Improved NMDA Receptor Activation by the Secreted Amyloid-Protein Precursor-α in Healthy Aging: A Role for D-Serine?
Abstract
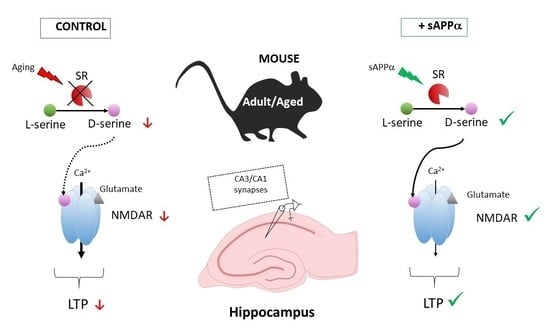
:1. Introduction
2. Results
2.1. Isolated NMDAR Activation
2.2. Theta-Burst-Induced Long-Term Potentiation (LTP)
2.3. Basal Neurotransmission
2.4. Isolated NMDAR Activation in SR-KO Mice
3. Discussion
4. Materials and Methods
4.1. Hippocampal Slices Electrophysiology
4.2. Preparation of Recombinant sAPPα
4.3. Pharmacology
4.4. Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jewett, B.E.; Thapa, B. Physiology, NMDA Receptor. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2022. [Google Scholar]
- Stroebel, D.; Paoletti, P. Architecture and function of NMDA receptors: An evolutionary perspective. J. Physiol. 2021, 599, 2615–2638. [Google Scholar] [CrossRef] [PubMed]
- Traynelis, S.F.; Wollmuth, L.P.; McBain, C.J.; Menniti, F.S.; Vance, K.M.; Ogden, K.K.; Hansen, K.B.; Yuan, H.; Myers, S.J.; Dingledine, R. Glutamate receptor ion channels: Structure, regulation, and function. Pharm. Rev. 2010, 62, 405–496. [Google Scholar] [CrossRef] [Green Version]
- Billard, J.M. Changes in Serine Racemase-Dependent Modulation of NMDA Receptor: Impact on Physiological and Pathological Brain Aging. Front. Mol. Biosci. 2018, 5, 106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coyle, J.T.; Balu, D.T. The Role of Serine Racemase in the Pathophysiology of Brain Disorders. Adv. Pharmacol. 2018, 82, 35–56. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Foster, T.C. Alteration in NMDA Receptor Mediated Glutamatergic Neurotransmission in the Hippocampus During Senescence. Neurochem. Res. 2019, 44, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Mira, R.G.; Cerpa, W. Building a Bridge Between NMDAR-Mediated Excitotoxicity and Mitochondrial Dysfunction in Chronic and Acute Diseases. Cell Mol. Neurobiol. 2021, 41, 1413–1430. [Google Scholar] [CrossRef] [PubMed]
- Billard, J.M. Ageing, hippocampal synaptic activity and magnesium. Magnes. Res. 2006, 19, 199–215. [Google Scholar]
- Burke, S.N.; Barnes, C.A. Neural plasticity in the ageing brain. Nat. Rev. Neurosci. 2006, 7, 30–40. [Google Scholar] [CrossRef]
- Foster, T.C. Biological markers of age-related memory deficits: Treatment of senescent physiology. CNS Drugs 2006, 20, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Ploux, E.; Freret, T.; Billard, J.M. D-serine in physiological and pathological brain aging. Biochim. Biophys. Acta. Proteins Proteom. 2021, 1869, 140542. [Google Scholar] [CrossRef]
- Samson, R.D.; Barnes, C.A. Impact of aging brain circuits on cognition. Eur. J. Neurosci. 2013, 37, 1903–1915. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.W.; Ascher, P. Glycine potentiates the NMDA response in cultured mouse brain neurons. Nature 1987, 325, 529–531. [Google Scholar] [CrossRef] [PubMed]
- McBain, C.J.; Kleckner, N.W.; Wyrick, S.; Dingledine, R. Structural requirements for activation of the glycine coagonist site of N-methyl-D-aspartate receptors expressed in Xenopus oocytes. Mol. Pharm. 1989, 36, 556–565. [Google Scholar]
- Basu, A.C.; Tsai, G.E.; Ma, C.L.; Ehmsen, J.T.; Mustafa, A.K.; Han, L.; Jiang, Z.I.; Benneyworth, M.A.; Froimowitz, M.P.; Lange, N.; et al. Targeted disruption of serine racemase affects glutamatergic neurotransmission and behavior. Mol. Psychiatry 2009, 14, 719–727. [Google Scholar] [CrossRef] [Green Version]
