Improved Locomotor Recovery in a Rat Model of Spinal Cord Injury by BioLuminescent-OptoGenetic (BL-OG) Stimulation with an Enhanced Luminopsin
Abstract
:1. Introduction
2. Results
2.1. LMO3.2 Induces Higher Photocurrents Compared to LMO3
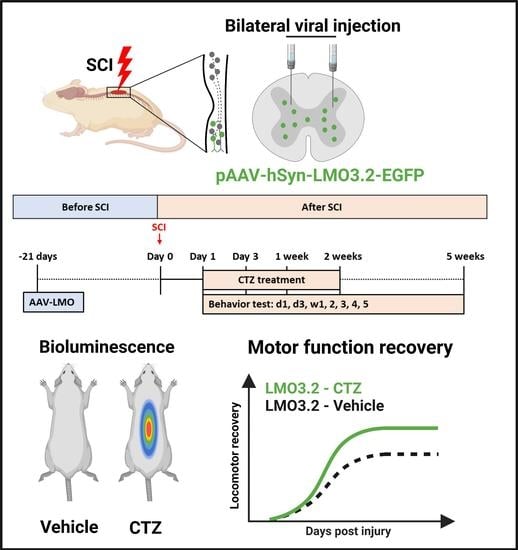
2.2. Intraperitoneal Injection of CTZ Leads to Measurable Bioluminescence Emission
2.3. Rats That Received Stimulation via LMO3.2 Showed Improved Locomotor Function after SCI
2.4. CatWalk Automated Gait Analysis Detected Difference in Stand between Vehicle- and CTZ-Treated Rats
2.5. Experimental and Control Groups Were Comparable Regarding Viral Transduction and Severity of Contusion Injury
3. Discussion
3.1. Relationship to Previous Studies
3.2. Limitations of the Study
4. Materials and Methods
4.1. Animals
4.2. Plasmids and Viruses
4.3. Electrophysiological Recordings from HEK293 Cells
4.4. Surgery
4.5. Viral Injections
4.6. Spinal Cord Injury
4.7. IVIS Imaging
4.8. Stimulating Treatment
4.9. Assessment
4.9.1. Basso, Beattie, and Bresnahan (BBB) Open-Field Locomotion Score
4.9.2. BBB Subscore
4.9.3. CatWalk Assessment
4.9.4. Viral Expression and Eriochrome Cyanine Staining
4.10. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yu, P.; Zhang, W.; Liu, Y.; Sheng, C.; So, K.F.; Zhou, L.; Zhu, H. The Effects and Potential Mechanisms of Locomotor Training on Improvements of Functional Recovery after Spinal Cord Injury, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2019; Volume 147. [Google Scholar]
- Park, E.H.; White, G.A.; Tieber, L.M. Mechanisms of injury and emergency care of acute spinal cord injury in dogs and cats. J. Vet. Emerg. Crit. Care 2012, 22, 160–178. [Google Scholar] [CrossRef] [PubMed]
- Cizkova, D.; Murgoci, A.N.; Cubinkova, V.; Humenik, F.; Mojzisova, Z.; Maloveska, M.; Cizek, M.; Fournier, I.; Salzet, M. Spinal Cord Injury: Animal Models, Imaging Tools and the Treatment Strategies. Neurochem. Res. 2020, 45, 134–143. [Google Scholar] [CrossRef] [PubMed]
- De Cassia Sampaio, O.; Defino, H.L.A.; del Bel Belluz Guimarães, E.A. Effect of hypovolemia on traumatic spinal cord injury. Spinal Cord 2016, 54, 742–745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dumont, R.J.; Okonkwo, D.O.; Verma, S.; Hurlbert, R.J.; Boulos, P.T.; Ellegala, D.B.; Dumont, A.S. Acute spinal cord injury, part I: Pathophysiologic mechanisms. Clin. Neuropharmacol. 2001, 24, 254–264. [Google Scholar] [CrossRef] [Green Version]
- Dell’Anno, M.T.; Wang, X.; Onorati, M.; Li, M.; Talpo, F.; Sekine, Y.; Ma, S.; Liu, F.; Cafferty, W.B.; Sestan, N.; et al. Human neuroepithelial stem cell regional specificity enables spinal cord repair through a relay circuit. Nat. Commun. 2018, 9, 3419. [Google Scholar] [CrossRef] [Green Version]
- Hilton, B.J.; Moulson, A.J.; Tetzlaff, W. Neuroprotection and secondary damage following spinal cord injury: Concepts and methods. Neurosci. Lett. 2017, 652, 3–10. [Google Scholar] [CrossRef]
- Li, Y.; Lucas-Osma, A.M.; Black, S.; Bandet, M.V.; Stephens, M.J.; Vavrek, R.; Sanelli, L.; Fenrich, K.K.; di Narzo, A.F.; Dracheva, S.; et al. Pericytes impair capillary blood flow and motor function after chronic spinal cord injury. Nat. Med. 2017, 23, 733–741. [Google Scholar] [CrossRef] [Green Version]
- Varma, A.K.; Das, A.; Wallace, G.; Barry, J.; Vertegel, A.A.; Ray, S.K.; Banik, N.L. Spinal cord injury: A review of current therapy, future treatments, and basic science frontiers. Neurochem. Res. 2013, 38, 895–905. [Google Scholar] [CrossRef] [Green Version]
