Effect of Air Exposure-Induced Hypoxia on Neurotransmitters and Neurotransmission Enzymes in Ganglia of the Scallop Azumapecten farreri
Abstract
:1. Introduction
2. Results
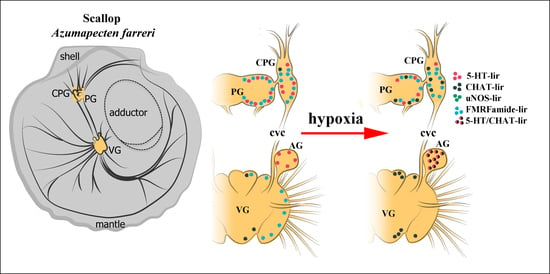
2.1. General Neuroanatomy of the Scallop Azumapecten farreri
2.2. HIF1alpha Protein Expression
2.3. Impact of Hypoxia on Neurotransmitter Distribution in Scallop CNS
2.3.1. 5-HT-1ir Distribution after Hypoxia Exposure
2.3.2. Choline Acetyltransferase-lir Distribution after Hypoxia Exposure
2.3.3. uNOS-lir Distribution after Hypoxia Exposure
2.3.4. FMRFamide-lir Distribution after Hypoxia Exposure
2.4. Colocalization of Neurotransmitters after Hypoxia
2.4.1. FMRF/5-HT-lir
2.4.2. NOS-/5-HT-lir
2.4.3. CHAT/5-HT-lir
3. Discussion
3.1. 5-HT-1ir in Bivalves
3.2. CHAT-lir in Bivalves
3.3. uNOS in Bivalves
3.4. FMRFamide in Bivalves
4. Materials and Methods
4.1. Experimental Animals and Establishment of Hypoxic Conditions
4.2. SDS and Western Blotting of HIF1alpha
4.3. Antibodies
4.4. Immunohistochemistry
4.5. Microscopy and Imaging
4.6. Quantification and Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, R.S.S. Hypoxia: From molecular responses to ecosystem responses. Mar. Pollut. Bull. 2002, 45, 35–45. [Google Scholar] [CrossRef]
- Larade, K.; Storey, K. Living without Oxygen: Anoxia-Responsive Gene Expression and Regulation. Curr. Genom. 2009, 10, 76–85. [Google Scholar] [CrossRef] [Green Version]
- Spicer, I.J. What can an ecophysiological approach tell us about the physiological responses of marine invertebrates to hypoxia? J. Exp. Biol. 2014, 217, 46–56. [Google Scholar] [CrossRef] [Green Version]
- Lacoste, A.; Malham, S.K.; Cueff, A.; Jalabert, F.; Gélébart, F.; Poulet, S.A. Evidence for a form of adrenergic response to stress in the mollusc Crassostrea gigas. J. Exp. Biol. 2001, 204, 1247–1255. [Google Scholar] [CrossRef]
- Lacoste, A.; Jalabert, F.; Malham, S.K.; Cueff, A.; Poulet, S.A. Stress and Stress-Induced Neuroendocrine Changes Increase the Susceptibility of Juvenile Oysters (Crassostrea gigas) to Vibrio splendidus. Appl. Environ. Microbiol. 2001, 67, 2304–2309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lacoste, A.; De Cian, M.C.; Cueff, A.; Poulet, S.A. Noradrenaline and α-adrenergic signaling induce the hsp70 gene promoter in mollusc immune cells. J. Cell Sci. 2001, 114, 3557–3564. [Google Scholar] [CrossRef]
- Song, L.; Wang, L.; Zhang, H.; Wang, M. The immune system and its modulation mechanism in scallop. Fish Shellfish. Immunol. 2015, 46, 65–78. [Google Scholar] [CrossRef]
- Liu, Z.; Zhou, Z.; Wang, L.; Dong, W.; Qiu, L.; Song, L. The cholinergic immune regulation mediated by a novel muscarinic acetylcholine receptor through TNF pathway in oyster Crassostrea gigas. Dev. Comp. Immunol. 2016, 65, 139–148. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Song, X.; Song, L. The oyster immunity. Dev. Comp. Immunol. 2018, 80, 99–118. [Google Scholar] [CrossRef]
- Lowy, J. Contraction and relaxation in the adductor muscles of Pecten maximus. J. Physiol. 1954, 124, 100–105. [Google Scholar] [CrossRef] [Green Version]
