Chrysin Induces Apoptosis via the MAPK Pathway and Regulates ERK/mTOR-Mediated Autophagy in MC-3 Cells
Abstract
:1. Introduction
2. Results
2.1. Chrysin Reduces the Viability of MC-3 Oral Cancer Cells
2.2. Chrysin INDUCES Morphological Changes in MC-3 Cells
2.3. Chrysin Induces Apoptosis in MC-3 Cells
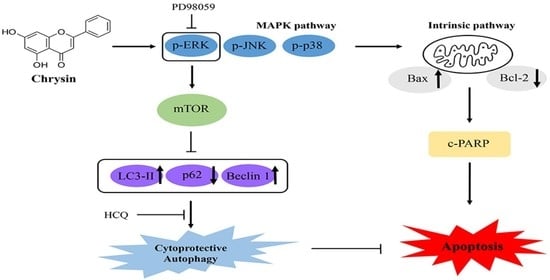
2.4. Chrysin-Induced Autophagy and Association with Apoptosis in Oral Cancer Cells
2.5. Chrysin Induces MAPK/ERK-Mediated Apoptosis and Autophagy in MC-3 Cells
3. Discussion
4. Materials and Methods
4.1. Cell Lines and Reagents
4.2. Cell Culture
4.3. MTT Assay
4.4. DAPI Staining
4.5. Annexin V-PI Staining
4.6. Acridine Orange Staining
4.7. Western Blot Analysis
4.8. Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brandwein, M.S.; Ivanov, K.; Wallace, D.I.; Hille, J.J.; Wang, B.; Fahmy, A.; Bodian, C.; Urken, M.L.; Gnepp, D.R.; Huvos, A.; et al. Mucoepidermoid carcinoma: A clinicopathologic study of 80 patients with special reference to histological grading. Am. J. Surg. Pathol. 2001, 25, 835–845. [Google Scholar] [CrossRef] [PubMed]
- Finger, P.T. Radiation therapy for orbital tumors: Concepts, current use, and ophthalmic radiation side effects. Surv. Ophthalmol. 2009, 54, 545–568. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.; Ullah, H.; Martorell, M.; Valdes, S.E.; Belwal, T.; Tejada, S.; Sureda, A.; Kamal, M.A. Flavonoids nanoparticles in cancer: Treatment, prevention and clinical prospects. Semin. Cancer Biol. 2021, 69, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, D.; Tuli, H.S.; Yerer, M.B.; Sharma, A.; Sak, K.; Srivastava, S.; Pandey, A.; Garg, V.K.; Sethi, G.; Bishayee, A. Natural product-based nanoformulations for cancer therapy: Opportunities and challenges. Semin. Cancer Biol. 2021, 69, 5–23. [Google Scholar] [CrossRef] [PubMed]
- Rapta, P.; Misík, V.; Stasko, A.; Vrábel, I. Redox intermediates of flavonoids and caffeic acid esters from propolis: An EPR spectroscopy and cyclic voltammetry study. Free Radic. Biol Med. 1995, 18, 901–908. [Google Scholar] [CrossRef] [PubMed]
- Zeinali, M.; Rezaee, S.A.; Hosseinzadeh, H. An overview on immunoregulatory and anti-inflammatory properties of chrysin and flavonoids substances. Biomed. Pharmacother. 2017, 92, 998–1009. [Google Scholar] [CrossRef] [PubMed]
- Halevas, E.; Mavroidi, B.; Pelecanou, M.; Hatzidimitriou, A.G. Structurally characterized zinc complexes of flavonoids chrysin and quercetin with antioxidant potential. Inorg. Chim. Acta 2021, 523, 120407. [Google Scholar] [CrossRef]
- Khoo, B.Y.; Chua, S.L.; Balaram, P. Apoptotic effects of chrysin in human cancer cell lines. Int. J. Mol. Sci. 2010, 11, 2188–2199. [Google Scholar] [CrossRef] [Green Version]
- Hassan, M.; Watari, H.; AbuAlmaaty, A.; Ohba, Y.; Sakuragi, N. Apoptosis and molecular targeting therapy in cancer. BioMed Res. Int. 2014, 2014, 150845. [Google Scholar] [CrossRef] [Green Version]
- Elumalai, P.; Gunadharini, D.N.; Senthilkumar, K.; Banudevi, S.; Arunkumar, R.; Benson, C.S.; Sharmila, G.; Arunakaran, J. Induction of apoptosis in human breast cancer cells by nimbolide through extrinsic and intrinsic pathway. Toxicol. Lett. 2012, 215, 131–142. [Google Scholar] [CrossRef]
- Levine, B.; Klionsky, D.J. Development by self-digestion: Molecular mechanisms and biological functions of autophagy. Dev. Cell 2004, 6, 463–477. [Google Scholar] [CrossRef] [PubMed]
