Arachidin-1, a Prenylated Stilbenoid from Peanut, Induces Apoptosis in Triple-Negative Breast Cancer Cells
Abstract
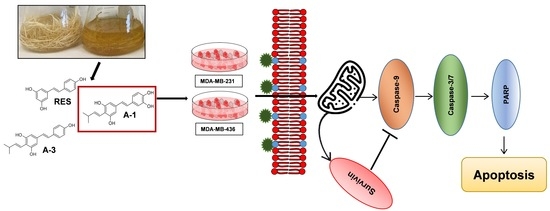
:1. Introduction
2. Results
2.1. Bioproduction and Purification of Prenylated Stilbenoids from Peanut Hairy Root Cultures
2.2. Prenylated Stilbenoid A-1 Shows the Highest Effect on Inhibition of Cell Proliferation
2.3. A-1 Blocks TNBC Cell Division Cycle in G2/M Phase
2.4. A-1 Induced Apoptosis in TNBC Cell Lines in a Dose-Dependent Manner
2.5. Impact of A-1 in Caspase-8 and Caspase-9, and PARP and Survivin Expression in TNBC Cell Lines
3. Discussion
4. Materials and Methods
4.1. Growth of Hairy Roots and Elicitation of Stilbenoids
4.2. Extraction and Purification of Stilbenoids by Semi-Preparative HPLC
4.3. Culture of Human Cell Lines
4.4. Cytotoxicity Assay
4.5. Cell Cycle Analysis with PI
4.6. Analysis of Apoptosis Using Annexin V-FITC Assay
4.7. Measuring of Caspase-3/7 Activity
4.8. Immunoblotting of Caspase-8 and Caspase-9
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Date, J.; Hon, C.; Singh, B.; Sahin, A.; Du, G.; Wang, J.; Wang, V.Y.; Deng, M.; Zhang, D.Y.; Monaco, M.E.; et al. Breast cancer molecular subtypes: From TNBC to QNBC. Am. J. Cancer Res. 2016, 6, 1864–1872. [Google Scholar]
- DeSantis, C.E.; Ma, J.; Gaudet, M.M.; Newman, L.A.; Miller, K.D.; Goding Sauer, A.; Jemal, A.; Siegel, R.L. Breast cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 211–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- André, F.; Zielinski, C.C. Optimal strategies for the treatment of metastatic triple-negative breast cancer with currently approved agents. Ann. Oncol. 2012, 23, 46–51. [Google Scholar] [CrossRef]
- Nedeljković, M.N.; Damjanović, A. Cells mechanisms of chemotherapy resistance in triple-negative breast cancer-how we can rise to the challenge. Cells 2019, 8, 957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sirerol, J.A.; Rodríguez, M.L.; Mena, S.; Asensi, M.A.; Estrela, J.M.; Ortega, A.L. Role of natural stilbenes in the prevention of cancer. Oxidative Med. Cell. Longev. 2016, 2016, 3128951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akinwumi, B.C.; Bordun, K.A.M.; Anderson, H.D. Biological activities of stilbenoids. Int. J. Mol. Sci. 2018, 19, 792. [Google Scholar] [CrossRef] [Green Version]
- Nandagopal, K.; Halder, M.; Dash, B.; Nayak, S.; Jha, S. Biotechnological approaches for production of anti-cancerous compounds resveratrol, podophyllotoxin and zerumbone. Curr. Med. Chem. 2017, 25, 4693–4717. [Google Scholar] [CrossRef]
- Medina-Bolivar, L.F.; Dolan, M.; Bennett, S.; Condori, J.M.; Hubstenberger, J.F. Production of Stilbenes in Plant Hairy Root Cultures. US Patent 7,666,677 B2, 23 February 2010. [Google Scholar]
- Yang, T.; Fang, L.; Sanders, S.; Jayanthi, S.; Rajan, G.; Podicheti, R.; Thallapuranam, S.K.; Mockaitis, K.; Medina-Bolivar, L.F. Stilbenoid prenyltransferases define key steps in the diversification of peanut phytoalexins. J. Biol. Chem. 2018, 293, 28–46. [Google Scholar] [CrossRef] [Green Version]
- Medina-Bolivar, F.L.; Condori, J.; Rimando, A.M.; Hubstenberger, J.; Shelton, K.; O’Keefe, S.F.; Bennett, S.; Dolan, M.C. Production and secretion of resveratrol in hairy root cultures of peanut. Phytochemistry 2007, 68, 1992–2003. [Google Scholar] [CrossRef]
