Differences in the Formation of Reactive Oxygen Species and Their Cytotoxicity between Thiols Combined with Aqua- and Cyanocobalamins
Abstract
:1. Introduction
2. Results
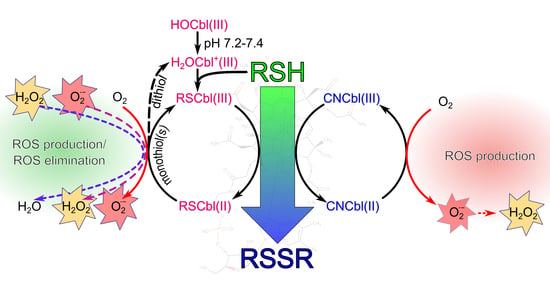
2.1. ROS Production during the Oxidation of Thiols Catalyzed by H2OCbl+/HOCbl and CNCbl
2.2. Kinetics of Thiol Oxidation Catalyzed by H2OCbl+/HOCbl and CNCbl
2.3. Examination of the Reaction between Cobalamins and Thiols by UV-Visible Spectroscopy
2.4. A Comparative Examination of the Cytotoxic Effect of the Combinations of Thiols with H2OCbl+/HOCbl and CNCbl
3. Discussion
3.1. Proposed Mechanisms for the Oxidation of Thiols Catalyzed by Cobalamins
3.2. Differences in ROS Production during the Oxidation of the Compounds Catalyzed by H2OCbl+/HOCbl and CNCbl
3.3. Reactive Oxygen Species and Their Cytotoxicity Induced by Combinations of Thiols with H2OCbl+/HOCbl and CNCbl
4. Materials and Methods
4.1. Chemicals
4.2. Cell Culture
4.3. Chemiluminescence Measurements
4.4. Kinetic Studies
4.4.1. Reactions of Cobalamins with GSH, NAC, and DTT
4.4.2. Reaction of Cobalamins with TNB
4.4.3. Reactions of Cobalamins with DDC and AA
4.4.4. Determination of Rate Constants
4.5. UV-Visible Spectral Studies
4.6. Cytotoxicity Assay
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Obeid, R.; Fedosov, S.N.; Nexo, E. Cobalamin Coenzyme Forms Are Not Likely to Be Superior to Cyano- and Hydroxyl-Cobalamin in Prevention or Treatment of Cobalamin Deficiency. Mol. Nutr. Food Res. 2015, 59, 1364–1372. [Google Scholar] [CrossRef] [PubMed]
- Fedosov, S.N.; Berglund, L.; Nexø, E.; Petersen, T.E. Tetrazole Derivatives and Matrices as Novel Cobalamin Coordinating Compounds. J. Organomet. Chem. 2007, 692, 1234–1242. [Google Scholar] [CrossRef]
- Cregan, A.G.; Brasch, N.E.; van Eldik, R. Thermodynamic and Kinetic Studies on the Reaction between the Vitamin B12 Derivative Beta-(N-Methylimidazolyl)Cobalamin and N-Methylimidazole: Ligand Displacement at the Alpha Axial Site of Cobalamins. Inorg. Chem. 2001, 40, 1430–1438. [Google Scholar] [CrossRef] [PubMed]
- Brayfield, A. Martindale: The Complete Drug Reference; PhP, Pharmaceutical Press: London, UK, 2014; ISBN 978-0-85711-139-5. [Google Scholar]
- Joint Formulary Committee (Great Britain). BNF 80: September 2020–March 2021; BMJ Group; Pharmaceutical Press: London, UK, 2020; ISBN 978-0-85711-369-6. [Google Scholar]
- Vidal-Alaball, J.; Butler, C.; Cannings-John, R.; Goringe, A.; Hood, K.; McCaddon, A.; McDowell, I.; Papaioannou, A. Oral Vitamin B12 versus Intramuscular Vitamin B12 for Vitamin B12 Deficiency. Cochrane Database Syst. Rev. 2005, 20, CD004655. [Google Scholar] [CrossRef]
- European Medicines Agency. European Public Assessment Report (EPAR) for Cyanokit. 2007. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Scientific_Discussion/human/000806/WC500036364.pdf (accessed on 3 August 2022).
