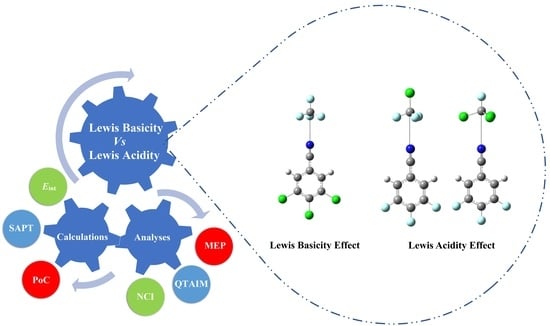
Effects of Lewis Basicity and Acidity on σ-Hole Interactions in Carbon-Bearing Complexes: A Comparative Ab Initio Study
Abstract
:1. Introduction
2. Results and Discussion
2.1. Electrostatic Potential (EP) Analysis
2.2. Point-of-Charge (PoC) Calculations
2.3. Interaction Energy
2.4. Quantum Theory of Atoms in Molecules (QTAIM) Analysis
2.5. Noncovalent Interaction (NCI) Analysis
2.6. Symmetry-Adapted Perturbation Theory (SAPT) Calculations
3. Methods and Materials
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gimeno, N.; Ros, M.B.; Serrano, J.L.; De la Fuente, M.R. Noncovalent interactions as a tool to design new bent-core liquid-crystal materials. Chem. Mater. 2008, 20, 1262–1271. [Google Scholar] [CrossRef]
- Munarriz, J.; Rabuffetti, F.A.; Contreras-Garcia, J. Building Fluorinated Hybrid Crystals: Understanding the Role of Noncovalent Interactions. Cryst. Growth Des. 2018, 18, 6901–6910. [Google Scholar] [CrossRef]
- Ariga, K.; Ito, H.; Hill, J.P.; Tsukube, H. Molecular recognition: From solution science to nano/materials technology. Chem. Soc. Rev. 2012, 41, 5800–5835. [Google Scholar] [CrossRef] [PubMed]
- Mazik, M. Molecular recognition of carbohydrates by acyclic receptors employing noncovalent interactions. Chem. Soc. Rev. 2009, 38, 935–956. [Google Scholar] [CrossRef] [PubMed]
- Riel, A.M.S.; Rowe, R.K.; Ho, E.N.; Carlsson, A.C.; Rappe, A.K.; Berryman, O.B.; Ho, P.S. Hydrogen Bond Enhanced Halogen Bonds: A Synergistic Interaction in Chemistry and Biochemistry. Acc. Chem. Res. 2019, 52, 2870–2880. [Google Scholar] [CrossRef]
- Choudhuri, K.; Pramanik, M.; Mal, P. Noncovalent Interactions in C-S Bond Formation Reactions. J. Org. Chem. 2020, 85, 11997–12011. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, D.; Cantu, D.C.; Koech, P.K.; Heldebrant, D.J.; Karkamkar, A.; Zheng, F.; Bearden, M.D.; Rousseau, R.; Glezakou, V.A. Directed Hydrogen Bond Placement: Low Viscosity Amine Solvents for CO2 Capture. ACS Sustain. Chem. Eng. 2019, 7, 7535–7542. [Google Scholar] [CrossRef]
- Stasyuk, O.A.; Jakubec, D.; Vondrasek, J.; Hobza, P. Noncovalent Interactions in Specific Recognition Motifs of Protein-DNA Complexes. J. Chem. Theory Comput. 2017, 13, 877–885. [Google Scholar] [CrossRef]
- Mani, D.; Arunan, E. The X-C...pi (X = F, Cl, Br, CN) carbon bond. J. Phys. Chem. A 2014, 118, 10081–10089. [Google Scholar] [CrossRef]
- Mahmoudi, G.; Bauza, A.; Amini, M.; Molins, E.; Mague, J.T.; Frontera, A. On the importance of tetrel bonding interactions in lead(ii) complexes with (iso)nicotinohydrazide based ligands and several anions. Dalton Trans. 2016, 45, 10708–10716. [Google Scholar] [CrossRef]