- Courtine, G.; Sofroniew, M.V. Spinal cord repair: Advances in biology and technology. Nat. Med. 2019, 25, 898–908. [Google Scholar] [CrossRef]
- Holmes, D. Spinal-cord injury: Spurring regrowth. Nature 2017, 552, 51. [Google Scholar] [CrossRef]
- Berglund, K.; Birkner, E.; Augustine, G.J.; Hochgeschwender, U. Light-Emitting Channelrhodopsins for Combined Optogenetic and Chemical-Genetic Control of Neurons. PLoS ONE 2013, 8, e59759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tung, J.K.; Berglund, K.; Gutekunst, C.-A.; Hochgeschwender, U.; Gross, R.E. Bioluminescence imaging in live cells and animals. Neurophotonics 2016, 3, 025001. [Google Scholar] [CrossRef] [PubMed]
- Birkner, E.; Berglund, K.; Klein, M.E.; Augustine, G.J.; Hochgeschwender, U. Non-invasive activation of optogenetic actuators. Opt. Tech. Neurosurg. Neurophotonics Optogenet. 2014, 8928, 9282F. [Google Scholar] [CrossRef] [Green Version]
- Park, S.Y.; Song, S.H.; Palmateer, B.; Pal, A.; Petersen, E.D.; Shall, G.P.; Welchko, R.M.; Ibata, K.; Miyawaki, A.; Augustine, G.J.; et al. Novel luciferase–opsin combinations for improved luminopsins. J. Neurosci. Res. 2020, 98, 410–421. [Google Scholar] [CrossRef]
- Tung, J.K.; Gutekunst, C.A.; Gross, R.E. Inhibitory luminopsins: Genetically-encoded bioluminescent opsins for versatile, scalable, and hardware-independent optogenetic inhibition. Sci. Rep. 2015, 5, 14366. [Google Scholar] [CrossRef] [Green Version]
- Tung, J.K.; Shiu, F.H.; Ding, K.; Gross, R.E. Chemically activated luminopsins allow optogenetic inhibition of distributed nodes in an epileptic network for non-invasive and multi-site suppression of seizure activity. Neurobiol. Dis. 2018, 109, 1–10. [Google Scholar] [CrossRef]
- Zenchak, J.R.; Palmateer, B.; Dorka, N.; Brown, T.M.; Wagner, L.M.; Medendorp, W.E.; Petersen, E.D.; Prakash, M.; Hochgeschwender, U. Bioluminescence-driven optogenetic activation of transplanted neural precursor cells improves motor deficits in a Parkinson’s disease mouse model. J. Neurosci. Res. 2020, 98, 458–468. [Google Scholar] [CrossRef]
- Cheng, K.P.; Kiernan, E.A.; Eliceiri, K.W.; Williams, J.C.; Watters, J.J. Blue Light Modulates Murine Microglial Gene Expression in the Absence of Optogenetic Protein Expression. Sci. Rep. 2016, 6, 21172. [Google Scholar] [CrossRef] [Green Version]
- Inagaki, S.; Tsutsui, H.; Suzuki, K.; Agetsuma, M.; Arai, Y.; Jinno, Y.; Bai, G.; Daniels, M.J.; Okamura, Y.; Matsuda, T.; et al. Genetically encoded bioluminescent voltage indicator for multi-purpose use in wide range of bioimaging. Sci. Rep. 2017, 7, 42398. [Google Scholar] [CrossRef]
- Samineni, V.K.; Yoon, J.; Crawford, K.E.; Jeong, Y.R.; McKenzie, K.C.; Shin, G.; Xie, Z.; Sundaram, S.S.; Li, Y.; Yang, M.Y.; et al. Fully implantable, battery-free wireless optoelectronic devices for spinal optogenetics. Pain 2017, 158, 2108–2116. [Google Scholar] [CrossRef]
- Stockley, J.H.; Evans, K.; Matthey, M.; Volbracht, K.; Agathou, S.; Mukanowa, J.; Burrone, J.; Káradóttir, R.T. Surpassing light-induced cell damage in vitro with novel cell culture media. Sci. Rep. 2017, 7, 849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stujenske, J.M.; Spellman, T.; Gordon, J.A. Modeling the Spatiotemporal Dynamics of Light and Heat Propagation for InVivo Optogenetics. Cell Rep. 2015, 12, 525–534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petersen, E.D.; Sharkey, E.D.; Pal, A.; Shafau, L.O.; Zenchak-Petersen, J.; Peña, A.J.; Aggarwal, A.; Prakash, M.; Hochgeschwender, U. Restoring Function After Severe Spinal Cord Injury Through BioLuminescent-OptoGenetics. Front. Neurol. 2022, 12, 792643. [Google Scholar] [CrossRef] [PubMed]