- Forsyth, J.C.; Mueller, P.D.; Becker, C.E.; Osterloh, J.; Benowitz, N.L.; Rumack, B.H.; Hall, A.H. Hydroxocobalamin as a Cyanide Antidote: Safety, Efficacy and Pharmacokinetics in Heavily Smoking Normal Volunteers. J. Toxicol. Clin. Toxicol. 1993, 31, 277–294. [Google Scholar] [CrossRef]
- Banerjee, R.; Gouda, H.; Pillay, S. Redox-Linked Coordination Chemistry Directs Vitamin B12 Trafficking. Acc. Chem. Res. 2021, 54, 2003–2013. [Google Scholar] [CrossRef]
- Rizzo, G.; Laganà, A.S. A Review of Vitamin B12. In Molecular Nutrition; Elsevier: Amsterdam, The Netherlands, 2020; pp. 105–129. ISBN 978-0-12-811907-5. [Google Scholar]
- Wingert, V.; Mukherjee, S.; Esser, A.J.; Behringer, S.; Tanimowo, S.; Klenzendorf, M.; Derevenkov, I.A.; Makarov, S.V.; Jacobsen, D.W.; Spiekerkoetter, U.; et al. Thiolatocobalamins Repair the Activity of Pathogenic Variants of the Human Cobalamin Processing Enzyme CblC. Biochimie 2021, 183, 108–125. [Google Scholar] [CrossRef]
- Dereven’kov, I.A.; Salnikov, D.S.; Silaghi-Dumitrescu, R.; Makarov, S.V.; Koifman, O.I. Redox Chemistry of Cobalamin and Its Derivatives. Coord. Chem. Rev. 2016, 309, 68–83. [Google Scholar] [CrossRef]
- Dereven’kov, I.A.; Hannibal, L.; Dürr, M.; Salnikov, D.S.; Bui Thi, T.T.; Makarov, S.V.; Koifman, O.I.; Ivanović-Burmazović, I. Redox Turnover of Organometallic B12 Cofactors Recycles Vitamin C: Sulfur Assisted Reduction of Dehydroascorbic Acid by Cob(II)Alamin. J. Organomet. Chem. 2017, 839, 53–59. [Google Scholar] [CrossRef]
- Li, Z.; Mascarenhas, R.; Twahir, U.T.; Kallon, A.; Deb, A.; Yaw, M.; Penner-Hahn, J.; Koutmos, M.; Warncke, K.; Banerjee, R. An Interprotein Co-S Coordination Complex in the B12-Trafficking Pathway. J. Am. Chem. Soc. 2020, 142, 16334–16345. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H.; Rinna, A. Glutathione: Overview of Its Protective Roles, Measurement, and Biosynthesis. Mol. Aspects Med. 2009, 30, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Paul, C.; Brady, D.M. Comparative Bioavailability and Utilization of Particular Forms of B12 Supplements with Potential to Mitigate B12-Related Genetic Polymorphisms. Integr. Med. Encinitas 2017, 16, 42–49. [Google Scholar] [PubMed]
- Zhang, Y.; Hodgson, N.; Trivedi, M.; Deth, R. Neuregulin 1 Promotes Glutathione-Dependent Neuronal Cobalamin Metabolism by Stimulating Cysteine Uptake. Oxid. Med. Cell. Longev. 2016, 2016, 1–13. [Google Scholar] [CrossRef]
- Pezacka, E.; Green, R.; Jacobsen, D.W. Glutathionylcobalamin as an Intermediate in the Formation of Cobalamin Coenzymes. Biochem. Biophys. Res. Commun. 1990, 169, 443–450. [Google Scholar] [CrossRef]
- Wang, X.; Yang, Y.; Li, X.; Li, C.; Wang, C. Distinct Clinical, Neuroimaging and Genetic Profiles of Late-Onset Cobalamin C Defects (Cb1C): A Report of 16 Chinese Cases. Orphanet J. Rare Dis. 2019, 14, 109. [Google Scholar] [CrossRef] [PubMed]
- Sloan, J.L.; Carrillo, N.; Adams, D.; Venditti, C.P. Disorders of Intracellular Cobalamin Metabolism. In GeneReviews®; Adam, M.P., Ardinger, H.H., Pagon, R.A., Wallace, S.E., Bean, L.J., Mirzaa, G., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1993. [Google Scholar]
- Andersson, H.C.; Shapira, E. Biochemical and Clinical Response to Hydroxocobalamin versus Cyanocobalamin Treatment in Patients with Methylmalonic Acidemia and Homocystinuria (CblC). J. Pediatr. 1998, 132, 121–124. [Google Scholar] [CrossRef]
