.; Eisner, D.A. Calcium Handling Defects and Cardiac Arrhythmia Syndromes. Front. Pharmacol. 2020, 11, 72. [Google Scholar] [CrossRef]
- Schmidt, C.; Wiedmann, F.; Voigt, N.; Zhou, X.B.; Heijman, J.; Lang, S.; Albert, V.; Kallenberger, S.; Ruhparwar, A.; Szabo, G.; et al. Upregulation of K(2P)3.1 K+ Current Causes Action Potential Shortening in Patients With Chronic Atrial Fibrillation. Circulation 2015, 132, 82–92. [Google Scholar] [CrossRef]
- Bosch, R.F.; Zeng, X.; Grammer, J.B.; Popovic, K.; Mewis, C.; Kuhlkamp, V. Ionic mechanisms of electrical remodeling in human atrial fibrillation. Cardiovasc. Res. 1999, 44, 121–131. [Google Scholar] [CrossRef]
- Morillo, C.A.; Klein, G.J.; Jones, D.L.; Guiraudon, C.M. Chronic rapid atrial pacing. Structural, functional, and electrophysiological characteristics of a new model of sustained atrial fibrillation. Circulation 1995, 91, 1588–1595. [Google Scholar] [CrossRef]
- Yorikane, R.; Koike, H.; Miyake, S. Electrophysiological effects of endothelin-1 on canine myocardial cells. J. Cardiovasc. Pharmacol. 1991, 17 (Suppl. S7), S159–S162. [Google Scholar] [CrossRef]
- Horinouchi, T.; Terada, K.; Higashi, T.; Miwa, S. Endothelin receptor signaling: New insight into its regulatory mechanisms. J. Pharmacol. Sci. 2013, 123, 85–101. [Google Scholar] [CrossRef]
- Imamura, T.; Huang, J.; Dalle, S.; Ugi, S.; Usui, I.; Luttrell, L.M.; Miller, W.E.; Lefkowitz, R.J.; Olefsky, J.M. Beta-Arrestin-mediated recruitment of the Src family kinase Yes mediates endothelin-1-stimulated glucose transport. J. Biol. Chem. 2001, 276, 43663–43667. [Google Scholar] [CrossRef]
- Berghausen, E.M.; Janssen, W.; Vantler, M.; Gnatzy-Feik, L.L.; Krause, M.; Behringer, A.; Joseph, C.; Zierden, M.; Freyhaus, H.T.; Klinke, A.; et al. Disrupted PI3K subunit p110alpha signaling protects against pulmonary hypertension and reverses established disease in rodents. J. Clin. Investig. 2021, 131, e136939. [Google Scholar] [CrossRef]
- Lin, R.Z.; Lu, Z.; Anyukhovsky, E.P.; Jiang, Y.P.; Wang, H.Z.; Gao, J.; Rosen, M.R.; Ballou, L.M.; Cohen, I.S. Regulation of heart rate and the pacemaker current by phosphoinositide 3-kinase signaling. J. Gen. Physiol. 2019, 151, 1051–1058. [Google Scholar] [CrossRef]
- Seccia, T.M.; Rigato, M.; Ravarotto, V.; Calo, L.A. ROCK (RhoA/Rho Kinase) in Cardiovascular-Renal Pathophysiology: A Review of New Advancements. J. Clin. Med. 2020, 9, 1328. [Google Scholar] [CrossRef] [PubMed]
- Loirand, G. Rho Kinases in Health and Disease: From Basic Science to Translational Research. Pharmacol. Rev. 2015, 67, 1074–1095. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.C.; Chang, J.P.; Chang, T.H.; Lin, Y.S.; Huang, Y.K.; Pan, K.L.; Fang, C.Y.; Chen, C.J.; Ho, W.C.; Chen, M.C. Enhanced expression of ROCK in left atrial myocytes of mitral regurgitation: A potential mechanism of myolysis. BMC Cardiovasc. Disord. 2015, 15, 33. [Google Scholar] [CrossRef] [PubMed]