- Mellon, D.F. Junctional physiology and motor nerve distribution in the fast adductor muscle of the scallop. Science 1968, 160, 1018–1020. [Google Scholar] [CrossRef]
- De Mellon, F. The reflex control of rhythmic motor output during swimming in the scallop. Z. Für Vgl. Physiol. 1969, 62, 318–336. [Google Scholar] [CrossRef]
- Mckenzie, J.D.; Caunce, M.; Hetherington, M.S.; Winlow, W. Serotonergic innervation of the foot of the pond snail Lymnaea stagnalis (L.). J. Neurocytol. 1998, 27, 459–470. [Google Scholar] [CrossRef]
- Gillette, R. Evolution and function in serotonergic systems. Integr. Comp. Biol. 2006, 46, 838–846. [Google Scholar] [CrossRef] [Green Version]
- Ranganathan, R.; Cannon, S.C.; Horvitz, H.R. MOD-1 is a serotonin-gated chloride channel that modulates locomotory behaviour in C. elegans. Nature 2000, 408, 470–475. [Google Scholar] [CrossRef]
- Crisp, K.M.; Mesce, K.A. To swim or not to swim: Regional effects of serotonin, octopamine and amine mixtures in the medicinal leech. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 2003, 189, 461–470. [Google Scholar] [CrossRef]
- Mesquita, S.R.; Guilhermino, L.; Guimarães, L. Biochemical and locomotor responses of Carcinus maenas exposed to the serotonin reuptake inhibitor fluoxetine. Chemosphere 2011, 85, 967–976. [Google Scholar] [CrossRef]
- Majeed, Z.R.; Abdeljaber, E.; Soveland, R.; Cornwell, K.; Bankemper, A.; Koch, F.; Cooper, R.L. Modulatory Action by the Serotonergic System: Behavior and Neurophysiology in Drosophila melanogaster. Neural Plast. 2016, 2016, 7291438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vitellaro-Zuccarello, L.; De Biasi, S.; Bernardi, P.; Oggioní, A. Distribution of serotonin-, gamma-aminobutyric acid- and substance P-like immunoreactivity in the central and peripheral nervous system of Mytilus galloprovincialis. Tissue Cell 1991, 23, 261–270. [Google Scholar] [CrossRef]
- Lewis, S.L.; Lyons, D.E.; Meekins, T.L.; Newcomb, J.M. Serotonin influences locomotion in the nudibranch mollusc Melibe leonina. Biol. Bull. 2011, 220, 155–160. [Google Scholar] [CrossRef] [Green Version]
- Tanabe, T.; Yuan, Y.; Nakamura, S.; Itoh, N.; Takahashi, K.G.; Osada, M. The role in spawning of a putative serotonin receptor isolated from the germ and ciliary cells of the gonoduct in the gonad of the Japanese scallop, Patinopecten yessoensis. Gen. Comp. Endocrinol. 2010, 166, 620–627. [Google Scholar] [CrossRef]
- Dyakonova, V.; Hernádi, L.; Ito, E.; Dyakonova, T.; Zakharov, I.; Sakharov, D. The activity of isolated snail neurons controlling locomotion is affected by glucose. Biophysics 2015, 11, 55–60. [Google Scholar] [CrossRef] [Green Version]
- Jia, Y.; Yang, B.; Dong, W.; Liu, Z.; Lv, Z.; Jia, Z.; Qiu, L.; Wang, L.; Song, L. A serotonin receptor (Cg5-HTR-1) mediating immune response in oyster Crassostrea gigas. Dev. Comp. Immunol. 2018, 82, 83–93. [Google Scholar] [CrossRef]