- Chun, Y.J.; Kim, M.Y.; Guengerich, F.P. Resveratrol is a selective human cytochrome P450 1A1 inhibitor. Biochem. Biophys. Res. Commun. 1999, 262, 20–24. [Google Scholar] [CrossRef]
- Ferraz da Costa, D.C.; Campos, N.P.C.; Santos, R.A.; Guedes-da-Silva, F.H.; Martins-Dinis, M.M.D.C.; Zanphorlin, L.; Ramos, C.; Rangel, L.P.; Silva, J.L. Resveratrol prevents P53 aggregation in vitro and in breast cancer cells. Oncotarget 2018, 9, 29112–29122. [Google Scholar] [CrossRef] [Green Version]
- Benitez, D.A.; Pozo-Guisado, E.; Alvarez-Barrientos, A.; Fernandez-Salguero, P.M.; Castellón, E.A. Mechanisms involved in resveratrol-induced apoptosis and cell cycle arrest in prostate cancer-derived cell lines. J. Androl. 2007, 28, 282–293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rai, G.; Mishra, S.; Suman, S.; Shukla, Y. Resveratrol improves the anticancer effects of doxorubicin in vitro and in vivo models: A mechanistic insight. Phytomedicine 2016, 23, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Pfeffer, C.M.; Singh, A.T.K. Molecular sciences apoptosis: A target for anticancer therapy. Int. J. Mol. Sci. 2018, 19, 448. [Google Scholar] [CrossRef] [Green Version]
- Pop, C.; Timmer, J.; Sperandio, S.; Salvesen, G.S. The apoptosome activates caspase-9 by dimerization. Mol. Cell 2006, 22, 269–275. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, D.; Wang, X.; Wang, Y.; Ren, F.; Chang, D.; Jia, B. Caspase 3 is activated through caspase 8 instead of caspase 9 during H2O2-induced apoptosis in HeLa cells. Cell. Physiol. Biochem. 2011, 27, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.N.; Cao, Y.N.; Sun, J.; Liang, z.; Wu, Q.; Cui, S.H.; Zhi, D.F.; Guo, S.T.; Zhen, Y.H.; Zhang, S.B. Anti-breast cancer activity of resveratrol encapsulated in liposomes. J. Mater. Chem. B. 2019, 8, 27–37. [Google Scholar] [CrossRef]
- Mirzapur, P.; Khazaei, M.R.; Moradi, M.T.; Khazaei, M. Apoptosis induction in human breast cancer cell lines by synergic effect of raloxifene and resveratrol through increasing proapoptotic genes. Life Sci. 2018, 205, 45–53. [Google Scholar] [CrossRef]
- Venkatadri, R.; Iyer, A.K.V.; Kaushik, V.; Neelam, A. A novel resveratrol-salinomycin combination sensitizes er-positive breast cancer cells to apoptosis. Pharmacol. Rep. 2017, 69, 788–797. [Google Scholar] [CrossRef] [PubMed]
- Rathore, R.; McCallum, J.E.; Varghese, E.; Florea, A.M.; Büsselberg, D. Novel combination of salinomycin and resveratrol synergistically enhances the anti-proliferative and pro-apoptotic effects on human breast cancer cells. Apoptosis 2017, 22, 898–919. [Google Scholar] [CrossRef]
- Yar Saglam, A.S.; Kayhan, H.; Alp, E.; Onen, H.I. Resveratrol enhances the sensitivity of FL118 in triple-negative breast cancer cell lines via suppressing epithelial to mesenchymal transition. Mol. Biol. Rep. 2021, 48, 475–489. [Google Scholar] [CrossRef]
- Alkhalaf, M. Resveratrol-induced growth inhibition in MDA-MB-231 breast cancer cells is associated with mitogen-activated protein kinase signaling and protein translation. Eur. J. Cancer Prev. 2007, 16, 334–341. [Google Scholar] [CrossRef]
