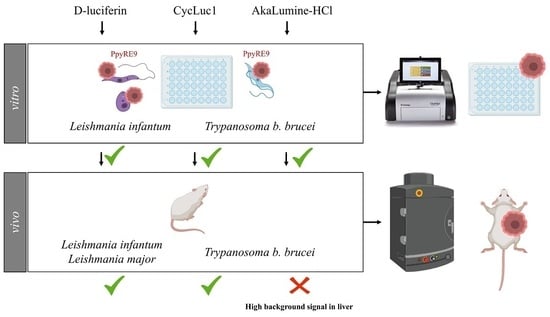
Comparison of Bioluminescent Substrates in Natural Infection Models of Neglected Parasitic Diseases
Abstract
:1. Introduction
2. Results
2.1. CycLuc1 Has a Moderately Higher Potency to Detect Parasites In Vitro
2.2. CycLuc1 Requires Lower Doses for Sensitive Leishmania Infantum Detection in Dermis and Visceral Organs
2.3. CycLuc1 Requires Lower Doses for Detection of Naturally Transmitted Leishmania and Trypanosoma Infections
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Parasites and Transfections
4.3. Infection of Sand Flies and Tsetse Flies for Transmission Studies
4.4. Comparison of Different Substrates for Parasite Detection In Vitro
4.5. Determination of the Detection Limit of the Different Substrates in the In Vivo L. Infantum Model
4.6. Comparison of Different Substrates for Infection Follow-Up in Mice
4.7. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Naylor, L.H. Reporter gene technology: The future looks bright. Biochem. Pharmacol. 1999, 58, 749–757. [Google Scholar] [CrossRef] [PubMed]
- De Niz, M.; Spadin, F.; Marti, M.; Stein, J.V.; Frenz, M.; Frischknecht, F. Toolbox for in vivo imaging of host-parasite interactions at multiple scales. Trends Parasitol. 2019, 35, 193–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaskova, Z.M.; Tsarkova, A.S.; Yampolsky, I.V. 1001 lights: Luciferins, luciferases, their mechanisms of action and applications in chemical analysis, biology and medicine. Chem. Soc. Rev. 2016, 45, 6048–6077. [Google Scholar] [CrossRef] [PubMed]
- Fraga, H. Firefly luminescence: A historical perspective and recent developments. Photochem. Photobiol. Sci. 2008, 7, 146–158. [Google Scholar] [CrossRef] [PubMed]
- Inouye, S. Firefly luciferase: An adenylate-forming enzyme for multicatalytic functions. Cell. Mol. Life Sci. 2010, 67, 387–404. [Google Scholar] [CrossRef]
- Weissleder, R.; Ntziachristos, V. Shedding light onto live molecular targets. Nat. Med. 2003, 9, 123–128. [Google Scholar] [CrossRef]
- Dawson, J.B.; Barker, D.J.; Ellis, D.J.; Grassam, E.; Cotterill, J.A.; Fisher, G.W.; Feather, J.W. A theoretical and experimental study of light absorption and scattering by In Vivo skin. Phys. Med. Biol. 1980, 25, 695–709. [Google Scholar] [CrossRef]
- Berger, F.; Paulmurugan, R.; Bhaumik, S.; Gambhir, S.S. Uptake kinetics and biodistribution of 14C-D-luciferin—A radiolabeled substrate for the firefly luciferase catalyzed bioluminescence reaction: Impact on bioluminescence based reporter gene imaging. Eur. J. Nucl. Med. Mol. Imaging 2008, 35, 2275–2285. [Google Scholar] [CrossRef]
- Kojima, R.; Takakura, H.; Ozawa, T.; Tada, Y.; Nagano, T.; Urano, Y. Rational design and development of near-infrared-emitting firefly luciferins available In Vivo. Angew. Chem. Int. Ed. Engl. 2013, 52, 1175–1179. [Google Scholar] [CrossRef]
- Shinde, R.; Perkins, J.; Contag, C.H. Luciferin derivatives for enhanced In Vitro and In Vivo bioluminescence assays. Biochemistry 2006, 45, 11103–11112. [Google Scholar] [CrossRef]