- Hochbaum, D.R.; Zhao, Y.; Farhi, S.L.; Klapoetke, N.; Werley, C.A.; Kapoor, V.; Zou, P.; Kralj, J.M.; Maclaurin, D.; Smedemark-Margulies, N.; et al. All-optical electrophysiology in mammalian neurons using engineered microbial rhodopsins. Nat. Methods 2014, 11, 825–833. [Google Scholar] [CrossRef] [Green Version]
- Timotius, I.K.; Bieler, L.; Couillard-Despres, S.; Sandner, B.; Garcia-Ovejero, D.; Labombarda, F.; Estrada, V.; Müller, H.W.; Winkler, J.; Klucken, J.; et al. Combination of defined catwalk gait parameters for predictive locomotion recovery in experimental spinal cord injury rat models. eNeuro 2021, 8. [Google Scholar] [CrossRef]
- Zheng, G.; Younsi, A.; Scherer, M.; Riemann, L.; Walter, J.; Skutella, T.; Unterberg, A.; Zweckberger, K. The catwalk XT® gait analysis is closely correlated with tissue damage after cervical spinal cord injury in rats. Appl. Sci. 2021, 11, 4097. [Google Scholar] [CrossRef]
- Mallory, G.W.; Grahn, P.J.; Hachmann, J.T.; Lujan, J.L.; Lee, K.H. Optical stimulation for restoration of motor function after spinal cord injury. Mayo Clin. Proc. 2015, 90, 300–307. [Google Scholar] [CrossRef] [Green Version]
- Nishimura, Y.; Perlmutter, S.I.; Fetz, E.E. Restoration of upper limb movement via artificial corticospinal and musculospinal connections in a monkey with spinal cord injury. Front. Neural Circuits 2013, 7, 57. [Google Scholar] [CrossRef] [Green Version]
- Towne, C.; Montgomery, K.L.; Iyer, S.M.; Deisseroth, K.; Delp, S.L. Optogenetic Control of Targeted Peripheral Axons in Freely Moving Animals. PLoS ONE 2013, 8, e72691. [Google Scholar] [CrossRef] [Green Version]
- Hägglund, M.; Dougherty, K.J.; Borgius, L.; Itohara, S.; Iwasato, T.; Kiehn, O. Optogenetic dissection reveals multiple rhythmogenic modules underlying locomotion. Proc. Natl. Acad. Sci. USA 2013, 110, 11589–11594. [Google Scholar] [CrossRef]
- Deng, W.W.; Wu, G.Y.; Min, L.X.; Feng, Z.; Chen, H.; Tan, M.L.; Sui, J.F.; Liu, H.L.; Hou, J.M. Optogenetic Neuronal Stimulation Promotes Functional Recovery After Spinal Cord Injury. Front. Neurosci. 2021, 15, 640255. [Google Scholar] [CrossRef] [PubMed]
- English, A.W.; Berglund, K.; Carrasco, D.; Goebel, K.; Gross, R.E.; Isaacson, R.; Mistretta, O.C.; Wynans, C. Bioluminescent optogenetics: A novel experimental therapy to promote axon regeneration after peripheral nerve injury. Int. J. Mol. Sci. 2021, 22, 7217. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.T.; Carpenter, E.J.; Tian, Z.; Bruskiewich, R.; Burris, J.N.; Carrigan, C.T.; Chase, M.W.; Clarke, N.D.; Covshoff, S.; Depamphilis, C.W.; et al. Evaluating Methods for Isolating Total RNA and Predicting the Success of Sequencing Phylogenetically Diverse Plant Transcriptomes. PLoS ONE 2012, 7, e50226. [Google Scholar] [CrossRef] [PubMed]
- Klapoetke, N.C.; Murata, Y.; Kim, S.S.; Pulver, S.R.; Birdsey-Benson, A.; Cho, Y.K.; Morimoto, T.K.; Chuong, A.S.; Carpenter, E.J.; Tian, Z.; et al. Independent optical excitation of distinct neural populations. Nat. Methods 2014, 11, 338–346. [Google Scholar] [CrossRef] [Green Version]