- Mizushima, N. Autophagy: Process and function. Genes Dev. 2007, 21, 2861–2873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Xu, H.L.; Liu, Y.X.; An, N.; Zhao, S.; Bao, J.K. Autophagy modulation as a target for anticancer drug discovery. Acta Pharmacol. Sin. 2013, 34, 612–624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- White, E.; DiPaola, R.S. The Double-Edged Sword of Autophagy Modulation in Cancer Autophagy in Cancer Therapy. Clin. Cancer Res. 2009, 15, 5308–5316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmitz, K.J.; Ademi, C.; Bertram, S.; Schmid, K.W.; Baba, H.A. Prognostic relevance of autophagy-related markers LC3, p62/sequestosome 1, Beclin-1 and ULK1 in colorectal cancer patients with respect to KRAS mutational status. World J. Surg. Oncol. 2016, 14, 189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mi, W.; Wang, C.; Luo, G.; Li, J.; Zhang, Y.; Jiang, M.; Zhang, C.; Liu, N.; Jiang, X.; Yang, G.; et al. Targeting ERK induced cell death and p53/ROS-dependent protective autophagy in colorectal cancer. Cell Death Discov. 2021, 7, 375. [Google Scholar] [CrossRef]
- Ba, L.; Gao, J.; Chen, Y.; Qi, H.; Dong, C.; Pan, H.; Zhang, Q.; Shi, P.; Song, C.; Guan, X.; et al. Allicin attenuates pathological cardiac hypertrophy by inhibiting autophagy via activation of PI3K/Akt/mTOR and MAPK/ERK/mTOR signaling pathways. Phytomedicine 2019, 58, 152765. [Google Scholar] [CrossRef]
- Yang, S.H.; Sharrocks, A.D.; Whitmarsh, A.J. MAP kinase signaling cascades and transcriptional regulation. Gene 2013, 513, 1–13. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, W.Z.; Liu, T.; Feng, X.; Yang, N.; Zhou, H.F. Signaling pathway of MAPK/ERK in cell proliferation, differentiation, migration, senescence and apoptosis. J. Recept. Signal Transduct. Res. 2015, 35, 600–604. [Google Scholar] [CrossRef]
- Yue, J.; López, J.M. Understanding MAPK signaling pathways in apoptosis. Int. J. Mol. Sci. 2020, 21, 2346. [Google Scholar] [CrossRef]
- Samarghandian, S.; Afshari, J.T.; Davoodi, S. Chrysin reduces proliferation and induces apoptosis in the human prostate cancer cell line pc-3. Clinics 2011, 66, 1073–1079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhong, X.; Liu, D.; Jiang, Z.; Li, C.; Chen, L.; Xia, Y.; Liu, D.; Yao, Q.; Wang, D. Chrysin induced cell apoptosis and inhibited invasion through regulation of TET1 expression in gastric cancer cells. OncoTargets Ther. 2020, 13, 3277–3287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Battistelli, M.; Falcieri, E. Apoptotic bodies: Particular extracellular vesicles involved in intercellular communication. Biology 2020, 9, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, R.; Saneja, A.; Panda, A.K. An Annexin V-FITC—Propidium Iodide-Based Method for Detecting Apoptosis in a Non-Small Cell Lung Cancer Cell Line. Methods Mol. Biol. 2021, 2279, 213–223. [Google Scholar] [PubMed]
- Mehdi, S.H.; Zafaryab, M.; Nafees, S.; Khan, A.; Ahmad, I.; Hafeez, Z.B.; Rizvi, M.A. Chrysin sensitizes human lung cancer cells to tumor necrosis factor related apoptosis-inducing ligand (TRAIL) mediated apoptosis. APJCB Asian Pac. J. Cancer Biol. 2019, 4, 27–33. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Ma, S.; Liu, B.; Liu, J.; Zhu, R.; Li, M. Chrysin induces cell apoptosis via activation of the p53/Bcl-2/caspase-9 pathway in hepatocellular carcinoma cells. Exp. Ther. Med. 2016, 12, 469–474. [Google Scholar] [CrossRef] [Green Version]