- Conley, N.R.; Dragulescu-Andrasi, A.; Rao, J.; Moerner, W.E. A Selenium analogue of firefly D-luciferin with red-shifted bioluminescence emission. Angew. Chem. Int. Ed. 2012, 51, 3350–3353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harwood, K.R.; Mofford, D.M.; Reddy, G.R.; Miller, S.C. Identification of mutant firefly luciferases that efficiently utilize aminoluciferins. Chem. Biol. 2011, 18, 1649–1657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, Y.; Walczak, P.; Bulte, J.W. Comparison of red-shifted firefly luciferase Ppy RE9 and conventional Luc2 as bioluminescence imaging reporter genes for In Vivo imaging of stem cells. J. Biomed. Opt. 2012, 17, 016004. [Google Scholar] [CrossRef] [PubMed]
- Adams, S.T., Jr.; Miller, S.C. Beyond D-luciferin: Expanding the scope of bioluminescence imaging In Vivo. Curr. Opin. Chem. Biol. 2014, 21, 112–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evans, M.S.; Chaurette, J.P.; Adams, S.T.; Reddy, G.R.; Paley, M.A.; Aronin, N.; Prescher, J.A.; Miller, S.C. A synthetic luciferin improves bioluminescence imaging in live mice. Nat. Methods 2014, 11, 393–395. [Google Scholar] [CrossRef] [PubMed]
- Iwano, S.; Obata, R.; Miura, C.; Kiyama, M.; Hama, K.; Nakamura, M.; Amano, Y.; Kojima, S.; Hirano, T.; Maki, S.; et al. Development of simple firefly luciferin analogs emitting blue, green, red, and near-infrared biological window light. Tetrahedron 2013, 69, 3847–3856. [Google Scholar] [CrossRef]
- Kuchimaru, T.; Iwano, S.; Kiyama, M.; Mitsumata, S.; Kadonosono, T.; Niwa, H.; Maki, S.; Kizaka-Kondoh, S. A luciferin analogue generating near-infrared bioluminescence achieves highly sensitive deep-tissue imaging. Nat. Commun. 2016, 7, 11856. [Google Scholar] [CrossRef] [Green Version]
- Lang, T.; Goyard, S.; Lebastard, M.; Milon, G. Bioluminescent Leishmania expressing luciferase for rapid and high throughput screening of drugs acting on amastigote-harbouring macrophages and for quantitative real-time monitoring of parasitism features in living mice. Cell. Microbiol. 2005, 7, 383–392. [Google Scholar] [CrossRef]
- Michel, G.; Ferrua, B.; Lang, T.; Maddugoda, M.P.; Munro, P.; Pomares, C.; Lemichez, E.; Marty, P. Luciferase-expressing Leishmania infantum allows the monitoring of amastigote population size, In Vivo, Ex Vivo and In Vitro. PLoS Negl. Trop. Dis. 2011, 5, e1323. [Google Scholar] [CrossRef] [Green Version]
- Melo, G.D.; Goyard, S.; Lecoeur, H.; Rouault, E.; Pescher, P.; Fiette, L.; Boissonnas, A.; Minoprio, P.; Lang, T. New insights into experimental visceral leishmaniasis: Real-time In Vivo imaging of Leishmania donovani virulence. PLoS Negl. Trop. Dis. 2017, 11, e0005924. [Google Scholar] [CrossRef]
- Tavares, J.; Santarém, N.; Cordeiro-da-Silva, A. Quantification of Leishmania parasites in murine models of visceral infection. Methods Mol. Biol. 2019, 1971, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Goyard, S.; Dutra, P.L.; Deolindo, P.; Autheman, D.; D’Archivio, S.; Minoprio, P. In Vivo imaging of trypanosomes for a better assessment of host–parasite relationships and drug efficacy. Parasitol. Int. 2014, 63, 260–268. [Google Scholar] [CrossRef] [Green Version]