- Alkhalaf, M.; El-Mowafy, A.; Renno, W.; Rachid, O.; Ali, A.; Al-Attyiah, R. Resveratrol-induced apoptosis in human breast cancer cells is mediated primarily through the caspase-3-dependent pathway. Arch. Med. Res. 2008, 39, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Siedlecka-Kroplewska, K.; Wrońska, A.; Kmieć, Z. Piceatannol, a structural analog of resveratrol, is an apoptosis inducer and a multidrug resistance modulator in HL-60 human acute myeloid leukemia cells. Int. J. Mol. Sci. 2021, 22, 10597. [Google Scholar] [CrossRef]
- Dörrie, J.; Gerauer, H.; Wachter, Y.; Zunino, S.J. Resveratrol induces extensive apoptosis by depolarizing mitochondrial membranes and activating caspase-9 in acute lymphoblastic leukemia cells. Cancer Res. 2001, 61, 4731–4739. [Google Scholar] [PubMed]
- Li, Y.; Liu, J.; Liu, X.; Xing, K.; Wang, Y.; Li, F.; Yao, L. Resveratrol-induced cell inhibition of growth and apoptosis in MCF7 human breast cancer cells are associated with modulation of phosphorylated Akt and caspase-9. Appl. Biochem. Biotechnol. 2006, 135, 181–192. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, X.; Chen, T. Resveratrol induces mitochondria-mediated AIF and to a lesser extent caspase-9-dependent apoptosis in human lung adenocarcinoma ASTC-a-1 cells. Mol. Cell. Biochem. 2011, 354, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.A.; Choi, B.T.; Lee, Y.T.; Park, D.I.L.; Rhee, S.H.; Park, K.Y.; Choi, Y.H. Resveratrol Inhibits cell proliferation and induces apoptosis of human breast carcinoma MCF-7 cells. Oncol. Rep. 2004, 11, 441–446. [Google Scholar] [CrossRef]
- Fu, Y.; Ye, Y.; Zhu, G.; Xu, Y.; Sun, J.; Wu, H.; Feng, F.; Wen, Z.; Jiang, S.; Li, Y.; et al. Resveratrol induces human colorectal cancer cell apoptosis by activating the mitochondrial pathway via increasing reactive oxygen species. Mol. Med. Rep. 2021, 23, 170. [Google Scholar] [CrossRef]
- Medina-Aguilar, R.; Pérez-Plasencia, C.; Gariglio, P.; Marchat, L.A.; Flores-Pérez, A.; López-Camarillo, C.; García Mena, J. DNA methylation data for identification of epigenetic targets of resveratrol in triple negative breast cancer cells. Data Br. 2017, 11, 169–182. [Google Scholar] [CrossRef] [PubMed]
- Brents, L.K.; Medina-Bolivar, L.F.; Seely, K.A.; Nair, V.; Bratton, S.M.; Opo-Olazabal, L.; Patel, R.Y.; Liu, H.; Doerksen, R.J.; Prather, P.L.; et al. Natural Prenylated Resveratrol analogs arachidin-1 and -3 demonstrate improved glucuronidation profiles and have affinity for cannabinoid receptors. Xenobiotica 2012, 42, 139–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, J.C.; Lai, Y.H.; Djoko, B.; Wu, P.L.; Liu, C.D.; Liu, Y.W.; Chiou, R.Y.Y. Biosynthesis enhancement and antioxidant and anti-inflammatory activities of peanut (Arachis hypogaea L.) arachidin-1, arachidin-3, and isopentadienylresveratrol. J. Agric. Food Chem. 2006, 54, 10281–10287. [Google Scholar] [CrossRef]
- Huang, C.P.; Au, L.C.; Chiou, R.Y.Y.; Chung, P.C.; Chen, S.Y.; Tang, W.C.; Chang, C.L.; Fang, W.H.; Lin, S.B. Arachidin-1, a peanut stilbenoid, induces programmed cell death in human leukemia HL-60 cells. J. Agric. Food Chem. 2010, 58, 12123–12129. [Google Scholar] [CrossRef]