- Bacqué-cazenave, J.; Bharatiya, R.; Barrière, G.; Delbecque, J.P.; Bouguiyoud, N.; Di Giovanni, G.; Cattaert, D.; De Deurwaerdère, P. Serotonin in animal cognition and behavior. Int. J. Mol. Sci. 2020, 21, 1649. [Google Scholar] [CrossRef] [Green Version]
- Zagoraiou, L.; Akay, T.; Martin, J.F.; Brownstone, R.M.; Jessell, T.M.; Miles, G.B. A Cluster of Cholinergic Premotor Interneurons Modulates Mouse Locomotor Activity. Neuron 2009, 64, 645–662. [Google Scholar] [CrossRef] [Green Version]
- Messenger, J.B. Neurotransmitters of cephalopods. Invertebr. Neurosci. 1996, 2, 95–114. [Google Scholar] [CrossRef]
- Gerschenfeld, H.M. Chemical transmission in invertebrate central nervous systems and neuromuscular junctions. Physiol. Rev. 1973, 53, 1–119. [Google Scholar] [CrossRef]
- D’Este, L.; Kimura, S.; Casini, A.; Matsuo, A.; Bellier, J.P.; Kimura, H.; Renda, T.G. First visualization of cholinergic cells and fibers by immunohistochemistry for choline acetyltransferase of the common type in the optic lobe and peduncle complex of Octopus vulgaris. J. Comp. Neurol. 2008, 509, 566–579. [Google Scholar] [CrossRef]
- Deiana, S.; Platt, B.; Riedel, G. The cholinergic system and spatial learning. Behav. Brain Res. 2011, 221, 389–411. [Google Scholar] [CrossRef]
- Bellier, J.P.; Casini, A.; Sakaue, Y.; Kimura, S.; Kimura, H.; Renda, T.G.; D’Este, L. Chemical neuroanatomy of the cholinergic neurons in the cephalopod octopus and the gastropod Limax. In Mollusks: Morphology, Behavior and Ecology; Nova Science Publishers: New York, NY, USA, 2012. [Google Scholar]
- Shi, X.; Zhou, Z.; Wang, L.; Yue, F.; Wang, M.; Yang, C.; Song, L. The immunomodulation of acetylcholinesterase in Zhikong Scallop Chlamys farreri. PLoS ONE 2012, 7, e30828. [Google Scholar] [CrossRef] [Green Version]
- Carew, T.J.; Pinsker, H.; Rubinson, K.; Kandel, E.R. Physiological and biochemical properties of neuromuscular transmission between identified motoneurons and gill muscle in Aplysia. J. Neurophysiol. 1974, 37, 1020–1040. [Google Scholar] [CrossRef]
- Wells, D.W.; Hill, R.B. The possible role of acetylcholine in the rhythmicity of the crop of Aplysia dactylomela (Opisthobranchia: Anaspidea). Comp. Biochem. Physiol. Part C Comp. 1980, 67, 97–106. [Google Scholar] [CrossRef]
- Murray, T.F.; Mpitsos, G.J. Evidence for heterogeneity of muscarinic receptors in the mollusc Pleurobranchaea. Brain Res. Bull. 1988, 21, 181–190. [Google Scholar] [CrossRef]
- Norekian, T.P. Coordination of startle and swimming neural systems in the pteropod mollusk Clione limacina: Role of the cerebral cholinergic interneuron. J. Neurophysiol. 1997, 78, 308–320. [Google Scholar] [CrossRef]
- Norekian, T.P.; Satterlie, R.A. Cholinergic activation of startle motoneurons by a pair of cerebral interneurons in the pteropod mollusk Clione limacina. J. Neurophysiol. 1997, 77, 281–288. [Google Scholar] [CrossRef] [Green Version]
- Newcomb, J.M.; Watson, W.H. Modulation of swimming in the gastropod Melibe leonina by nitric oxide. J. Exp. Biol. 2002, 205, 397–403. [Google Scholar] [CrossRef]