- Chapados, R.; Abe, K.; Ihida-Stansbury, K.; McKean, D.; Gates, A.T.; Kern, M.; Merklinger, S.; Elliott, J.; Plant, A.; Shimokawa, H.; et al. ROCK controls matrix synthesis in vascular smooth muscle cells: Coupling vasoconstriction to vascular remodeling. Circ. Res. 2006, 99, 837–844. [Google Scholar] [CrossRef]
- Xie, X.; Chen, C.; Huang, K.; Wang, S.; Hao, J.; Huang, J.; Huang, H. RhoA/rho kinase signaling reduces connexin43 expression in high glucose-treated glomerular mesangial cells with zonula occludens-1 involvement. Exp. Cell Res. 2014, 327, 276–286. [Google Scholar] [CrossRef]
- Gomez-Arroyo, J.G.; Farkas, L.; Alhussaini, A.A.; Farkas, D.; Kraskauskas, D.; Voelkel, N.F.; Bogaard, H.J. The monocrotaline model of pulmonary hypertension in perspective. Am. J. Physiol. Lung Cell. Mol. Physiol. 2012, 302, L363–L369. [Google Scholar] [CrossRef]
- Chen, L.; Xiao, J.; Li, Y.; Ma, H. Ang-(1-7) might prevent the development of monocrotaline induced pulmonary arterial hypertension in rats. Eur. Rev. Med. Pharmacol. Sci. 2011, 15, 1–7. [Google Scholar]
- Chen, Y.C.; Lu, Y.Y.; Cheng, C.C.; Lin, Y.K.; Chen, S.A.; Chen, Y.J. Sinoatrial node electrical activity modulates pulmonary vein arrhythmogenesis. Int. J. Cardiol. 2014, 173, 447–452. [Google Scholar] [CrossRef]
- Kao, Y.H.; Chen, Y.C.; Cheng, C.C.; Lee, T.I.; Chen, Y.J.; Chen, S.A. Tumor necrosis factor-alpha decreases sarcoplasmic reticulum Ca2+-ATPase expressions via the promoter methylation in cardiomyocytes. Crit. Care Med. 2010, 38, 217–222. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Y.-Y.; Lin, F.-J.; Chen, Y.-C.; Kao, Y.-H.; Higa, S.; Chen, S.-A.; Chen, Y.-J. Role of Endothelin-1 in Right Atrial Arrhythmogenesis in Rabbits with Monocrotaline-Induced Pulmonary Arterial Hypertension. Int. J. Mol. Sci. 2022, 23, 10993. https://doi.org/10.3390/ijms231910993
Lu Y-Y, Lin F-J, Chen Y-C, Kao Y-H, Higa S, Chen S-A, Chen Y-J. Role of Endothelin-1 in Right Atrial Arrhythmogenesis in Rabbits with Monocrotaline-Induced Pulmonary Arterial Hypertension. International Journal of Molecular Sciences. 2022; 23(19):10993. https://doi.org/10.3390/ijms231910993
Chicago/Turabian StyleLu, Yen-Yu, Fong-Jhih Lin, Yao-Chang Chen, Yu-Hsun Kao, Satoshi Higa, Shih-Ann Chen, and Yi-Jen Chen. 2022. "Role of Endothelin-1 in Right Atrial Arrhythmogenesis in Rabbits with Monocrotaline-Induced Pulmonary Arterial Hypertension" International Journal of Molecular Sciences 23, no. 19: 10993. https://doi.org/10.3390/ijms231910993
APA StyleLu, Y. -Y., Lin, F. -J., Chen, Y. -C., Kao, Y. -H., Higa, S., Chen, S. -A., & Chen, Y. -J. (2022). Role of Endothelin-1 in Right Atrial Arrhythmogenesis in Rabbits with Monocrotaline-Induced Pulmonary Arterial Hypertension. International Journal of Molecular Sciences, 23(19), 10993. https://doi.org/10.3390/ijms231910993