- Bodamer, O.A.F.; Rosenblatt, D.S.; Appel, S.H.; Beaudet, A.L. Adult-Onset Combined Methylmalonic Aciduria and Homocystinuria (CblC). Neurology 2001, 56, 1113. [Google Scholar] [CrossRef] [PubMed]
- Huemer, M.; Diodato, D.; Schwahn, B.; Schiff, M.; Bandeira, A.; Benoist, J.-F.; Burlina, A.; Cerone, R.; Couce, M.L.; Garcia-Cazorla, A.; et al. Guidelines for Diagnosis and Management of the Cobalamin-Related Remethylation Disorders CblC, CblD, CblE, CblF, CblG, CblJ and MTHFR Deficiency. J. Inherit. Metab. Dis. 2017, 40, 21–48. [Google Scholar] [CrossRef] [PubMed]
- Almannai, M.; Marom, R.; Divin, K.; Scaglia, F.; Sutton, V.R.; Craigen, W.J.; Lee, B.; Burrage, L.C.; Graham, B.H. Milder Clinical and Biochemical Phenotypes Associated with the c.482G > A (p.Arg161Gln) Pathogenic Variant in Cobalamin C Disease: Implications for Management and Screening. Mol. Genet. Metab. 2017, 122, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Higashimoto, T.; Kim, A.Y.; Ogawa, J.T.; Sloan, J.L.; Almuqbil, M.A.; Carlson, J.M.; Manoli, I.; Venditti, C.P.; Gunay-Aygun, M.; Wang, T. High-dose Hydroxocobalamin Achieves Biochemical Correction and Improvement of Neuropsychiatric Deficits in Adults with Late Onset Cobalamin C Deficiency. JIMD Rep. 2020, 51, 17–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fischer, S.; Huemer, M.; Baumgartner, M.; Deodato, F.; Ballhausen, D.; Boneh, A.; Burlina, A.B.; Cerone, R.; Garcia, P.; Gökçay, G.; et al. Clinical Presentation and Outcome in a Series of 88 Patients with the CblC Defect. J. Inherit. Metab. Dis. 2014, 37, 831–840. [Google Scholar] [CrossRef] [PubMed]
- Carrillo-Carrasco, N.; Sloan, J.; Valle, D.; Hamosh, A.; Venditti, C.P. Hydroxocobalamin dose escalation improves metabolic control in cblC. J. Inherit. Metab. Dis. 2009, 32, 728–731. [Google Scholar] [CrossRef] [PubMed]
- Van Hove, J.L.K.; Van Damme-Lombaerts, R.; Grünewald, S.; Peters, H.; Van Damme, B.; Fryns, J.-P.; Arnout, J.; Wevers, R.; Baumgartner, E.R.; Fowler, B. Cobalamin disorder Cbl-C presenting with late-onset thrombotic microangiopathy. Am. J. Med. Genet. 2002, 111, 195–201. [Google Scholar] [CrossRef]
- Matos, I.V.; Castejón, E.; Meavilla, S.; O’Callaghan, M.; Garcia-Villoria, J.; López-Sala, A.; Ribes, A.; Artuch, R.; Garcia-Cazorla, A. Clinical and biochemical outcome after hydroxocobalamin dose escalation in a series of patients with cobalamin C deficiency. Mol. Genet. Metab. 2013, 109, 360–365. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, D.W.; Troxell, L.S.; Brown, K.L. Catalysis of Thiol Oxidation by Cobalamins and Cobinamides: Reaction Products and Kinetics. Biochemistry 1984, 23, 2017–2025. [Google Scholar] [CrossRef]
- Solov’eva, M.E.; Solov’ev, V.V.; Faskhutdinova, A.A.; Kudryavtsev, A.A.; Akatov, V.S. Prooxidant and Cytotoxic Action of N-Acetylcysteine and Glutathione in Combinations with Vitamin B12b. Cell Tissue Biol. 2007, 1, 40–49. [Google Scholar] [CrossRef]
- Solovieva, M.E.; Solovyev, V.V.; Kudryavtsev, A.A.; Trizna, Y.A.; Akatov, V.S. Vitamin B12b Enhances the Cytotoxicity of Dithiothreitol. Free Radic. Biol. Med. 2008, 44, 1846–1856. [Google Scholar] [CrossRef]
- Gherasim, C.; Ruetz, M.; Li, Z.; Hudolin, S.; Banerjee, R. Pathogenic Mutations Differentially Affect the Catalytic Activities of the Human B12-Processing Chaperone CblC and Increase Futile Redox Cycling. J. Biol. Chem. 2015, 290, 11393–11402. [Google Scholar] [CrossRef]