- Phan, T.; Yu, X.M.; Kunnimalaiyaan, M.; Chen, H. Antiproliferative effect of chrysin on anaplastic thyroid cancer. J. Surg. Res. 2011, 170, 84–88. [Google Scholar] [CrossRef]
- Lin, Y.M.; Chen, C.I.; Hsiang, Y.P.; Hsu, Y.C.; Cheng, K.C.; Chien, P.H.; Pan, H.L.; Lu, C.C.; Chen, Y.J. Chrysin attenuates cell viability of human colorectal cancer cells through autophagy induction unlike 5-fluorouracil/oxaliplatin. Int. J. Mol. Sci. 2018, 19, 1763. [Google Scholar] [CrossRef] [Green Version]
- He, Y.; Shi, Y.; Yang, Y.; Huang, H.; Feng, Y.; Wang, Y.; Zhan, L.; Wei, B. Chrysin induces autophagy through the inactivation of the ROS-mediated Akt/mTOR signaling pathway in endometrial cancer. Int. J. Mol. Med. 2021, 48, 172. [Google Scholar] [CrossRef]
- Jo, M.H.; Kim, Y.T.; Park, S.J. Dieckol inhibits autophagic flux and induces apoptotic cell death in A375 human melanoma cells via lysosomal dysfunction and mitochondrial membrane impairment. Int. J. Mol. Sci. 2022, 23, 14149. [Google Scholar] [CrossRef]
- Braicu, C.; Buse, M.; Busuioc, C.; Drula, R.; Gulei, D.; Raduly, L.; Rusu, A.; Irimie, A.; Atanasov, A.G.; Slaby, O.; et al. A comprehensive review on MAPK: A promising therapeutic target in cancer. Cancers 2019, 11, 1618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Wang, H.; Sun, K.; Wang, X.; Pan, H.; Zhu, J.; Ji, X.; Li, X. Chrysin suppresses proliferation, migration, and invasion in glioblastoma cell lines via mediating the ERK/Nrf2 signaling pathway. Drug Des. Dev. Ther. 2018, 12, 721–733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, C.H.; Ro, S.H.; Cao, J.; Otto, N.M.; Kim, D.H. mTOR regulation of autophagy. FEBS Lett. 2010, 584, 1287–1295. [Google Scholar] [CrossRef] [PubMed]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, G.-H.; Lee, J.-H.; Han, S.-H.; Woo, J.-S.; Choi, E.-Y.; Jeon, S.-J.; Han, E.-J.; Jung, S.-H.; Park, Y.-S.; Park, B.-K.; et al. Chrysin Induces Apoptosis via the MAPK Pathway and Regulates ERK/mTOR-Mediated Autophagy in MC-3 Cells. Int. J. Mol. Sci. 2022, 23, 15747. https://doi.org/10.3390/ijms232415747
Jung G-H, Lee J-H, Han S-H, Woo J-S, Choi E-Y, Jeon S-J, Han E-J, Jung S-H, Park Y-S, Park B-K, et al. Chrysin Induces Apoptosis via the MAPK Pathway and Regulates ERK/mTOR-Mediated Autophagy in MC-3 Cells. International Journal of Molecular Sciences. 2022; 23(24):15747. https://doi.org/10.3390/ijms232415747
Chicago/Turabian StyleJung, Gi-Hwan, Jae-Han Lee, So-Hee Han, Joong-Seok Woo, Eun-Young Choi, Su-Ji Jeon, Eun-Ji Han, Soo-Hyun Jung, Young-Seok Park, Byung-Kwon Park, and et al. 2022. "Chrysin Induces Apoptosis via the MAPK Pathway and Regulates ERK/mTOR-Mediated Autophagy in MC-3 Cells" International Journal of Molecular Sciences 23, no. 24: 15747. https://doi.org/10.3390/ijms232415747
APA StyleJung, G. -H., Lee, J. -H., Han, S. -H., Woo, J. -S., Choi, E. -Y., Jeon, S. -J., Han, E. -J., Jung, S. -H., Park, Y. -S., Park, B. -K., Kim, B. -S., Kim, S. -K., & Jung, J. -Y. (2022). Chrysin Induces Apoptosis via the MAPK Pathway and Regulates ERK/mTOR-Mediated Autophagy in MC-3 Cells. International Journal of Molecular Sciences, 23(24), 15747. https://doi.org/10.3390/ijms232415747