- Zeng, R.; Gong, Z.; Chen, L.; Yan, Q. Solution Self-Assembly of Chalcogen-Bonding Polymer Partners. ACS Macro Lett. 2020, 9, 1102–1107. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.Y.C.; Beer, P.D. Sigma-Hole Interactions in Anion Recognition. Chem 2018, 4, 731–783. [Google Scholar] [CrossRef]
- Murray, J.S.; Lane, P.; Clark, T.; Riley, K.E.; Politzer, P. Sigma-holes, pi-holes and electrostatically-driven interactions. J. Mol. Model. 2012, 18, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Politzer, P.; Murray, J.S.; Concha, M.C. Sigma-hole bonding between like atoms; a fallacy of atomic charges. J. Mol. Model. 2008, 14, 659–665. [Google Scholar] [CrossRef]
- Murray, J.S.; Lane, P.; Clark, T.; Politzer, P. Sigma-hole bonding: Molecules containing group VI atoms. J. Mol. Model. 2007, 13, 1033–1038. [Google Scholar] [CrossRef] [PubMed]
- Clark, T.; Hennemann, M.; Murray, J.S.; Politzer, P. Halogen bonding: The sigma-hole. Proceedings of “Modeling interactions in biomolecules II”, Prague, September 5th–9th, 2005. J. Mol. Model. 2007, 13, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.A.A.; Ahmed, O.A.M.; El-Taher, S.; Al-Fahemi, J.H.; Moussa, N.A.M.; Moustafa, H. Cospatial sigma-Hole and Lone Pair Interactions of Square-Pyramidal Pentavalent Halogen Compounds with pi-Systems: A Quantum Mechanical Study. ACS Omega 2021, 6, 3319–3329. [Google Scholar] [CrossRef]
- Ibrahim, M.A.A.; Ahmed, O.A.M.; Moussa, N.A.M.; El-Taher, S.; Moustafa, H. Comparative investigation of interactions of hydrogen, halogen and tetrel bond donors with electron-rich and electron-deficient π-systems. RSC Adv. 2019, 9, 32811–32820. [Google Scholar] [CrossRef] [Green Version]
- McDowell, S.A.; Joseph, J.A. The effect of atomic ions on model sigma-hole bonded complexes of AH3Y (A = C, Si, Ge; Y = F, Cl, Br). Phys. Chem. Chem. Phys. 2014, 16, 10854–10860. [Google Scholar] [CrossRef]
- Clark, T.; Hesselmann, A. The coulombic sigma-hole model describes bonding in CX3IY(-) complexes completely. Phys. Chem. Chem. Phys. 2018, 20, 22849–22855. [Google Scholar] [CrossRef]
- Li, Q.; Guo, X.; Yang, X.; Li, W.; Cheng, J.; Li, H.B. A sigma-hole interaction with radical species as electron donors: Does single-electron tetrel bonding exist? Phys. Chem. Chem. Phys. 2014, 16, 11617–11625. [Google Scholar] [CrossRef]
- Scheiner, S. The ditetrel bond: Noncovalent bond between neutral tetrel atoms. Phys. Chem. Chem. Phys. 2020, 22, 16606–16614. [Google Scholar] [CrossRef] [PubMed]
- Scheiner, S. Competition between a Tetrel and Halogen Bond to a Common Lewis Acid. J. Phys. Chem. A 2021, 125, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Scheiner, S. Origins and properties of the tetrel bond. Phys. Chem. Chem. Phys. 2021, 23, 5702–5717. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, H.; Li, Q.; Li, W.; Cheng, J. The dual role of pnicogen as Lewis acid and base and the unexpected interplay between the pnicogen bond and coordination interaction in H3N⋯FH2X⋯MCN (X = P and As; M = Cu, Ag, and Au). New J. Chem. 2015, 39, 2067–2074. [Google Scholar] [CrossRef]