- Scheinker, V.; Fiore, G.; Di Cristo, C.; Di Cosmo, A.; D’Ischia, M.; Enikolopov, G.; Palumbo, A. Nitric oxide synthase in the nervous system and ink gland of the cuttlefish Sepia officinalis: Molecular cloning and expression. Biochem. Biophys. Res. Commun. 2005, 338, 1204–1215. [Google Scholar] [CrossRef]
- Korneev, S.A.; Straub, V.; Kemenes, I.; Korneeva, E.I.; Ott, S.R.; Benjamin, P.R.; O’Shea, M. Timed and targeted differential regulation of nitric oxide synthase (NOS) and anti-NOS genes by reward conditioning leading to long-term memory formation. J. Neurosci. 2005, 25, 1188–1192. [Google Scholar] [CrossRef] [Green Version]
- Matsuo, R.; Ito, E. A novel nitric oxide synthase expressed specifically in the olfactory center. Biochem. Biophys. Res. Commun. 2009, 386, 724–728. [Google Scholar] [CrossRef]
- Cioni, C.; Di Patti, M.C.B.; Venturini, G.; Modica, M.V.; Scarpa, F.; Oliverio, M.; Toni, M. Cellular, biochemical, and molecular characterization of nitric oxide synthase expressed in the nervous system of the prosobranch Stramonita haemastoma (Gastropoda, Neogastropoda). J. Comp. Neurol. 2012, 520, 364–383. [Google Scholar] [CrossRef]
- Jiang, Q.; Zhou, Z.; Wang, L.; Wang, L.; Yue, F.; Wang, J.; Song, L. A Scallop Nitric Oxide Synthase (NOS) with Structure Similar to Neuronal NOS and Its Involvement in the Immune Defense. PLoS ONE 2013, 8, e69158. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Q.; Liu, Z.; Zhou, Z.; Wang, L.; Wang, L.; Yue, F.; Wang, J.; Wang, H.; Song, L. Transcriptional activation and translocation of ancient NOS during immune response. FASEB J. 2016, 30, 3527–3540. [Google Scholar] [CrossRef] [Green Version]
- Conte, A.; Ottaviani, E. Nitric oxide synthase activity in molluscan hemocytes. FEBS Lett. 1995, 365, 120–124. [Google Scholar] [CrossRef] [Green Version]
- Jacklet, J.W. Nitric oxide signaling in invertebrates. Invertebr. Neurosci. 1997, 3, 1–14. [Google Scholar] [CrossRef]
- Moroz, L.L.; Chen, D.; Gillette, M.U.; Gillette, R. Nitric oxide synthase activity in the molluscan CNS. J. Neurochem. 1996, 66, 873–876. [Google Scholar] [CrossRef] [Green Version]
- González, P.M.; Abele, D.; Puntarulo, S. Iron and radical content in Mya arenaria. Possible sources of NO generation. Aquat. Toxicol. 2008, 89, 122–128. [Google Scholar] [CrossRef] [Green Version]
- Giovine, M.; Pozzolini, M.; Favre, A.; Bavestrello, G.; Cerrano, C.; Ottaviani, F.; Chiarantini, L.; Cerasi, A.; Cangiotti, M.; Zocchi, E.; et al. Heat stress-activated, calcium-dependent nitric oxide synthase in sponges. Nitric Oxide Biol. Chem. 2001, 5, 427–431. [Google Scholar] [CrossRef]
- Mantione, K.J.; Cadet, P.; Casares, F.; Zhu, W.; Stefano, G.B. Seasonal variations in mu opiate receptor signaling in the nervous system of the blue mussel, Mytilus edulis: Temperature controls physiological processes. Invertebr. Surviv. J. 2010, 7, 141–145. [Google Scholar]
- Vaschenko, M.A.; Kotsyuba, E.P. NADPH-diaphorase activity in the central nervous system of the Gray mussel Crenomytilus grayanus (Dunker) under stress conditions: A histochemical study. Mar. Environ. Res. 2008, 66, 249–258. [Google Scholar] [CrossRef] [Green Version]