- Li, Z.; Shanmuganathan, A.; Ruetz, M.; Yamada, K.; Lesniak, N.A.; Kräutler, B.; Brunold, T.C.; Koutmos, M.; Banerjee, R. Coordination Chemistry Controls the Thiol Oxidase Activity of the B12-Trafficking Protein CblC. J. Biol. Chem. 2017, 292, 9733–9744. [Google Scholar] [CrossRef]
- Pastore, A.; Martinelli, D.; Piemonte, F.; Tozzi, G.; Boenzi, S.; Di Giovamberardino, G.; Petrillo, S.; Bertini, E.; Dionisi-Vici, C. Glutathione Metabolism in Cobalamin Deficiency Type C (CblC). J. Inherit. Metab. Dis. 2014, 37, 125–129. [Google Scholar] [CrossRef]
- Birch, C.S.; Brasch, N.E.; McCaddon, A.; Williams, J.H.H. A Novel Role for Vitamin B12: Cobalamins Are Intracellular Antioxidants in Vitro. Free Radic. Biol. Med. 2009, 47, 184–188. [Google Scholar] [CrossRef] [PubMed]
- Solovieva, M.E.; Soloviev, V.V.; Akatov, V.S. Vitamin B12b increases the cytotoxicity of short-time exposure to ascorbic acid, inducing oxidative burst and iron-dependent DNA damage. Eur. J. Pharmacol. 2007, 566, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Solovieva, M.; Shatalin, Y.; Odinokova, I.; Krestinina, O.; Baburina, Y.; Mishukov, A.; Lomovskaya, Y.; Pavlik, L.; Mikheeva, I.; Holmuhamedov, E.; et al. Disulfiram oxy-derivatives induce entosis or paraptosis-like death in breast cancer MCF-7 cells depending on the duration of treatment. Biochim. Biophys. Acta BBA Gen. Subj. 2022, 1866, 130184. [Google Scholar] [CrossRef] [PubMed]
- Solovieva, M.; Shatalin, Y.; Fadeev, R.; Krestinina, O.; Baburina, Y.; Kruglov, A.; Kharechkina, E.; Kobyakova, M.; Rogachevsky, V.; Shishkova, E.; et al. Vitamin B12b Enhances the Cytotoxicity of Diethyldithiocarbamate in a Synergistic Manner, Inducing the Paraptosis-Like Death of Human Larynx Carcinoma Cells. Biomolecules 2020, 10, 69. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Cregan, A.G.; Berben, L.A.; Brasch, N.E. Studies on the Formation of Glutathionylcobalamin: Any Free Intracellular Aquacobalamin Is Likely to Be Rapidly and Irreversibly Converted to Glutathionylcobalamin. Inorg. Chem. 2004, 43, 6848–6857. [Google Scholar] [CrossRef]
- Salnikov, D.S.; Makarov, S.V.; van Eldik, R.; Kucherenko, P.N.; Boss, G.R. Kinetics and Mechanism of the Reaction of Hydrogen Sulfide with Diaquacobinamide in Aqueous Solution. Eur. J. Inorg. Chem. 2014, 2014, 4123–4133. [Google Scholar] [CrossRef]
- Brasch, N.E.; Hsu, T.-L.C.; Doll, K.M.; Finke, R.G. Synthesis and Characterization of Isolable Thiolatocobalamin Complexes Relevant to Coenzyme B12-Dependent Ribonucleoside Triphosphate Reductase. J. Inorg. Biochem. 1999, 76, 197–209. [Google Scholar] [CrossRef]
- Suarez-Moreira, E.; Hannibal, L.; Smith, C.A.; Chavez, R.A.; Jacobsen, D.W.; Brasch, N.E. A Simple, Convenient Method to Synthesize Cobalamins: Synthesis of Homocysteinylcobalamin, N-Acetylcysteinylcobalamin, 2-N-Acetylamino-2-Carbomethoxyethanethiolatocobalamin, Sulfitocobalamin and Nitrocobalamin. Dalton Trans. Camb. Engl. 2006, 44, 5269–5277. [Google Scholar] [CrossRef]
- Mukherjee, R.; McCaddon, A.; Smith, C.A.; Brasch, N.E. Synthesis, Synchrotron X-Ray Diffraction, and Kinetic Studies on the Formation of a Novel Thiolatocobalamin of Captopril: Evidence for Cis-Trans Isomerization in the Beta-Axial Ligand. Inorg. Chem. 2009, 48, 9526–9534. [Google Scholar] [CrossRef]