- Bauza, A.; Quinonero, D.; Deya, P.M.; Frontera, A. Pnicogen-pi complexes: Theoretical study and biological implications. Phys. Chem. Chem. Phys. 2012, 14, 14061–14066. [Google Scholar] [CrossRef] [PubMed]
- Alkorta, I.; Elguero, J.; Solimannejad, M. Single electron pnicogen bonded complexes. J. Phys. Chem. A 2014, 118, 947–953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, X.; An, X.; Li, Q. Se...N chalcogen bond and Se...X halogen bond involving F2C horizontal line Se: Influence of hybridization, substitution, and cooperativity. J. Phys. Chem. A 2015, 119, 3518–3527. [Google Scholar] [CrossRef]
- Varadwaj, P.R.; Varadwaj, A.; Marques, H.M.; MacDougall, P.J. The chalcogen bond: Can it be formed by oxygen? Phys. Chem. Chem. Phys. 2019, 21, 19969–19986. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Ji, B.; Zhang, Y. Chalcogen bond: A sister noncovalent bond to halogen bond. J. Phys. Chem. A 2009, 113, 8132–8135. [Google Scholar] [CrossRef] [PubMed]
- Riley, K.E.; Murray, J.S.; Fanfrlik, J.; Rezac, J.; Sola, R.J.; Concha, M.C.; Ramos, F.M.; Politzer, P. Halogen bond tunability I: The effects of aromatic fluorine substitution on the strengths of halogen-bonding interactions involving chlorine, bromine, and iodine. J. Mol. Model. 2011, 17, 3309–3318. [Google Scholar] [CrossRef] [PubMed]
- Cavallo, G.; Metrangolo, P.; Milani, R.; Pilati, T.; Priimagi, A.; Resnati, G.; Terraneo, G. The halogen bond. Chem. Rev. 2016, 116, 2478–2601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hauchecorne, D.; van der Veken, B.J.; Moiana, A.; Herrebout, W.A. The C–Cl⋯N halogen bond, the weaker relative of the C–I and C–Br⋯N halogen bonds, finally characterized in solution. Chem. Phys. 2010, 374, 30–36. [Google Scholar] [CrossRef]
- Ibrahim, M.A.A.; Saeed, R.R.A.; Shehata, M.N.I.; Ahmed, M.N.; Shawky, A.M.; Khowdiary, M.M.; Elkaeed, E.B.; Soliman, M.E.S.; Moussa, N.A.M. Type I-IV Halogen...Halogen Interactions: A Comparative Theoretical Study in Halobenzene...Halobenzene Homodimers. Int. J. Mol. Sci. 2022, 23, 3114. [Google Scholar] [CrossRef] [PubMed]
- Hennemann, M.; Murray, J.S.; Politzer, P.; Riley, K.E.; Clark, T. Polarization-induced sigma-holes and hydrogen bonding. J. Mol. Model. 2012, 18, 2461–2469. [Google Scholar] [CrossRef] [PubMed]
- Politzer, P.; Murray, J.S. σ-Hole Interactions: Perspectives and Misconceptions. Crystals 2017, 7, 212. [Google Scholar] [CrossRef] [Green Version]
- Politzer, P.; Murray, J.S. Halogen bonding: An interim discussion. ChemPhysChem 2013, 14, 278–294. [Google Scholar] [CrossRef]
- Bauza, A.; Mooibroek, T.J.; Frontera, A. Tetrel Bonding Interactions. Chem. Rec. 2016, 16, 473–487. [Google Scholar] [CrossRef] [PubMed]