- Walker, R.J.; Papaioannou, S.; Holden-Dye, L. A review of FMRFamide- and RFamide-like peptides in metazoa. Invertebr. Neurosci. 2009, 9, 111–153. [Google Scholar] [CrossRef]
- Krajniak, K.G. Invertebrate FMRFamide Related Peptides. Protein Pept. Lett. 2013, 20, 647–670. [Google Scholar] [CrossRef]
- Zatylny-Gaudin, C.; Favrel, P. Diversity of the RFamide peptide family in mollusks. Front. Endocrinol. 2014, 5, 178. [Google Scholar] [CrossRef]
- Boer, H.H.; Schot, L.P.C.; Veenstra, J.A.; Reichelt, D. Immunocytochemical identification of neural elements in the central nervous systems of a snail, some insects, a fish, and a mammal with an antiserum to the molluscan cardio-excitatory tetrapeptide FMRF-amide. Cell Tissue Res. 1980, 213, 21–27. [Google Scholar] [CrossRef]
- Schot, L.P.C.; Boer, H.H. Immunocytochemical demonstration of peptidergic cells in the pond snail Lymnaea stagnalis with an antiserum to the molluscan cardioactive tetrapeptide FMRF-amide. Cell Tissue Res. 1982, 225, 347–354. [Google Scholar] [CrossRef]
- Buchberger, A.R.; Sauer, C.S.; Vu, N.Q.; DeLaney, K.; Li, L. Temporal study of the perturbation of crustacean neuropeptides due to severe hypoxia using 4-Plex reductive dimethylation. J. Proteome Res. 2020, 19, 1548–1555. [Google Scholar] [CrossRef]
- Long, W.C.; Brylawski, B.J.; Seitz, R.D. Behavioral effects of low dissolved oxygen on the bivalve Macoma balthica. J. Exp. Mar. Biol. Ecol. 2008, 359, 34–39. [Google Scholar] [CrossRef]
- Band, E. The visceral ganglion of Pecten, with some notes on the physiology of the nervous system, and an inquiry into the innervation of the osphradium in Lamellibranchia. Mitth. Aus Der Zool. Stn. Zu Neapel: Zugleich Ein Repert. Für Mittelmeerkunde 1910, 20, 1–40. [Google Scholar]
- Matsutani, T.; Nomura, T. Localization of monoamines in the central nervous system and gonad of the scallop Patinopecten yessoensis. Nippon Suisan Gakkaishi Ni 1984, 50, 425–430. [Google Scholar] [CrossRef]
- Paulet, Y.M.; Donval, A.; Bekhadra, F. Monoamines and reproduction in Pecten maximus, a preliminary approach. Invertebr. Reprod. Dev. 1993, 23, 89–94. [Google Scholar] [CrossRef]
- Matsutani, T.; Nomura, T. Serotonin-like immunoreactivity in the central nervous system and gonad of the scallop, Patinopecten yessoensis. Cell Tissue Res. 1986, 244, 515–517. [Google Scholar] [CrossRef]
- Bullock, T.H.; Horridge, G.A. Structure and Function in the Nervous Systems of Invertebrates; W.H. Freeman: San Francisco, CA, USA, 1965. [Google Scholar]
- Vitellaro-Zuccarello, L.; De Biasi, S.; Bairati, A. Subcellular localization of serotonin-immunoreactivity in the pedal ganglion of Mytilus galloprovincialis (Mollusca, Bivalvia). J. Submicrosc. Cytol. Pathol. 1988, 20, 109–113. [Google Scholar]
- Pani, A.K.; Croll, R.P. Distribution of catecholamines, indoleamines, and their precursors and metabolites in the scallop, Placopecten magellanicus (Bivalvia, Pectinidae). Cell. Mol. Neurobiol. 1995, 15, 371–386. [Google Scholar] [CrossRef]