- Bulté, D.; Van Bockstal, L.; Dirkx, L.; Van den Kerkhof, M.; De Trez, C.; Timmermans, J.P.; Hendrickx, S.; Maes, L.; Caljon, G. Miltefosine enhances infectivity of a miltefosine-resistant Leishmania infantum strain by attenuating its innate immune recognition. PLoS Negl. Trop. Dis. 2021, 15, e0009622. [Google Scholar] [CrossRef] [PubMed]
- Mendes Costa, D.; Cecílio, P.; Santarém, N.; Cordeiro-da-Silva, A.; Tavares, J. Murine infection with bioluminescent Leishmania infantum axenic amastigotes applied to drug discovery. Sci. Rep. 2019, 9, 18989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Álvarez-Velilla, R.; Gutiérrez-Corbo, M.d.C.; Punzón, C.; Pérez-Pertejo, M.Y.; Balaña-Fouce, R.; Fresno, M.; Reguera, R.M. A chronic bioluminescent model of experimental visceral leishmaniasis for accelerating drug discovery. PLoS Negl. Trop. Dis. 2019, 13, e0007133. [Google Scholar] [CrossRef] [PubMed]
- Lecoeur, H.; Buffet, P.; Morizot, G.; Goyard, S.; Guigon, G.; Milon, G.; Lang, T. Optimization of topical therapy for Leishmania major localized cutaneous leishmaniasis using a reliable C57BL/6 model. PLoS Negl. Trop. Dis. 2007, 1, e34. [Google Scholar] [CrossRef] [Green Version]
- Costa, D.M.; Cecílio, P.; Santarém, N.; Cordeiro-da-Silva, A.; Tavares, J. Whole-mouse In Vivo bioluminescence imaging applied to drug screening against Leishmania infantum: A reliable method to evaluate efficacy and optimize treatment regimens. bioRxiv 2018. [Google Scholar] [CrossRef] [Green Version]
- Ritchie, R.; Barrett, M.P.; Mottram, J.C.; Myburgh, E. In Vivo bioluminescence imaging to assess compound efficacy against Trypanosoma brucei. In Trypanosomatids: Methods and Protocols; Michels, P.A.M., Ginger, M.L., Zilberstein, D., Eds.; Springer: New York, NY, USA, 2020; pp. 801–817. [Google Scholar]
- Lewis, M.D.; Francisco, A.F.; Taylor, M.C.; Kelly, J.M. A new experimental model for assessing drug efficacy against Trypanosoma cruzi infection based on highly sensitive In Vivo imaging. J. Biomol. Screen. 2015, 20, 36–43. [Google Scholar] [CrossRef] [Green Version]
- McLatchie, A.P.; Burrell-Saward, H.; Myburgh, E.; Lewis, M.D.; Ward, T.H.; Mottram, J.C.; Croft, S.L.; Kelly, J.M.; Taylor, M.C. Highly sensitive In Vivo imaging of Trypanosoma brucei expressing “red-shifted” luciferase. PLoS Negl. Trop. Dis. 2013, 7, e2571. [Google Scholar] [CrossRef] [Green Version]
- Eberhardt, E.; Bulte, D.; Van Bockstal, L.; Van den Kerkhof, M.; Cos, P.; Delputte, P.; Hendrickx, S.; Maes, L.; Caljon, G. Miltefosine enhances the fitness of a non-virulent drug-resistant Leishmania infantum strain. J. Antimicrob. Chemother. 2019, 74, 395–406. [Google Scholar] [CrossRef]
- Domínguez-Asenjo, B.; Gutiérrez-Corbo, C.; Pérez-Pertejo, Y.; Iborra, S.; Balaña-Fouce, R.; Reguera, R.M. Bioluminescent imaging identifies thymus, as overlooked colonized organ, in a chronic model of Leishmania donovani mouse visceral leishmaniasis. ACS Infect. Dis. 2021, 7, 871–883. [Google Scholar] [CrossRef] [PubMed]