- Murray, J.S.; Lane, P.; Politzer, P. Expansion of the sigma-hole concept. J. Mol. Model. 2009, 15, 723–729. [Google Scholar] [CrossRef] [PubMed]
- Bauza, A.; Frontera, A. Competition between Halogen Bonding and pi-Hole Interactions Involving Various Donors: The Role of Dispersion Effects. ChemPhysChem 2015, 16, 3108–3113. [Google Scholar] [CrossRef] [PubMed]
- Frontera, A. Tetrel bonding interactions involving carbon at work: Recent advances in crystal engineering and catalysis. C.-J. Carbon Res. 2020, 6, 60. [Google Scholar] [CrossRef]
- Karim, A.; Schulz, N.; Andersson, H.; Nekoueishahraki, B.; Carlsson, A.C.; Sarabi, D.; Valkonen, A.; Rissanen, K.; Grafenstein, J.; Keller, S.; et al. Carbon’s three-center, four-electron tetrel bond, treated experimentally. J. Am. Chem. Soc. 2018, 140, 17571–17579. [Google Scholar] [CrossRef] [PubMed]
- Afkhami, F.A.; Mahmoudi, G.; Qu, F.R.; Gupta, A.; Kose, M.; Zangrando, E.; Zubkov, F.I.; Alkorta, I.; Safin, D.A. Supramolecular lead (II) architectures engineered by tetrel bonds. Crystengcomm 2020, 22, 2389–2396. [Google Scholar] [CrossRef]
- Bauza, A.; Mooibroek, T.J.; Frontera, A. Tetrel-bonding interaction: Rediscovered supramolecular force? Angew. Chem. Int. Ed. 2013, 52, 12317–12321. [Google Scholar] [CrossRef] [PubMed]
- Grabowski, S. Lewis Acid Properties of Tetrel Tetrafluorides—The Coincidence of the σ-Hole Concept with the QTAIM Approach. Crystals 2017, 7, 43–56. [Google Scholar] [CrossRef] [Green Version]
- Scheiner, S. Systematic elucidation of factors that influence the strength of tetrel bonds. J. Phys. Chem. A 2017, 121, 5561–5568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibrahim, M.A.A.; Moussa, N.A.M.; Safy, M.E.A. Quantum-mechanical investigation of tetrel bond characteristics based on the point-of-charge (PoC) approach. J. Mol. Model. 2018, 24, 219. [Google Scholar] [CrossRef]
- Hou, M.C.; Yang, S.B.; Li, Q.Z.; Cheng, J.B.; Li, H.B.; Liu, S.F. Tetrel Bond between 6-OTX(3)-Fulvene and NH(3): Substituents and Aromaticity. Molecules 2018, 24, 10. [Google Scholar] [CrossRef] [Green Version]
- Karpfen, A. On the interaction of propynal with HNO, HF, HCl, H2O, CH3OH, and NH3: Red- and blue-shifting hydrogen bonds and tetrel bonds. Comput. Theor. Chem. 2019, 1160, 1–13. [Google Scholar] [CrossRef]
- Ibrahim, M.A.A.; Mahmoud, A.H.M.; Moussa, N.A.M. Comparative investigation of ±σ–hole interactions of carbon-containing molecules with Lewis bases, acids and di-halogens. Chem. Pap. 2020, 74, 3569–3580. [Google Scholar] [CrossRef]
- Michalczyk, M.; Zierkiewicz, W.; Wysokinski, R.; Scheiner, S. Hexacoordinated Tetrel-Bonded Complexes between TF4 (T = Si, Ge, Sn, Pb) and NCH: Competition between sigma- and pi-Holes. ChemPhysChem 2019, 20, 959–966. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, M.A.A.; Moussa, N.A.M.; Kamel, A.A.K.; Shehata, M.N.I.; Ahmed, M.N.; Taha, F.; Abourehab, M.A.S.; Shawky, A.M.; Elkaeed, E.B.; Soliman, M.E.S. External Electric Field Effect on the Strength of sigma-Hole Interactions: A Theoretical Perspective in Likecdots, three dots, centeredLike Carbon-Containing Complexes. Molecules 2022, 27, 2963. [Google Scholar] [CrossRef] [PubMed]