- Smith, S.A.; Nason, J.; Croll, R.P. Distribution of catecholamines in the sea scallop, Placopecten magellanicus. Can. J. Zool. 1998, 76, 1254–1262. [Google Scholar] [CrossRef]
- Alavi, S.M.H.; Nagasawa, K.; Takahashi, K.G.; Osada, M. Structure-Function of Serotonin in Bivalve Molluscs. In Serotonin—A Chemical Messenger Between All Types of Living Cells; InTech: London, UK, 2017. [Google Scholar]
- Stephens, P.J.; Boyle, P.R. Escape responses of the queen scallop Chlamys opercularis (L.) (Mollusca: Bivalvia). Mar. Behav. Physiol. 1978, 5, 103–113. [Google Scholar] [CrossRef]
- Wilkens, L.A. Neurobiology of the scallop. I. Starfish-mediated escape behaviors. Proc. R. Soc. Lond. Biol. Sci. 1981, 211, 341–372. [Google Scholar] [CrossRef]
- Ciocco, N.F. Anatomia de la “vieira tehuelche”, Aequipecten tehuelchus (d’Orbigny, 1846) (=Chlamys tehuelcha). IV. Sistema nervioso y estructuras sensoriales (Bivalvia, Pectinidae). Rev. De Biol. Mar. Y Oceanogr. 1998, 33, 25–42. [Google Scholar]
- Aiello, E.; Guideri, G. Nervous Control of Ciliary Activity. Science 1964, 146, 1692–1693. [Google Scholar] [CrossRef]
- Stephens, R.E.; Prior, G. Dynein from serotonin-activated cilia and flagella: Extraction characteristics and distinct sites for cAMP-dependent protein phosphorylation. J. Cell Sci. 1992, 103, 999–1012. [Google Scholar] [CrossRef]
- Gainey, L.F.; Greenberg, M.J. Nitric oxide mediates seasonal muscle potentiation in clam gills. J. Exp. Biol. 2003, 206, 3507–3520. [Google Scholar] [CrossRef] [Green Version]
- Bayne, B.L. Ventilation, the heart beat and oxygen uptake by Mytilus edulis L. in declining oxygen tension. Comp. Biochem. Physiol. Part A Physiol. 1971, 40, 1065–1085. [Google Scholar] [CrossRef]
- Aiello, E.; Guideri, G. Distribution and function of the branchial nerve in the mussel. Biol. Bull. 1965, 129, 431–438. [Google Scholar] [CrossRef] [PubMed]
- González, P.M.; Rocchetta, I.; Abele, D.; Rivera-Ingraham, G.A. Hypoxically induced nitric oxide: Potential Role as a Vasodilator in Mytilus edulis Gills. Front. Physiol. 2019, 10, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Kuang, S.; Doran, S.A.; Wilson, R.J.A.; Goss, G.G.; Goldberg, J.I. Serotonergic sensory-motor neurons mediate a behavioral response to hypoxia in pond snail embryos. J. Neurobiol. 2002, 52, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, J.I.; Rich, D.R.; Muruganathan, S.P.; Liu, M.B.; Pon, J.R.; Tam, R.; Diefenbach, T.J.; Kuang, S. Identification and evolutionary implications of neurotransmitter-ciliary interactions underlying the behavioral response to hypoxia in Lymnaea stagnalis embryos. J. Exp. Biol. 2011, 214, 2660–2670. [Google Scholar] [CrossRef] [Green Version]
- Berlind, A. Neurohumoral and reflex control of scaphognathite beating in the crab Carcinus maenas. J. Comp. Physiol. A 1977, 116, 77–90. [Google Scholar] [CrossRef]
- Fu, X.W.; Nurse, C.A.; Wong, V.; Cutz, E. Hypoxia-induced secretion of serotonin from intact pulmonary neuroepithelial bodies in neonatal rabbit. J. Physiol. 2002, 539, 503–510. [Google Scholar] [CrossRef]