- Claes, F.; Vodnala, S.K.; van Reet, N.; Boucher, N.; Lunden-Miguel, H.; Baltz, T.; Goddeeris, B.M.; Büscher, P.; Rottenberg, M.E. Bioluminescent imaging of Trypanosoma brucei shows preferential testis dissemination which may hamper drug efficacy in sleeping sickness. PLoS Negl. Trop. Dis. 2009, 3, e486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dirkx, L.; Hendrickx, S.; Merlot, M.; Bulté, D.; Starick, M.; Elst, J.; Bafica, A.; Ebo, D.G.; Maes, L.; Van Weyenbergh, J.; et al. Long-term hematopoietic stem cells as a parasite niche during treatment failure in visceral leishmaniasis. Commun. Biol. 2022, 5, 626. [Google Scholar] [CrossRef]
- Agostino, V.S.; Trinconi, C.M.; Galuppo, M.K.; Price, H.; Uliana, S.R.B. Evaluation of NanoLuc, RedLuc and Luc2 as bioluminescent reporters in a cutaneous leishmaniasis model. Acta Trop. 2020, 206, 105444. [Google Scholar] [CrossRef]
- Van Reet, N.; Van de Vyver, H.; Pyana, P.P.; Van der Linden, A.M.; Büscher, P. A panel of Trypanosoma brucei strains tagged with blue and red-shifted luciferases for bioluminescent imaging in murine infection models. PLoS Negl. Trop. Dis. 2014, 8, e3054. [Google Scholar] [CrossRef] [PubMed]
- Branchini, B.R.; Fontaine, D.M.; Kohrt, D.; Huta, B.P.; Racela, A.R.; Fort, B.R.; Southworth, T.L.; Roda, A. Systematic comparison of beetle luciferase-luciferin pairs as sources of near-infrared light for In Vitro and In Vivo applications. Int. J. Mol. Sci. 2022, 23, 2451. [Google Scholar] [CrossRef]
- Su, Y.; Walker, J.R.; Park, Y.; Smith, T.P.; Liu, L.X.; Hall, M.P.; Labanieh, L.; Hurst, R.; Wang, D.C.; Encell, L.P.; et al. Novel NanoLuc substrates enable bright two-population bioluminescence imaging in animals. Nat. Methods 2020, 17, 852–860. [Google Scholar] [CrossRef]
- Nakayama, J.; Saito, R.; Hayashi, Y.; Kitada, N.; Tamaki, S.; Han, Y.; Semba, K.; Maki, S.A. High sensitivity In Vivo imaging of cancer metastasis using a near-infrared luciferin analogue seMpai. Int. J. Mol. Sci. 2020, 21, 7896. [Google Scholar] [CrossRef]
- Caljon, G.; Mabille, D.; Stijlemans, B.; De Trez, C.; Mazzone, M.; Tacchini-Cottier, F.; Malissen, M.; A Van Ginderachter, J.; Magez, S.; De Baetselier, P.; et al. Neutrophils enhance early Trypanosoma brucei infection onset. Sci. Rep. 2018, 8, 11203. [Google Scholar] [CrossRef] [Green Version]
- Volf, P.; Volfova, V. Establishment and maintenance of sand fly colonies. J. Vector Ecol. 2011, 36, S1–S9. [Google Scholar] [CrossRef]
- Hendrickx, S.; Van Bockstal, L.; Aslan, H.; Sadlova, J.; Maes, L.; Volf, P.; Caljon, G. Transmission potential of paromomycin-resistant Leishmania infantum and Leishmania donovani. J. Antimicrob. Chemother. 2019, 75, 951–957. [Google Scholar] [CrossRef] [PubMed]
- Hendrickx, S.; Van Bockstal, L.; Bulté, D.; Mondelaers, A.; Aslan, H.; Rivas, L.; Maes, L.; Caljon, G. Phenotypic adaptations of Leishmania donovani to recurrent miltefosine exposure and impact on sand fly infection. Parasites Vectors 2020, 13, 96. [Google Scholar] [CrossRef]