- Fossat, P.; Turpin, F.R.; Sacchi, S.; Dulong, J.; Shi, T.; Rivet, J.M.; Sweedler, J.V.; Pollegioni, L.; Millan, M.J.; Oliet, S.H.; et al. Glial D-Serine Gates NMDA Receptors at Excitatory Synapses in Prefrontal Cortex. Cereb Cortex 2012, 22, 595–606. [Google Scholar] [CrossRef] [Green Version]
- Le Bail, M.; Martineau, M.; Sacchi, S.; Yatsenko, N.; Radzishevsky, I.; Conrod, S.; Ait Ouares, K.; Wolosker, H.; Pollegioni, L.; Billard, J.M.; et al. Identity of the NMDA receptor coagonist is synapse specific and developmentally regulated in the hippocampus. Proc. Natl. Acad. Sci. USA 2015, 112, E204–E213. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Sacchi, S.; Pollegioni, L.; Basu, A.C.; Coyle, J.T.; Bolshakov, V.Y. Identity of endogenous NMDAR glycine site agonist in amygdala is determined by synaptic activity level. Nat. Commun. 2013, 4, 1760. [Google Scholar] [CrossRef] [Green Version]
- Papouin, T.; Ladepeche, L.; Ruel, J.; Sacchi, S.; Labasque, M.; Hanini, M.; Groc, L.; Pollegioni, L.; Mothet, J.P.; Oliet, S.H. Synaptic and Extrasynaptic NMDA Receptors Are Gated by Different Endogenous Coagonists. Cell 2012, 150, 633–646. [Google Scholar] [CrossRef] [Green Version]
- Billard, J.M.; Freret, T. Asc-1 transporter activation: An alternative to rescue age-related alterations in functional plasticity at rat hippocampal CA3/CA1 synapses. J. Neurochem. 2018, 147, 514–525. [Google Scholar] [CrossRef] [Green Version]
- Mothet, J.P.; Rouaud, E.; Sinet, P.M.; Potier, B.; Jouvenceau, A.; Dutar, P.; Videau, C.; Epelbaum, J.; Billard, J.M. A critical role for the glial-derived neuromodulator D-serine in the age-related deficits of cellular mechanisms of learning and memory. Aging Cell 2006, 5, 267–274. [Google Scholar] [CrossRef]
- Nava-Gómez, L.; Calero-Vargas, I.; Higinio-Rodríguez, F.; Vázquez-Prieto, B.; Olivares-Moreno, R.; Ortiz-Retana, J.; Aranda, P.; Hernández-Chan, N.; Rojas-Piloni, G.; Alcauter, S.; et al. Aging-Associated Cognitive Decline Is Reversed by D-Serine Supplementation. ENeuro 2022, 9, E.NEURO.0176-22.2022. [Google Scholar] [CrossRef] [PubMed]
- Orzylowski, M.; Fujiwara, E.; Mousseau, D.D.; Baker, G.B. An Overview of the Involvement of D-Serine in Cognitive Impairment in Normal Aging and Dementia. Front. Psychiatry 2021, 12, 754032. [Google Scholar] [CrossRef] [PubMed]
- Panizzutti, R.; Scoriels, L.; Avellar, M. The co-agonist Site of NMDA-glutamate receptors: A novel therapeutic target for age-related cognitive decline. Curr. Pharm. Des. 2014, 20, 5160–5168. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Yaku, K.; Nakagawa, T. Simultaneous Measurement of Amino Acid Enantiomers in Aged Mouse Brain Samples by LC/MS/MS Combined with Derivatization Using N (α)-(5-Fluoro-2,4-dinitrophenyl)-l-leucinamide (l-FDLA). Metabolites 2021, 11, 57. [Google Scholar] [CrossRef] [PubMed]
- Ganote, C.E.; Peterson, D.R.; Carone, F.A. The nature of D-serine--induced nephrotoxicity. Am. J. Pathol. 1974, 77, 269–282. [Google Scholar] [PubMed]
- Krug, A.W.; Volker, K.; Dantzler, W.H.; Silbernagl, S. Why is D-serine nephrotoxic and alpha-aminoisobutyric acid protective? Am. J. Physiol Ren. Physiol. 2007, 293, F382–F390. [Google Scholar] [CrossRef] [Green Version]