- Condori, J.; Sivakumar, G.; Hubstenberger, J.; Dolan, M.C.; Sobolev, V.S.; Medina-Bolivar, L.F. Induced biosynthesis of resveratrol and the prenylated stilbenoids arachidin-1 and arachidin-3 in hairy root cultures of peanut: Effects of culture medium and growth stage. Plant Physiol. Biochem. 2010, 48, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Yang, T.; Medina-Bolivar, L.F. Production of prenylated stilbenoids in hairy root cultures of peanut (Arachis hypogaea) and its wild relatives A. ipaensis and A. duranensis via an optimized elicitation procedure. Molecules 2020, 25, 509. [Google Scholar] [CrossRef] [Green Version]
- Yang, T.; Fang, L.; Nopo-Olazabal, C.; Condori, J.; Nopo-Olazabal, L.; Balmaceda, C.; Medina-Bolivar, L.F. Enhanced production of resveratrol, piceatannol, arachidin-1, and arachidin-3 in hairy root cultures of peanut co-treated with methyl jasmonate and cyclodextrin. J. Agric. Food Chem. 2015, 63, 3942–3950. [Google Scholar] [CrossRef]
- American Cancer Society. Cancer Facts and Figures; American Cancer Society: Atlanta, GA, USA, 2017; pp. 1–38. [Google Scholar]
- Lowenthal, R.M.; Eaton, K. Toxicity of chemotherapy. Hematol. Oncol. Clin. North Am. 1996, 10, 967–990. [Google Scholar] [CrossRef]
- Paller, C.J.; Rudek, M.A.; Zhou, X.C.; Wagner, W.D.; Hudson, T.S.; Anders, N.; Hammers, H.J.; Dowling, D.; King, S.; Antonarakis, E.S.; et al. A Phase I study of muscadine grape skin extract in men with biochemically recurrent prostate cancer: Safety, tolerability, and dose determination. Prostate 2015, 75, 1518–1525. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. WHO Global Report on Traditional and Complementary Medicine 2019; World Health Organization: Geneva, Switzerland, 2019; pp. 1–226. [Google Scholar]
- Cragg, G.M.; Kingston, D.G.; Newman, D.J. Anticancer Agents from Natural Products; CRC Press: Boca Raton, FL, USA, 2005; pp. 676–686. [Google Scholar]
- Mattio, L.M.; Catinella, G.; Pinto, A.; Dallavalle, S. Natural and nature-inspired stilbenoids as antiviral agents. Eur. J. Med. Chem. 2020, 202, 112541. [Google Scholar] [CrossRef]
- Meng, X.; Zhou, J.; Zhao, C.N.; Gan, R.Y.; Li, H.B. Health benefits and molecular mechanisms of resveratrol: A narrative review. Foods 2020, 9, 340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Springer, M.; Moco, S. Resveratrol and its human metabolites—Effects on metabolic health and obesity. Nutrients 2019, 11, 143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sobolev, V.S. Localized production of phytoalexins by peanut (Arachis hypogaea) kernels in response to invasion by Aspergillus species. J. Agric. Food Chem. 2008, 56, 1949–1954. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, B.; Ferreira, C.; Luan Alves Passos, C.; Lima Silva, J.; Fialho, E. Resveratrol, curcumin and piperine alter human glyoxalase 1 in MCF-7 breast cancer cells. Int. J. Mol. Sci. 2020, 21, 5244. [Google Scholar] [CrossRef]
- Özdemiír, F.; Sever, A.; Keçecí, Y.Ö.; Incesu, Z. Resveratrol increases the sensitivity of breast cancer MDA-MB-231 cell line to cisplatin by regulating intrinsic apoptosis. Iran. J. Basic Med. Sci. 2021, 24, 66. [Google Scholar]
- Sobolev, V.S.; Khan, S.I.; Tabanca, N.; Wedge, D.E.; Manly, S.P.; Cutler, S.J.; Coy, M.R.; Becnel, J.J.; Neff, S.A.; Gloer, J.B. Biological activity of peanut (Arachis hypogaea) phytoalexins and selected natural and synthetic stilbenoids. J. Agric. Food Chem. 2011, 59, 1673–1682. [Google Scholar] [CrossRef] [Green Version]