- Van Bockstal, L.; Bulté, D.; Hendrickx, S.; Sadlova, J.; Volf, P.; Maes, L.; Caljon, G. Impact of clinically acquired miltefosine resistance by Leishmania infantum on mouse and sand fly infection. Int. J. Parasitol. Drugs Drug Resist. 2020, 13, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Van den Kerkhof, M.; Van Bockstal, L.; Gielis, J.F.; Delputte, P.; Cos, P.; Maes, L.; Caljon, G.; Hendrickx, S. Impact of primary mouse macrophage cell types on Leishmania infection and In Vitro drug susceptibility. Parasitol. Res. 2018, 117, 3601–3612. [Google Scholar] [CrossRef] [PubMed]
- Tegazzini, D.; Diaz, R.; Aguilar, F.; Pena, I.; Presa, J.L.; Yardley, V.; Martin, J.J.; Coteron, J.M.; Croft, S.L.; Cantizani, J. A Replicative In Vitro assay for drug discovery against Leishmania donovani. Antimicrob. Agents Chemother. 2016, 60, 3524–3532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simonyan, H.; Hurr, C.; Young, C.N. A synthetic luciferin improves In Vivo bioluminescence imaging of gene expression in cardiovascular brain regions. Physiol. Genom. 2016, 48, 762–770. [Google Scholar] [CrossRef] [Green Version]
- Zambito, G.; Gaspar, N.; Ridwan, Y.; Hall, M.P.; Shi, C.; Kirkland, T.A.; Encell, L.P.; Löwik, C.; Mezzanotte, L. Evaluating brightness and spectral properties of click beetle and firefly luciferases using luciferin analogues: Identification of preferred pairings of luciferase and substrate for In Vivo bioluminescence imaging. Mol. Imaging Biol. 2020, 22, 1523–1531. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hendrickx, S.; Bulté, D.; Mabille, D.; Mols, R.; Claes, M.; Ilbeigi, K.; Ahmad, R.; Dirkx, L.; Van Acker, S.I.; Caljon, G. Comparison of Bioluminescent Substrates in Natural Infection Models of Neglected Parasitic Diseases. Int. J. Mol. Sci. 2022, 23, 16074. https://doi.org/10.3390/ijms232416074
Hendrickx S, Bulté D, Mabille D, Mols R, Claes M, Ilbeigi K, Ahmad R, Dirkx L, Van Acker SI, Caljon G. Comparison of Bioluminescent Substrates in Natural Infection Models of Neglected Parasitic Diseases. International Journal of Molecular Sciences. 2022; 23(24):16074. https://doi.org/10.3390/ijms232416074
Chicago/Turabian StyleHendrickx, Sarah, Dimitri Bulté, Dorien Mabille, Roxanne Mols, Mathieu Claes, Kayhan Ilbeigi, Rokaya Ahmad, Laura Dirkx, Sara I. Van Acker, and Guy Caljon. 2022. "Comparison of Bioluminescent Substrates in Natural Infection Models of Neglected Parasitic Diseases" International Journal of Molecular Sciences 23, no. 24: 16074. https://doi.org/10.3390/ijms232416074
APA StyleHendrickx, S., Bulté, D., Mabille, D., Mols, R., Claes, M., Ilbeigi, K., Ahmad, R., Dirkx, L., Van Acker, S. I., & Caljon, G. (2022). Comparison of Bioluminescent Substrates in Natural Infection Models of Neglected Parasitic Diseases. International Journal of Molecular Sciences, 23(24), 16074. https://doi.org/10.3390/ijms232416074