- Broclawik, E.; Borowski, T.; Radoń, M. (Eds.) Transition Metals in Coordination Environments: Computational Chemistry and Catalysis Viewpoints; Challenges and Advances in Computational Chemistry and Physics; Springer International Publishing: Cham, Switzerland, 2019; Volume 29, ISBN 978-3-030-11713-9. [Google Scholar]
- Suarez-Moreira, E.; Yun, J.; Birch, C.S.; Williams, J.H.H.; McCaddon, A.; Brasch, N.E. Vitamin B12 and Redox Homeostasis: Cob(II)Alamin Reacts with Superoxide at Rates Approaching Superoxide Dismutase (SOD). J. Am. Chem. Soc. 2009, 131, 15078–15079. [Google Scholar] [CrossRef]
- Salnikov, D.S.; Makarov, S.V.; Koifman, O.I. The Radical versus Ionic Mechanisms of Reduced Cobalamin Inactivation by Tert -Butyl Hydroperoxide and Hydrogen Peroxide in Aqueous Solution. New J. Chem. 2021, 45, 535–543. [Google Scholar] [CrossRef]
- Dereven’kov, I.A.; Makarov, S.V.; Shpagilev, N.I.; Salnikov, D.S.; Koifman, O.I. Studies on Reaction of Glutathionylcobalamin with Hypochlorite. Evidence of Protective Action of Glutathionyl-Ligand against Corrin Modification by Hypochlorite. Biometals Int. J. Role Met. Ions Biol. Biochem. Med. 2017, 30, 757–764. [Google Scholar] [CrossRef]
- Ramasamy, S.; Kundu, T.K.; Antholine, W.; Manoharan, P.T.; Rifkind, J.M. Internal Spin Trapping of Thiyl Radical during the Complexation and Reduction of Cobalamin with Glutathione and Dithiothrietol. J. Porphyr. Phthalocyanines 2012, 16, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, D.W.; Pezacka, E.H.; Brown, K.L. The Inhibition of Corrinoid-Catalyzed Oxidation of Mercaptoethanol by Methyl Iodide: Mechanistic Implications. J. Inorg. Biochem. 1993, 50, 47–63. [Google Scholar] [CrossRef]
- Qin, W.; Zhang, Z.; Liu, H. Chemiluminescence Flow Sensor for the Determination of Vitamin B12. Anal. Chim. Acta 1997, 357, 127–132. [Google Scholar] [CrossRef]
- Kumar, S.S.; Chouhan, R.S.; Thakur, M.S. Enhancement of Chemiluminescence for Vitamin B12 Analysis. Anal. Biochem. 2009, 388, 312–316. [Google Scholar] [CrossRef]
- Song, Z.; Hou, S. Sub-Picogram Determination of Vitamin B12 in Pharmaceuticals and Human Serum Using Flow Injection with Chemiluminescence Detection. Anal. Chim. Acta 2003, 488, 71–79. [Google Scholar] [CrossRef]
- Peel, J.L. The catalysis of the auto-oxidation of 2-mercaptoethanol and other thiols by vitamin B12 derivatives. Polarographic and other investigations. Biochem. J. 1963, 88, 296–308. [Google Scholar] [CrossRef]
- Schrauzer, G.N.; Sibert, J.W. Electron transfer reactions catalyzed by vitamin B12 and related compounds: The reduction of dyes and of riboflavin by thiols. Arch. Biochem. Biophys. 1969, 130, 257–266. [Google Scholar] [CrossRef]
- George, P.; Irvine, D.H.; Glauser, S.C. The Influence of Chelation in Determining the Reactivity of the Iron in Hemoproteins. and the Cobalt in Vitamin B12 Derivatives. Ann. N. Y. Acad. Sci. 1960, 88, 393–415. [Google Scholar] [CrossRef]
- Brown, K.L.; Peck-Siler, S. Heteronuclear NMR Studies of Cobalamins. 9. Temperature-Dependent NMR of Organocobalt Corrins Enriched in Carbon-13 in the Organic Ligand and the Thermodynamics of the Base-on/Base-off Reaction. Inorg. Chem. 1988, 27, 3548–3555. [Google Scholar] [CrossRef]