- Nurse, C.A. Neurotransmitter and neuromodulatory mechanisms at peripheral arterial chemoreceptors. Exp. Physiol. 2010, 95, 657–667. [Google Scholar] [CrossRef]
- Dong, W.; Liu, Z.; Qiu, L.; Wang, W.; Song, X.; Wang, X.; Li, Y.; Xin, L.; Wang, L.; Song, L. The modulation role of serotonin in Pacific oyster Crassostrea gigas in response to air exposure. Fish Shellfish. Immunol. 2017, 62, 341–348. [Google Scholar] [CrossRef] [Green Version]
- Beninger, P.G.; Donval, A.; Le Pennec, M. The osphradium in Placopecten magellanicus and Pecten maximus (Bivalvia, Pectinidae): Histology, ultrastructure, and implications for spawning synchronisation. Mar. Biol. 1995, 123, 121–129. [Google Scholar] [CrossRef]
- Siniscalchi, A.; Cavallini, S.; Sonetti, D.; Sbrenna, G.; Capuano, S.; Barbin, L.; Turolla, E.; Rossi, R. Serotonergic neurotransmission in the bivalve Venus verrucosa (Veneridae): A neurochemical and immunohistochemical study of the visceral ganglion and gonads. Mar. Biol. 2004, 144, 1205–1212. [Google Scholar] [CrossRef]
- Matsutani, T.; Nomura, T. Induction of Spawning by Serotonin in the Scallop Patinopecten-Yessoensis. Mar. Biol. Lett. 1982, 3, 353–358. [Google Scholar]
- Gibbons, M.C.; Goodsell, J.G.; Castagna, M.; Lutz, R.A. Chemical induction of spawning by serotonin in the ocean quahog Arctica islandica (Linne). J. Shellfish. Res. 1983, 3, 203–205. [Google Scholar]
- Gibbons, M.C.; Castagna, M. Serotonin as an inducer of spawning in six bivalve species. Aquaculture 1984, 40, 189–191. [Google Scholar] [CrossRef]
- Stephens, P.J. The Sensitivity and Control of the Scallop Mantle Edge. J. Exp. Biol. 1978, 75, 203–221. [Google Scholar] [CrossRef]
- The eyes of pecten, spondylus, amussium and allied lamellibranchs, with a short discussion on their evolution. Proc. R. Soc. London Ser. B Contain. Pap. A Biol. Character 1928, 103, 355–365. [CrossRef] [Green Version]
- Wilkens, L.A.; Ache, B.W. Visual responses in the central nervous system of the scallop Pecten ziczac. Experientia 1977, 33, 1338–1340. [Google Scholar] [CrossRef]
- Spagnolia, T.; Wilkens, L.A. Neurobiology of the scallop. II. structure of the parietovisceral ganglion lateral lobes in relation to afferent projections from the mantle eyes. Mar. Behav. Physiol. 1983, 10, 23–55. [Google Scholar] [CrossRef]
- Nezlin, L.; Moroz, L.; Elofsson, R.; Sakharov, D. Immunolabeled neuroactive substances in the osphradium of the pond snail Lymnaea stagnalis. Cell Tissue Res. 1994, 275, 269–275. [Google Scholar] [CrossRef]
- Beley, A.; Bertrand, N.; Beley, P. Cerebral ischemia: Changes in brain choline, acetylcholine, and other monoamines as related to energy metabolism. Neurochem. Res. 1991, 16, 555–561. [Google Scholar] [CrossRef]
- Jope, R.S.; Jenden, D.J. Choline and phospholipid metabolism and the synthesis of acetylcholine in rat brain. J. Neurosci. Res. 1979, 4, 69–82. [Google Scholar] [CrossRef]