- Maekawa, M.; Okamura, T.; Kasai, N.; Hori, Y.; Summer, K.H.; Konno, R. D-amino-acid oxidase is involved in D-serine-induced nephrotoxicity. Chem Res. Toxicol. 2005, 18, 1678–1682. [Google Scholar] [CrossRef]
- Okada, A.; Nangaku, M.; Jao, T.M.; Maekawa, H.; Ishimono, Y.; Kawakami, T.; Inagi, R. D-serine, a novel uremic toxin, induces senescence in human renal tubular cells via GCN2 activation. Sci. Rep. 2017, 7, 11168. [Google Scholar] [CrossRef] [Green Version]
- Williams, R.E.; Lock, E.A. D-serine-induced nephrotoxicity: Possible interaction with tyrosine metabolism. Toxicology 2004, 201, 231–238. [Google Scholar] [CrossRef]
- Guercio, G.D.; Panizzutti, R. Potential and Challenges for the Clinical Use of d-Serine as a Cognitive Enhancer. Front. Psychiatry 2018, 9, 14. [Google Scholar] [CrossRef] [Green Version]
- Meftah, A.; Hasegawa, H.; Kantrowitz, J.T. D-Serine: A Cross Species Review of Safety. Front. Psychiatry 2021, 12, 726365. [Google Scholar] [CrossRef] [PubMed]
- Koga, R.; Miyoshi, Y.; Sakaue, H.; Hamase, K.; Konno, R. Mouse d-Amino-Acid Oxidase: Distribution and Physiological Substrates. Front. Mol. Biosci. 2017, 4, 82. [Google Scholar] [CrossRef] [PubMed]
- Krebs, H.A. Metabolism of amino-acids: Deamination of amino-acids. Biochem. J. 1935, 29, 1620–1644. [Google Scholar] [CrossRef] [PubMed]
- Ohide, H.; Miyoshi, Y.; Maruyama, R.; Hamase, K.; Konno, R. D-Amino acid metabolism in mammals: Biosynthesis, degradation and analytical aspects of the metabolic study. J. Chromatogr B Anal. Technol Biomed. Life Sci. 2012, 879, 3162–3168. [Google Scholar] [CrossRef] [PubMed]
- Pollegioni, L.; Piubelli, L.; Sacchi, S.; Pilone, M.S.; Molla, G. Physiological functions of D-amino acid oxidases: From yeast to humans. Cell Mol. Life Sci. 2007, 64, 1373–1394. [Google Scholar] [CrossRef]
- Pollegioni, L.; Sacchi, S.; Murtas, G. Human D-Amino Acid Oxidase: Structure, Function, and Regulation. Front. Mol. Biosci. 2018, 5, 107. [Google Scholar] [CrossRef] [Green Version]
- Hopkins, S.C.; Campbell, U.C.; Heffernan, M.L.; Spear, K.L.; Jeggo, R.D.; Spanswick, D.C.; Varney, M.A.; Large, T.H. Effects of D-amino acid oxidase inhibition on memory performance and long-term potentiation in vivo. Pharmacol. Res. Perspect. 2013, 1, e00007. [Google Scholar] [CrossRef]
- Howley, E.; Bestwick, M.; Fradley, R.; Harrison, H.; Leveridge, M.; Okada, K.; Fieldhouse, C.; Farnaby, W.; Canning, H.; Sykes, A.P.; et al. Assessment of the Target Engagement and D-Serine Biomarker Profiles of the D-Amino Acid Oxidase Inhibitors Sodium Benzoate and PGM030756. Neurochem. Res. 2017, 42, 3279–3288. [Google Scholar] [CrossRef]
- Lane, H.Y.; Tu, C.H.; Lin, W.C.; Lin, C.H. Brain Activity of Benzoate, a D-Amino Acid Oxidase Inhibitor, in Patients With Mild Cognitive Impairment in a Randomized, Double-Blind, Placebo Controlled Clinical Trial. Int. J. Neuropsychopharmacol. 2021, 24, 392–399. [Google Scholar] [CrossRef]
- Nagy, L.V.; Bali, Z.K.; Kapus, G.; Pelsőczi, P.; Farkas, B.; Lendvai, B.; Lévay, G.; Hernádi, I. Converging Evidence on D-Amino Acid Oxidase-Dependent Enhancement of Hippocampal Firing Activity and Passive Avoidance Learning in Rats. Int. J. Neuropsychopharmacol. 2021, 24, 434–445. [Google Scholar] [CrossRef]