- Salnikov, D.S.; Dereven’kov, I.A.; Artyushina, E.N.; Makarov, S.V. Interaction of Cyanocobalamin with Sulfur-Containing Reducing Agents in Aqueous Solutions. Russ. J. Phys. Chem. A 2013, 87, 44–48. [Google Scholar] [CrossRef]
- Dereven’kov, I.A.; Ugodin, K.A.; Makarov, S.V. Mechanism of the Reaction between Cyanocobalamin and Reduced Flavin Mononucleotide. Russ. J. Phys. Chem. A 2021, 95, 2020–2024. [Google Scholar] [CrossRef]
- Lexa, D.; Sayeant, J.M.; Zickler, J. Electrochemistry of Vitamin B12. 5. Cyanocobalamins. J. Am. Chem. Soc. 1980, 102, 2654–2663. [Google Scholar] [CrossRef]
- Abel, P.J.; Wilkinson, R.; Pratt, E.W.; Whelan, J.M. The Mechanism of Oxidation of Vitamin B12r by Oxygen. South Afr. J. Chem. 1977, 30, 1–12. [Google Scholar] [CrossRef]
- Solovieva, M.E.; Shatalin, Y.V.; Solovyev, V.V.; Sazonov, A.V.; Kutyshenko, V.P.; Akatov, V.S. Hydroxycobalamin Catalyzes the Oxidation of Diethyldithiocarbamate and Increases Its Cytotoxicity Independently of Copper Ions. Redox Biol. 2019, 20, 28–37. [Google Scholar] [CrossRef]
- Nazhat, N.B.; Golding, B.T.; Johnson, G.R.A.; Jones, P. Destruction of Vitamin B12 by Reaction with Ascorbate: The Role of Hydrogen Peroxide and the Oxidation State of Cobalt. J. Inorg. Biochem. 1989, 36, 75–81. [Google Scholar] [CrossRef]
- Li, Z.; Greenhalgh, E.D.; Twahir, U.T.; Kallon, A.; Ruetz, M.; Warncke, K.; Brunold, T.C.; Banerjee, R. Chlorocob(II)Alamin Formation Which Enhances the Thiol Oxidase Activity of the B12-Trafficking Protein CblC. Inorg. Chem. 2020, 59, 16065–16072. [Google Scholar] [CrossRef]
- Feoktistova, M.; Geserick, P.; Leverkus, M. Crystal Violet Assay for Determining Viability of Cultured Cells. Cold Spring Harb. Protoc. 2016, 2016, pdb.prot087379. [Google Scholar] [CrossRef]







| Compounds | HOCbl | CNCbl |
|---|---|---|
| DTT | 6.33 × 10−3 | 5.14 × 10−3 |
| GSH | 3.99 × 10−4 | 1.53 × 10−4 |
| NAC | 1.47 × 10−4 | 0.75 × 10−4 |
| DDC 1 | 1.70 × 10−4 | 0.50 × 10−4 |
| Cycle of the oxidation of TNB | ||
| 1 | 6.42 × 10−5 | 1.02 × 10−3 |
| 2 | 0.99 × 10−3 | 1.33 × 10−3 |
| 3 | 1.28 × 10−3 | 1.38 × 10−3 |
| 4 | 1.89 × 10−3 | 1.40 × 10−3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shatalin, Y.V.; Shubina, V.S.; Solovieva, M.E.; Akatov, V.S. Differences in the Formation of Reactive Oxygen Species and Their Cytotoxicity between Thiols Combined with Aqua- and Cyanocobalamins. Int. J. Mol. Sci. 2022, 23, 11032. https://doi.org/10.3390/ijms231911032
Shatalin YV, Shubina VS, Solovieva ME, Akatov VS. Differences in the Formation of Reactive Oxygen Species and Their Cytotoxicity between Thiols Combined with Aqua- and Cyanocobalamins. International Journal of Molecular Sciences. 2022; 23(19):11032. https://doi.org/10.3390/ijms231911032
Chicago/Turabian StyleShatalin, Yuri V., Victoria S. Shubina, Marina E. Solovieva, and Vladimir S. Akatov. 2022. "Differences in the Formation of Reactive Oxygen Species and Their Cytotoxicity between Thiols Combined with Aqua- and Cyanocobalamins" International Journal of Molecular Sciences 23, no. 19: 11032. https://doi.org/10.3390/ijms231911032
APA StyleShatalin, Y. V., Shubina, V. S., Solovieva, M. E., & Akatov, V. S. (2022). Differences in the Formation of Reactive Oxygen Species and Their Cytotoxicity between Thiols Combined with Aqua- and Cyanocobalamins. International Journal of Molecular Sciences, 23(19), 11032. https://doi.org/10.3390/ijms231911032