- Berglund, K.; Clissold, K.; Li, H.E.; Wen, L.; Park, S.Y.; Gleixner, J.; Klein, M.E.; Lu, D.; Barter, J.W.; Rossi, M.A.; et al. Luminopsins integrate opto- and chemogenetics by using physical and biological light sources for opsin activation. Proc. Natl. Acad. Sci. USA 2016, 113, E358–E367. [Google Scholar] [CrossRef] [Green Version]
- Basso, D.M.; Beattie, M.S.; Bresnahan, J.C. Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight-drop device versus transection. Exp. Neurol. 1996, 139, 244–256. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Cheng, J.; Yin, J.; Yang, Y.; Guo, J.; Zhang, W.; Xie, B.; Lu, H.; Hao, D. Effects of sacral nerve electrical stimulation on 5-HT and 5-HT3AR/5-HT4R levels in the colon and sacral cord of acute spinal cord injury rat models. Mol. Med. Rep. 2020, 22, 763–773. [Google Scholar] [CrossRef]
- Basso, D.M.; Beattie, M.S.; Bresnahan, J.C. A Sensitive and Reliable Locomotor Rating Scale for Open Field Testing in Rats. J. Neurotrauma 1995, 12, 1–21. [Google Scholar] [CrossRef]
- Basso, D.M. Behavioral Testing after Spinal Cord Injury: Congruities, Complexities, and Controversies. J. Neurotrauma 2004, 21, 395–404. [Google Scholar] [CrossRef]
- Lankhorst, A.J.; Verzijl, M.R.; Hamers, F.P.T. Experimental spinal cord contusion injury: Comparison of different outcome parameters. Neurosci. Res. Commun. 1999, 24, 135–148. [Google Scholar] [CrossRef]
- Lee, J.K.; Emch, G.S.; Johnson, C.S.; Wrathall, J.R. Effect of spinal cord injury severity on alterations of the H-reflex. Exp. Neurol. 2005, 196, 430–440. [Google Scholar] [CrossRef] [PubMed]
- Rabchevsky, A.G.; Fugaccia, I.; Sullivan, P.G.; Scheff, S.W. Cyclosporin A Treatment Following Spinal Cord Injury to the Rat: Behavioral Effects and Stereological Assessment of Tissue Sparing; Mary Ann Liebert, Inc.: Larchmont, NY, USA, 2001. [Google Scholar]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ikefuama, E.C.; Kendziorski, G.E.; Anderson, K.; Shafau, L.; Prakash, M.; Hochgeschwender, U.; Petersen, E.D. Improved Locomotor Recovery in a Rat Model of Spinal Cord Injury by BioLuminescent-OptoGenetic (BL-OG) Stimulation with an Enhanced Luminopsin. Int. J. Mol. Sci. 2022, 23, 12994. https://doi.org/10.3390/ijms232112994
Ikefuama EC, Kendziorski GE, Anderson K, Shafau L, Prakash M, Hochgeschwender U, Petersen ED. Improved Locomotor Recovery in a Rat Model of Spinal Cord Injury by BioLuminescent-OptoGenetic (BL-OG) Stimulation with an Enhanced Luminopsin. International Journal of Molecular Sciences. 2022; 23(21):12994. https://doi.org/10.3390/ijms232112994
Chicago/Turabian StyleIkefuama, Ebenezer C., Griffin E. Kendziorski, Kevin Anderson, Lateef Shafau, Mansi Prakash, Ute Hochgeschwender, and Eric D. Petersen. 2022. "Improved Locomotor Recovery in a Rat Model of Spinal Cord Injury by BioLuminescent-OptoGenetic (BL-OG) Stimulation with an Enhanced Luminopsin" International Journal of Molecular Sciences 23, no. 21: 12994. https://doi.org/10.3390/ijms232112994
APA StyleIkefuama, E. C., Kendziorski, G. E., Anderson, K., Shafau, L., Prakash, M., Hochgeschwender, U., & Petersen, E. D. (2022). Improved Locomotor Recovery in a Rat Model of Spinal Cord Injury by BioLuminescent-OptoGenetic (BL-OG) Stimulation with an Enhanced Luminopsin. International Journal of Molecular Sciences, 23(21), 12994. https://doi.org/10.3390/ijms232112994