- Grabarz, A.; Michalczyk, M.; Zierkiewicz, W.; Scheiner, S. Noncovalent bonds between tetrel atoms. ChemPhysChem 2020, 21, 1934–1944. [Google Scholar] [CrossRef]
- Ibrahim, M.A.A.; Mohamed, Y.A.M.; Abuelliel, H.A.A.; Rady, A.-S.S.M.; Soliman, M.E.S.; Ahmed, M.N.; Mohamed, L.A.; Moussa, N.A.M. σ-Hole Interactions of Tetrahedral Group IV–VIII Lewis Acid Centers with Lewis Bases: A Comparative Study. ChemistrySelect 2021, 6, 11856–11864. [Google Scholar] [CrossRef]
- Weiner, P.K.; Langridge, R.; Blaney, J.M.; Schaefer, R.; Kollman, P.A. Electrostatic potential molecular surfaces. Proc. Natl. Acad. Sci. USA 1982, 79, 3754–3758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murray, J.S.; Politzer, P. The electrostatic potential: An overview. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2011, 1, 153–163. [Google Scholar] [CrossRef]
- Ibrahim, M.A.A.; Shehata, M.N.I.; Soliman, M.E.S.; Moustafa, M.F.; El-Mageed, H.R.A.; Moussa, N.A.M. Unusual chalcogen∙∙∙chalcogen interactions in like∙∙∙like and unlike Y=C=Y∙∙∙Y=C=Y complexes (Y = O, S, and Se). Phys. Chem. Chem. Phys. 2021, 24, 3386–3399. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.A.A.; Rady, A.-S.S.M.; Al-Fahemi, J.H.; Telb, E.M.Z.; Ahmed, S.A.; Shawky, A.M.; Moussa, N.A.M. ±π-Hole Interactions: A Comparative Investigation Based on Boron-Containing Molecules. ChemistrySelect 2020, 5, 13223–13231. [Google Scholar] [CrossRef]
- Ibrahim, M.A.A.; Saeed, R.R.A.; Shehata, M.N.I.; Mohamed, E.E.B.; Soliman, M.E.S.; Al-Fahemi, J.H.; El-Mageed, H.R.A.; Ahmed, M.N.; Shawky, A.M.; Moussa, N.A.M. Unexplored σ-hole and π-hole interactions in (X2CY)2 complexes (X = F, Cl; Y = O, S). J. Mol. Struct. 2022, 1265, 133232. [Google Scholar] [CrossRef]
- Ibrahim, M.A.A.; Saad, S.M.A.; Al-Fahemi, J.H.; Mekhemer, G.A.H.; Ahmed, S.A.; Shawky, A.M.; Moussa, N.A.M. External electric field effects on the σ-hole and lone-pair hole interactions of group V elements: A comparative investigation. RSC Adv. 2021, 11, 4022–4034. [Google Scholar] [CrossRef]
- Ibrahim, M.A.A.; Mohamed, Y.A.M.; Abd Elhafez, H.S.M.; Shehata, M.N.I.; Soliman, M.E.S.; Ahmed, M.N.; El-Mageed, A.H.R.; Moussa, N.A.M. R•-hole interactions of group IV-VII radical-containing molecules: A comparative study. J. Mol. Graph. Model. 2022, 111. [Google Scholar] [CrossRef] [PubMed]