- Mockett, B.G.; Richter, M.; Abraham, W.C.; Müller, U.C. Therapeutic Potential of Secreted Amyloid Precursor Protein APPsα. Front. Mol. Neurosci. 2017, 10, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chasseigneaux, S.; Allinquant, B. Functions of Aβ, sAPPα and sAPPβ: Similarities and differences. J. Neurochem. 2012, 120 (Suppl. 1), 99–108. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.J.; Wallace, M.S.; Hawver, D.B.; Kusiak, J.W.; Wallace, W.C. Characterization of the neurotrophic interaction between nerve growth factor and secreted alpha-amyloid precursor protein. J. Neurosci. Res. 2001, 63, 410–420. [Google Scholar] [CrossRef] [PubMed]
- Mattson, M.P.; Cheng, B.; Culwell, A.R.; Esch, F.S.; Lieberburg, I.; Rydel, R.E. Evidence for excitoprotective and intraneuronal calcium-regulating roles for secreted forms of the beta-amyloid precursor protein. Neuron 1993, 10, 243–254. [Google Scholar] [CrossRef]
- Anderson, J.J.; Holtz, G.; Baskin, P.P.; Wang, R.; Mazzarelli, L.; Wagner, S.L.; Menzaghi, F. Reduced cerebrospinal fluid levels of alpha-secretase-cleaved amyloid precursor protein in aged rats: Correlation with spatial memory deficits. Neuroscience 1999, 93, 1409–1420. [Google Scholar] [CrossRef]
- Xiong, M.; Jones, O.D.; Peppercorn, K.; Ohline, S.M.; Tate, W.P.; Abraham, W.C. Secreted amyloid precursor protein-alpha can restore novel object location memory and hippocampal LTP in aged rats. Neurobiol. Learn. Mem. 2017, 138, 291–299. [Google Scholar] [CrossRef]
- Fol, R.; Braudeau, J.; Ludewig, S.; Abel, T.; Weyer, S.W.; Roederer, J.P.; Brod, F.; Audrain, M.; Bemelmans, A.P.; Buchholz, C.J.; et al. Viral gene transfer of APPsalpha rescues synaptic failure in an Alzheimer’s disease mouse model. Acta Neuropathol. 2016, 131, 247–266. [Google Scholar] [CrossRef]
- Hick, M.; Herrmann, U.; Weyer, S.W.; Mallm, J.P.; Tschäpe, J.A.; Borgers, M.; Mercken, M.; Roth, F.C.; Draguhn, A.; Slomianka, L.; et al. Acute function of secreted amyloid precursor protein fragment APPsα in synaptic plasticity. Acta Neuropathol. 2015, 129, 21–37. [Google Scholar] [CrossRef]
- Morrissey, J.A.; Mockett, B.G.; Singh, A.; Kweon, D.; Ohline, S.M.; Tate, W.P.; Hughes, S.M.; Abraham, W.C. A C-terminal peptide from secreted amyloid precursor protein-α enhances long-term potentiation in rats and a transgenic mouse model of Alzheimer’s disease. Neuropharmacology 2019, 157, 107670. [Google Scholar] [CrossRef]
- Richter, M.C.; Ludewig, S.; Winschel, A.; Abel, T.; Bold, C.; Salzburger, L.R.; Klein, S.; Han, K.; Weyer, S.W.; Fritz, A.K.; et al. Distinct in vivo roles of secreted APP ectodomain variants APPsα and APPsβ in regulation of spine density, synaptic plasticity, and cognition. Embo J. 2018, 37, e98335. [Google Scholar] [CrossRef]
- Tan, V.T.Y.; Mockett, B.G.; Ohline, S.M.; Parfitt, K.D.; Wicky, H.E.; Peppercorn, K.; Schoderboeck, L.; Yahaya, M.F.B.; Tate, W.P.; Hughes, S.M.; et al. Lentivirus-mediated expression of human secreted amyloid precursor protein-alpha prevents development of memory and plasticity deficits in a mouse model of Alzheimer’s disease. Mol. Brain 2018, 11, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tyan, S.H.; Shih, A.Y.; Walsh, J.J.; Maruyama, H.; Sarsoza, F.; Ku, L.; Eggert, S.; Hof, P.R.; Koo, E.H.; Dickstein, D.L. Amyloid precursor protein (APP) regulates synaptic structure and function. Mol. Cell Neurosci. 