- Thomas, E.; Gopalakrishnan, V.; Hegde, M.; Kumar, S.; Karki, S.S.; Raghavan, S.C.; Choudhary, B. A novel resveratrol based tubulin inhibitor induces mitotic arrest and activates apoptosis in cancer cells. Sci. Rep. 2016, 6, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takashina, M.; Inoue, S.; Tomihara, K.; Tomita, K.; Hattori, K.; Zhao, Q.-L.; Suzuki, T.; Noguchi, M.; Ohashi, W.; Hattori, Y. Different effect of resveratrol to induction of apoptosis depending on the type of human cancer cells. Int. J. Oncol. 2017, 50, 787–797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, Y.; Ma, Z.; Dang, X.; Li, W.; Ma, Q. Effect of resveratrol on proliferation and apoptosis of human pancreatic cancer MIA PaCa-2 cells may involve inhibition of the hedgehog signaling pathway. Mol. Med. Rep. 2014, 10, 2563–2567. [Google Scholar] [CrossRef] [Green Version]
- Pucci, B.; Kasten, M.; Giordano, A. Cell cycle and apoptosis. Neoplasia 2000, 2, 291–299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, B.; Zhou, Z.; Zhou, W.; Liu, J.; Zhang, Q.; Xia, J.; Zhu, R. Resveratrol inhibits proliferation in human colorectal carcinoma cells by inducing G1/S-phase cell cycle arrest and apoptosis through caspase/cyclin-CDK pathways. Mol. Med. Rep. 2014, 10, 1697–1717. [Google Scholar] [CrossRef] [Green Version]
- Horgan, X.J.; Tatum, H.; Brannan, E.; Paull, D.H.; Rhodes, L.V. Resveratrol analogues surprisingly effective against triple-negative breast cancer, independent of ERα. Oncol. Rep. 2019, 41, 3517–3526. [Google Scholar] [CrossRef] [PubMed]
- Ko, H.S.; Lee, H.-J.; Kim, S.-H.; Lee, E.-O. Piceatannol suppresses breast cancer cell invasion through the inhibition of MMP-9: Involvement of PI3K/AKT and NF-ΚB pathways. J. Agric. Food Chem. 2012, 60, 24. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.K.; Park, Y.S.; Park, B.K.; Kim, B.S.; Kim, S.K.; Jung, J.Y. Resveratrol induces apoptosis through PI3K/Akt and P53 signal pathway in MDA-MB-231 breast cancer cells. Korean J. Food Sci. Technol. 2012, 44, 452–459. [Google Scholar] [CrossRef] [Green Version]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohammadhosseinpour, S.; Ho, L.-C.; Fang, L.; Xu, J.; Medina-Bolivar, F. Arachidin-1, a Prenylated Stilbenoid from Peanut, Induces Apoptosis in Triple-Negative Breast Cancer Cells. Int. J. Mol. Sci. 2022, 23, 1139. https://doi.org/10.3390/ijms23031139
Mohammadhosseinpour S, Ho L-C, Fang L, Xu J, Medina-Bolivar F. Arachidin-1, a Prenylated Stilbenoid from Peanut, Induces Apoptosis in Triple-Negative Breast Cancer Cells. International Journal of Molecular Sciences. 2022; 23(3):1139. https://doi.org/10.3390/ijms23031139
Chicago/Turabian StyleMohammadhosseinpour, Sepideh, Linh-Chi Ho, Lingling Fang, Jianfeng Xu, and Fabricio Medina-Bolivar. 2022. "Arachidin-1, a Prenylated Stilbenoid from Peanut, Induces Apoptosis in Triple-Negative Breast Cancer Cells" International Journal of Molecular Sciences 23, no. 3: 1139. https://doi.org/10.3390/ijms23031139
APA StyleMohammadhosseinpour, S., Ho, L. -C., Fang, L., Xu, J., & Medina-Bolivar, F. (2022). Arachidin-1, a Prenylated Stilbenoid from Peanut, Induces Apoptosis in Triple-Negative Breast Cancer Cells. International Journal of Molecular Sciences, 23(3), 1139. https://doi.org/10.3390/ijms23031139