- Cukrowski, I.; de Lange, J.H.; Adeyinka, A.S.; Mangondo, P. Evaluating common QTAIM and NCI interpretations of the electron density concentration through IQA interaction energies and 1D cross-sections of the electron and deformation density distributions. Comput. Theor. Chem. 2015, 1053, 60–76. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, M.A.A.; Kamel, A.A.K.; Soliman, M.E.S.; Moustafa, M.F.; El-Mageed, H.R.A.; Taha, F.; Mohamed, L.A.; Moussa, N.A.M. Effect of external electric field on tetrel bonding interactions in (FTF3...FH) complexes (T = C, Si, Ge, and Sn). ACS Omega 2021, 6, 25476–25485. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.R.; Keinan, S.; Mori-Sanchez, P.; Contreras-Garcia, J.; Cohen, A.J.; Yang, W. Revealing noncovalent interactions. J. Am. Chem. Soc. 2010, 132, 6498–6506. [Google Scholar] [CrossRef] [Green Version]
- Jeziorski, B.; Moszynski, R.; Szalewicz, K. Perturbation-theory approach to intermolecular potential-energy surfaces of van der Waals complexes. Chem. Rev. 1994, 94, 1887–1930. [Google Scholar] [CrossRef]
- Woon, D.E.; Dunning, T.H. Gaussian basis sets for use in correlated molecular calculations. III. The atoms aluminum through argon. J. Chem. Phys. 1993, 98, 1358–1371. [Google Scholar] [CrossRef] [Green Version]
- Woon, D.E.; Dunning, T.H. Gaussian basis sets for use in correlated molecular calculations. IV. Calculation of static electrical response properties. J. Chem. Phys. 1994, 100, 2975–2988. [Google Scholar] [CrossRef] [Green Version]
- Møller, C.; Plesset, M.S. Note on an approximation treatment for many-electron systems. Phys. Rev. 1934, 46, 618–622. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, M.A.A. Molecular mechanical perspective on halogen bonding. J. Mol. Model. 2012, 18, 4625–4638. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Ibrahim, M.A.A.; Moussa, N.A.M.; Soliman, M.E.S.; Moustafa, M.F.; Al-Fahemi, J.H.; El-Mageed, H.R.A. On the potentiality of X-T-X3 compounds (T = C, Si, and Ge, and X = F, Cl, and Br) as tetrel- and halogen-bond donors. ACS Omega 2021, 6, 19330–19341. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.A.A.; Rady, A.S.S.M.; Soliman, M.E.S.; Moustafa, M.F.; El-Mageed, H.R.A.; Moussa, N.A.M. π-hole interactions of group III–VI elements with π-systems and Lewis bases: A comparative study. Struct. Chem. 2022, 33, 9–21. [Google Scholar] [CrossRef]
- Ibrahim, M.A.A.; Moussa, N.A.M. Unconventional Type III Halogen...Halogen Interactions: A Quantum Mechanical Elucidation of sigma-Hole...sigma-Hole and Di-sigma-Hole Interactions. ACS Omega 2020, 5, 21824–21835. [Google Scholar] [CrossRef] [PubMed]
- Boys, S.F.; Bernardi, F. The calculation of small molecular interactions by the differences of separate total energies. Some procedures with reduced errors. Mol. Phys. 1970, 19, 553–566. [Google Scholar] [CrossRef]