2012, 51, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Moreno, L.; Rose, C.; Mohanraj, A.; Allinquant, B.; Billard, J.M.; Dutar, P. sAbetaPPalpha Improves Hippocampal NMDA-Dependent Functional Alterations Linked to Healthy Aging. J. Alzheimers Dis. 2015, 48, 927–935. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.J.; Ireland, D.R.; Ballagh, I.; Bourne, K.; Marechal, N.M.; Turner, P.R.; Bilkey, D.K.; Tate, W.P.; Abraham, W.C. Endogenous secreted amyloid precursor protein-alpha regulates hippocampal NMDA receptor function, long-term potentiation and spatial memory. Neurobiol. Dis. 2008, 31, 250–260. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, K.; Mattson, M.P. Secreted amyloid precursor protein alpha selectively suppresses N-methyl-D-aspartate currents in hippocampal neurons: Involvement of cycl.lic GMP. Neuroscience 1998, 83, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Basile, A.S.; Barger, S.W. Induction of serine racemase expression and D-serine release from microglia by secreted amyloid precursor protein (sAPP). Curr. Alzheimer Res. 2007, 4, 243–251. [Google Scholar]
- Wu, S.; Zhou, J.; Zhang, H.; Barger, S.W. Serine racemase expression differentiates aging from Alzheimer’s brain. Curr. Alzheimer Res. 2022, 19, 494–502. [Google Scholar] [CrossRef]
- Billard, J.M. Serine racemase as a prime target for age-related memory deficits. Eur. J. Neurosci. 2013, 37, 1931–1938. [Google Scholar] [CrossRef]
- Junjaud, G.; Rouaud, E.; Turpin, F.; Mothet, J.P.; Billard, J.M. Age-related effects of the neuromodulator D-serine on neurotransmission and synaptic potentiation in the CA1 hippocampal area of the rat. J. Neurochem. 2006, 98, 1159–1166. [Google Scholar] [CrossRef]
- Bliss, T.V.; Collingridge, G.L. A synaptic model of memory: Long-term potentiation in the hippocampus. Nature 1993, 361, 31–39. [Google Scholar] [CrossRef]
- Collingridge, G.L.; Bliss, T.V. Memories of NMDA receptors and LTP. Trends Neurosci. 1995, 18, 54–56. [Google Scholar] [CrossRef] [PubMed]
- Malenka, R.C.; Nicoll, R.A. NMDA-receptor-dependent synaptic plasticity: Multiple forms and mechanisms. Trends Neurosci. 1993, 16, 521–527. [Google Scholar] [CrossRef] [PubMed]
- Creager, R.; Dunwiddie, T.; Lynch, G. Paired-pulse and frequency facilitation in the CA1 region of the in vitro rat hippocampus. J. Physiol. 1980, 299, 409–424. [Google Scholar] [CrossRef] [PubMed]
- Wigström, H.; Gustafsson, B. Two types of synaptic facilitation recorded in pyramidal cells of in vitro hippocampal slices from guinea pigs. Neurosci. Lett. 1981, 26, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Alvares Pereira, G.; Silva Nunes, M.V.; Alzola, P.; Contador, I. Cognitive reserve and brain maintenance in aging and dementia: An integrative review. Appl. Neuropsychol. Adult 2022, 29, 1615–1625. [Google Scholar] [CrossRef]
- Damoiseaux, J.S. Effects of aging on functional and structural brain connectivity. NeuroImage 2017, 160, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Deery, H.A.; Di Paolo, R.; Moran, C.; Egan, G.F.; Jamadar, S.D. The older adult brain is less modular, more integrated, and less efficient at rest: A systematic review of large-scale resting-state functional brain networks in aging. Psychophysiology 2023, 60, e14159. [Google Scholar] [CrossRef]
- Eichenbaum, H. Learning from LTP: A comment on recent attempts to identify cellular and molecular mechanisms of memory. Learn. Mem. 1996, 3, 61–73. [Google Scholar] [CrossRef] [Green Version]
- Izquierdo, I. Role of NMDA receptors in memory. Trends Pharm. Sci. 1991, 12, 128–129. [Google Scholar] [CrossRef]
- Kim, S.J.; Linden, D.J. Ubiquitous plasticity and memory storage. Neuron 2007, 56, 582–592. [Google Scholar] [CrossRef] [Green Version]
- Lynch, M.A. Long-term potentiation and memory. Physiol. Rev. 2004, 84, 87–136. [Google Scholar] [CrossRef] [PubMed]
- Foster, A.C.; Rangel-Diaz, N.; Staubli, U.; Yang, J.Y.; Penjwini, M.; Viswanath, V.; Li, Y.X. Phenylglycine analogs are inhibitors of the neutral amino acid transporters ASCT1 and ASCT2 and enhance NMDA receptor-mediated LTP in rat visual cortex slices. Neuropharmacology 2017, 126, 70–83. [Google Scholar] [CrossRef] [PubMed]
- Paoletti, P.; Bellone, C.; Zhou, Q. NMDA receptor subunit diversity: Impact on receptor properties, synaptic plasticity and disease. Nat. Rev. Neurosci. 2013, 14, 383–400. [Google Scholar] [CrossRef] [PubMed]
- Ishida, A.; Furukawa, K.; Keller, J.N.; Mattson, M.P. Secreted form of beta-amyloid precursor protein shifts the frequency dependency for induction of LTD, and enhances LTP in hippocampal slices. Neuroreport 1997, 8, 2133–2137. [Google Scholar] [CrossRef] [PubMed]
- Livingstone, R.W.; Elder, M.K.; Singh, A.; Westlake, C.M.; Tate, W.P.; Abraham, W.C.; Williams, J.M. Secreted Amyloid Precursor Protein-Alpha Enhances LTP Through the Synthesis and Trafficking of Ca(2+)-Permeable AMPA Receptors. Front. Mol. Neurosci. 2021, 14, 660208. [Google Scholar] [CrossRef]
- Mockett, B.G.; Guévremont, D.; Elder, M.K.; Parfitt, K.D.; Peppercorn, K.; Morrissey, J.; Singh, A.; Hintz, T.J.; Kochen, L.; Tom Dieck, S.; et al. Glutamate Receptor Trafficking and Protein Synthesis Mediate the Facilitation of LTP by Secreted Amyloid Precursor Protein-Alpha. J. Neurosci. 2019, 39, 3188–3203. [Google Scholar] [CrossRef] [Green Version]
- Miya, K.; Inoue, R.; Takata, Y.; Abe, M.; Natsume, R.; Sakimura, K.; Hongou, K.; Miyawaki, T.; Mori, H. Serine racemase is predominantly localized in neurons in mouse brain. J. Comp. Neurol. 2008, 510, 641–654. [Google Scholar] [CrossRef]
- Ploux, E.; Bouet, V.; Radzishevsky, I.; Wolosker, H.; Freret, T.; Billard, J.M. Serine Racemase Deletion Affects the Excitatory/Inhibitory Balance of the Hippocampal CA1 Network. Int. J. Mol. Sci. 2020, 21, 9447. [Google Scholar] [CrossRef]
- Potier, B.; Turpin, F.R.; Sinet, P.M.; Rouaud, E.; Mothet, J.P.; Videau, C.; Epelbaum, J.; Dutar, P.; Billard, J.M. Contribution of the d-Serine-Dependent Pathway to the Cellular Mechanisms Underlying Cognitive Aging. Front. Aging Neurosci. 2010, 2, 1. [Google Scholar] [CrossRef] [Green Version]
- Turpin, F.R.; Potier, B.; Dulong, J.R.; Sinet, P.M.; Alliot, J.; Oliet, S.H.; Dutar, P.; Epelbaum, J.; Mothet, J.P.; Billard, J.M. Reduced serine racemase expression contributes to age-related deficits in hippocampal cognitive function. Neurobiol. Aging 2011, 32, 1495–1504. [Google Scholar] [CrossRef]