- Bader, R.F.W. Atoms in molecules. Acc. Chem. Res. 1985, 18, 9–15. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Revision E01; Gaussian Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Hohenstein, E.G.; Sherrill, C.D. Efficient evaluation of triple excitations in symmetry-adapted perturbation theory via second-order Moller-Plesset perturbation theory natural orbitals. J. Chem. Phys. 2010, 133, 104107. [Google Scholar] [CrossRef]
- Turney, J.M.; Simmonett, A.C.; Parrish, R.M.; Hohenstein, E.G.; Evangelista, F.A.; Fermann, J.T.; Mintz, B.J.; Burns, L.A.; Wilke, J.J.; Abrams, M.L.; et al. PSI4: An open-source ab initio electronic structure program. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2012, 2, 556–565. [Google Scholar] [CrossRef]
- Hohenstein, E.G.; Sherrill, C.D. Wavefunction methods for noncovalent interactions. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2012, 2, 304–326. [Google Scholar] [CrossRef]
- Hohenstein, E.G.; Sherrill, C.D. Density fitting and Cholesky decomposition approximations in symmetry-adapted perturbation theory: Implementation and application to probe the nature of pi-pi interactions in linear acenes. J. Chem. Phys. 2010, 132, 184111–184120. [Google Scholar] [CrossRef]
- Parker, T.M.; Burns, L.A.; Parrish, R.M.; Ryno, A.G.; Sherrill, C.D. Levels of symmetry adapted perturbation theory (SAPT). I. Efficiency and performance for interaction energies. J. Chem. Phys. 2014, 140, 094106. [Google Scholar] [CrossRef] [PubMed]







| Molecular Energies (i.e., Edestabilization and Estabilization, in kcal/mol) | ||||
|---|---|---|---|---|
| Lewis base∙∙∙PoC systems | ||||
| System | (a) NC-C6H2-X3∙∙∙PoC | (b) NCX∙∙∙PoC | ||
| −0.50 au | +0.50 au | −0.50 au | +0.50 au | |
| F | 7.41 | −13.06 | 7.13 | −11.20 |
| Cl | 7.44 | −13.39 | 7.62 | −11.40 |
| Br | 7.59 | −13.64 | 7.85 | −12.90 |
| I | 7.72 | −13.92 | 8.15 | −13.51 |
| Lewis acid∙∙∙PoC systems | ||||
| System | (c) W-C-F3∙∙∙PoC | (d) F-C-X3∙∙∙PoC | ||
| −0.50 au | +0.50 au | −0.50 au | +0.50 au | |
| F | −5.58 | 0.30 | −5.58 | 0.30 |
| Cl | −4.98 | −1.34 | −7.88 | −5.81 |
| Br | −4.96 | −1.75 | −9.11 | −8.18 |
| I | −4.62 | −2.66 | −12.12 | −10.41 |
| W/X | Complexation Parameters | Complexation Parameters | ||
|---|---|---|---|---|
| Distance (Å) | EMP2/aug−cc−pVTZ(PP) (kcal/mol) | Distance (Å) | EMP2/aug−cc−pVTZ(PP) (kcal/mol) | |
| (a) Set I complexes | ||||
| F-C-F3∙∙∙NC-C6H2-X3 | F-C-F3∙∙∙NCX | |||
| F | 3.33 | −1.20 | 3.35 | −1.05 |
| Cl | 3.33 | −1.21 | 3.34 | −1.13 |
| Br | 3.31 | −1.21 | 3.31 | −1.13 |
| I | 3.32 | −1.23 | 3.30 | −1.16 |
| (b) Set II complexes | ||||
| W-C-F3∙∙∙NC-C6H2-F3 | W-C-F3∙∙∙NCF | |||
| F | 3.33 | −1.20 | 3.35 | −1.05 |
| Cl | 3.37 | −1.07 | 3.38 | −0.91 |
| Br | 3.35 | −1.07 | 3.37 | −0.91 |
| I | 3.36 | −0.97 | 3.38 | −0.82 |
| F-C-X3∙∙∙NC-C6H2-F3 | F-C-X3∙∙∙NCF | |||
| F | 3.33 | −1.20 | 3.35 | –1.05 |
| Cl | 3.55 | –1.55 | 3.60 | –1.25 |
| Br | 3.72 a | –1.66 a | 3.57 | –1.25 |
| I | 3.75 a | –2.08 a | 3.58 | –1.46 |