- Bai, L.; Hof, P.R.; Standaert, D.G.; Xing, Y.; Nelson, S.E.; Young, A.B.; Magnusson, K.R. Changes in the expression of the NR2B subunit during aging in macaque monkeys. Neurobiol. Aging 2004, 25, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Brim, B.L.; Haskell, R.; Awedikian, R.; Ellinwood, N.M.; Jin, L.; Kumar, A.; Foster, T.C.; Magnusson, K.R. Memory in aged mice is rescued by enhanced expression of the GluN2B subunit of the NMDA receptor. Behav. Brain Res. 2013, 238, 211–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clayton, D.A.; Browning, M.D. Deficits in the expression of the NR2B subunit in the hippocampus of aged Fisher 344 rats. Neurobiol. Aging 2001, 22, 165–168. [Google Scholar] [CrossRef] [PubMed]
- Magnusson, K.R. Aging of glutamate receptors: Correlations between binding and spatial memory performance in mice. Mech. Ageing Dev. 1998, 104, 227–248. [Google Scholar] [CrossRef] [PubMed]
- Magnusson, K.R.; Nelson, S.E.; Young, A.B. Age-related changes in the protein expression of subunits of the NMDA receptor. Brain Res. Mol. Brain Res. 2002, 99, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Ryan, M.M.; Morris, G.P.; Mockett, B.G.; Bourne, K.; Abraham, W.C.; Tate, W.P.; Williams, J.M. Time-dependent changes in gene expression induced by secreted amyloid precursor protein-alpha in the rat hippocampus. BMC Genom. 2013, 14, 376. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.Z.; Bodles, A.M.; Porter, M.M.; Griffin, W.S.; Basile, A.S.; Barger, S.W. Induction of serine racemase expression and D-serine release from microglia by amyloid beta-peptide. J. Neuroinflamm. 2004, 1, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mustafa, A.K.; Kumar, M.; Selvakumar, B.; Ho, G.P.; Ehmsen, J.T.; Barrow, R.K.; Amzel, L.M.; Snyder, S.H. Nitric oxide S-nitrosylates serine racemase, mediating feedback inhibition of D-serine formation. Proc. Natl. Acad. Sci. USA 2007, 104, 2950–2955. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Barger, S.W. Cross-linking of serine racemase dimer by reactive oxygen species and reactive nitrogen species. J. Neurosci. Res. 2012, 90, 1218–1229. [Google Scholar] [CrossRef] [Green Version]
- Hefter, D.; Draguhn, A. APP as a Protective Factor in Acute Neuronal Insults. Front. Mol. Neurosci. 2017, 10, 22. [Google Scholar] [CrossRef] [Green Version]
- Mattson, M.P. Cellular actions of beta-amyloid precursor protein and its soluble and fibrillogenic derivatives. Physiol. Rev. 1997, 77, 1081–1132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mattson, M.P.; Gary, D.S.; Chan, S.L.; Duan, W. Perturbed endoplasmic reticulum function, synaptic apoptosis and the pathogenesis of Alzheimer’s disease. In Biochemical Society Symposia; Portland Press: London, UK, 2001; pp. 151–162. [Google Scholar] [CrossRef]
- Mattson, M.P.; Liu, D. Energetics and oxidative stress in synaptic plasticity and neurodegenerative disorders. Neuromolecular Med. 2002, 2, 215–231. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, D.; Artoul, S.; Segal, A.C.; Kolodney, G.; Radzishevsky, I.; Dikopoltsev, E.; Foltyn, V.N.; Inoue, R.; Mori, H.; Billard, J.M.; et al. Neuronal D-Serine and Glycine Release Via the Asc-1 Transporter Regulates NMDA Receptor-Dependent Synaptic Activity. J. Neurosci. 2013, 33, 3533–3544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aura, J.; Riekkinen, P., Jr. Pre-training blocks the improving effect of tetrahydroaminoacridine and D-cycloserine on spatial navigation performance in aged rats. Eur. J. Pharm. 2000, 390, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Baxter, M.G.; Lanthorn, T.H.; Frick, K.M.; Golski, S.; Wan, R.Q.; Olton, D.S. D-cycloserine, a novel cognitive enhancer, improves spatial memory in aged rats. Neurobiol. Aging 1994, 15, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Billard, J.M.; Rouaud, E. Deficit of NMDA receptor activation in CA1 hippocampal area of aged rats is rescued by D-cycloserine. Eur. J. Neurosci. 2007, 25, 2260–2268. [Google Scholar] [CrossRef]
- Donzis, E.J.; Thompson, L.T. D-cycloserine enhances both intrinsic excitability of CA1 hippocampal neurons and expression of activity-regulated cytoskeletal (Arc) protein. Neurosci. Lett. 2014, 571, 50–54. [Google Scholar] [CrossRef]
- Rouaud, E.; Billard, J.M. D-cycloserine facilitates synaptic plasticity but impairs glutamatergic neurotransmission in rat hippocampal slices. Br. J. Pharm. 2003, 140, 1051–1056. [Google Scholar] [CrossRef] [Green Version]
- Zlomuzica, A.; De Souza Silva, M.A.; Huston, J.P.; Dere, E. NMDA receptor modulation by D-cycloserine promotes episodic-like memory in mice. Psychopharmacology 2007, 193, 503–509. [Google Scholar] [CrossRef]
- Solntseva, S.V.; Kozyrev, S.A.; Nikitin, V.P. A Study of the Participation of NMDA Glutamate Receptors in the Mechanisms of Specific Anterograde Amnesia Reversion. Bull. Exp. Biol. Med. 2020, 170, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Anderson, W.W.; Collingridge, G.L. The LTP Program: A data acquisition program for on-line analysis of long-term potentiation and other synaptic events. J. Neurosci. Methods 2001, 108, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Anderson, W.W.; Collingridge, G.L. Capabilities of the WinLTP data acquisition program extending beyond basic LTP experimental functions. J. Neurosci. Methods 2007, 162, 346–356. [Google Scholar] [CrossRef] [PubMed]
- Chasseigneaux, S.; Dinc, L.; Rose, C.; Chabret, C.; Coulpier, F.; Topilko, P.; Mauger, G.; Allinquant, B. Secreted amyloid precursor protein β and secreted amyloid precursor protein α induce axon outgrowth in vitro through Egr1 signaling pathway. PLoS ONE 2011, 6, e16301. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Billard, J.-M.; Freret, T. Improved NMDA Receptor Activation by the Secreted Amyloid-Protein Precursor-α in Healthy Aging: A Role for D-Serine? Int. J. Mol. Sci. 2022, 23, 15542. https://doi.org/10.3390/ijms232415542
Billard J-M, Freret T. Improved NMDA Receptor Activation by the Secreted Amyloid-Protein Precursor-α in Healthy Aging: A Role for D-Serine? International Journal of Molecular Sciences. 2022; 23(24):15542. https://doi.org/10.3390/ijms232415542
Chicago/Turabian StyleBillard, Jean-Marie, and Thomas Freret. 2022. "Improved NMDA Receptor Activation by the Secreted Amyloid-Protein Precursor-α in Healthy Aging: A Role for D-Serine?" International Journal of Molecular Sciences 23, no. 24: 15542. https://doi.org/10.3390/ijms232415542
APA StyleBillard, J. -M., & Freret, T. (2022). Improved NMDA Receptor Activation by the Secreted Amyloid-Protein Precursor-α in Healthy Aging: A Role for D-Serine? International Journal of Molecular Sciences, 23(24), 15542. https://doi.org/10.3390/ijms232415542