| W/X | ρb (au) | ∇2ρb (au) | Hb (au) | ρb (au) | ∇2ρb (au) | Hb (au) |
|---|---|---|---|---|---|---|
| (a) Set I complexes | ||||||
| F-C-F3∙∙∙NC-C6H2-X3 | F-C-F3∙∙∙NCX | |||||
| F | 0.005043 | 0.020899 | 0.001060 | 0.004768 | 0.019982 | 0.001032 |
| Cl | 0.005062 | 0.020980 | 0.001064 | 0.004907 | 0.020402 | 0.001041 |
| Br | 0.005260 | 0.021898 | 0.001106 | 0.005265 | 0.021980 | 0.001110 |
| I | 0.005130 | 0.021289 | 0.001078 | 0.005317 | 0.022116 | 0.001111 |
| (b) Set II complexes | ||||||
| W-C-F3∙∙∙NC-C6H2-F3 | W-C-F3∙∙∙NCF | |||||
| F | 0.005033 | 0.020836 | 0.001056 | 0.004768 | 0.019982 | 0.001032 |
| Cl | 0.004860 | 0.019906 | 0.001005 | 0.004558 | 0.018900 | 0.000974 |
| Br | 0.004977 | 0.020428 | 0.001028 | 0.004660 | 0.019332 | 0.000993 |
| I | 0.005258 | 0.022034 | 0.000912 | 0.004624 | 0.019122 | 0.000981 |
| F-C-X3∙∙∙NC-C6H2-F3 | F-C-X3∙∙∙NCF | |||||
| F | 0.005033 | 0.020836 | 0.001056 | 0.004768 | 0.019982 | 0.001032 |
| Cl | 0.005739 | 0.017131 | 0.000847 | 0.005088 | 0.018951 | 0.001156 |
| Br | 0.005030 a | 0.016791 a | 0.000928 a | 0.006197 | 0.021593 | 0.001184 |
| I | 0.005591 a | 0.020600 a | 0.001220 a | 0.006986 | 0.021434 | 0.001017 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibrahim, M.A.A.; Shehata, M.N.I.; Rady, A.-s.S.M.; Abuelliel, H.A.A.; Abd Elhafez, H.S.M.; Shawky, A.M.; Oraby, H.F.; Hasanin, T.H.A.; Soliman, M.E.S.; Moussa, N.A.M. Effects of Lewis Basicity and Acidity on σ-Hole Interactions in Carbon-Bearing Complexes: A Comparative Ab Initio Study. Int. J. Mol. Sci. 2022, 23, 13023. https://doi.org/10.3390/ijms232113023
Ibrahim MAA, Shehata MNI, Rady A-sSM, Abuelliel HAA, Abd Elhafez HSM, Shawky AM, Oraby HF, Hasanin THA, Soliman MES, Moussa NAM. Effects of Lewis Basicity and Acidity on σ-Hole Interactions in Carbon-Bearing Complexes: A Comparative Ab Initio Study. International Journal of Molecular Sciences. 2022; 23(21):13023. https://doi.org/10.3390/ijms232113023
Chicago/Turabian StyleIbrahim, Mahmoud A. A., Mohammed N. I. Shehata, Al-shimaa S. M. Rady, Hassan A. A. Abuelliel, Heba S. M. Abd Elhafez, Ahmed M. Shawky, Hesham Farouk Oraby, Tamer H. A. Hasanin, Mahmoud E. S. Soliman, and Nayra A. M. Moussa. 2022. "Effects of Lewis Basicity and Acidity on σ-Hole Interactions in Carbon-Bearing Complexes: A Comparative Ab Initio Study" International Journal of Molecular Sciences 23, no. 21: 13023. https://doi.org/10.3390/ijms232113023
APA StyleIbrahim, M. A. A., Shehata, M. N. I., Rady, A. -s. S. M., Abuelliel, H. A. A., Abd Elhafez, H. S. M., Shawky, A. M., Oraby, H. F., Hasanin, T. H. A., Soliman, M. E. S., & Moussa, N. A. M. (2022). Effects of Lewis Basicity and Acidity on σ-Hole Interactions in Carbon-Bearing Complexes: A Comparative Ab Initio Study. International Journal of Molecular Sciences, 23(21), 13023. https://doi.org/10.3390/ijms232113023