- Blusztajn, J.K.; Liscovitch, M.; Richardson, U.I. Synthesis of acetylcholine from choline derived from phosphatidylcholine in a human neuronal cell line. Proc. Natl. Acad. Sci. USA 1987, 84, 5474–5477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trommer, B.A.; Schmidt, D.E.; Wecker, L. Exogenous Choline Enhances the Synthesis of Acetylcholine Only Under Conditions of Increased Cholinergic Neuronal Activity. J. Neurochem. 1982, 39, 1704–1709. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Wang, R.H.; Wang, H.; Long, C.L.; Wang, H. Brain protection against ischemic stroke using choline as a new molecular bypass treatment. Acta Pharmacol. Sin. 2015, 36, 1416–1425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dyuizen, I.V.; Annikova, L.V.; Motavkin, P.A. NO-synthase localization in the central nervous system of the bivalve mollusks Mizuhopecten yessoensis and Modiolus kurilensis. Russ. J. Mar. Biol. 1999, 25, 277–279. [Google Scholar]
- Larade, K.; Storey, K.B. Chapter 3 A profile of the metabolic responses to anoxia in marine invertebrates. In Cell and Molecular Response to Stress; Elsevier: Amsterdam, The Netherlands, 2002; Volume 3, pp. 27–46. [Google Scholar]
- Gorr, T.A.; Gassmann, M.; Wappner, P. Sensing and responding to hypoxia via HIF in model invertebrates. J. Insect Physiol. 2006, 52, 349–364. [Google Scholar] [CrossRef]
- Hochachka, P.W. Defense strategies against hypoxia and hypothermia. Science 1986, 231, 234–241. [Google Scholar] [CrossRef]
- Ballantyne, J.S. Mitochondria: Aerobic and anaerobic design—Lessons from molluscs and fishes. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2004, 139, 461–467. [Google Scholar] [CrossRef]
- David, E.; Tanguy, A.; Pichavant, K.; Moraga, D. Response of the Pacific oyster Crassostrea gigas to hypoxia exposure under experimental conditions. FEBS J. 2005, 272, 310–317. [Google Scholar] [CrossRef]
- Turrens, J.F. Mitochondrial formation of reactive oxygen species. J. Physiol. 2003, 552, 335–344. [Google Scholar] [CrossRef]
- Strahl, J.; Abele, D. Nitric oxide mediates metabolic functions in the bivalve Arctica islandica under hypoxia. PLoS ONE 2020, 15, e0232360. [Google Scholar] [CrossRef]
- Letendre, J.; Leboulenger, F.; Durand, F. Oxidative Challenge and Redox Sensing in Mollusks: Effects of Natural and Anthropic Stressors. Oxidative Stress Vertebr. Invertebr. Mol. Asp. Cell Signal. 2012, 26, 361–376. [Google Scholar]
- Bennet-Clark, H.C. Marine Mussels: Their Ecology and Physiology. International Biological Programme 10 Edited by B. L. Bayne, Cambridge University Press, 1976. Pp. xvii+506. £22.00. Q. J. Exp. Physiol. Cogn. Med. Sci. 1976, 61, 359. [Google Scholar] [CrossRef] [Green Version]
- Livingstone, D.R. Origins and evolution of pathways of anaerobic metabolism in the animal kingdom. Integr. Comp. Biol. 1991, 31, 522–534. [Google Scholar] [CrossRef] [Green Version]
- Artigaud, S.; Thorne, M.A.S.; Richard, J.; Lavaud, R.; Jean, F.; Flye-Sainte-Marie, J.; Peck, L.S.; Pichereau, V.; Clark, M.S. Deep sequencing of the mantle transcriptome of the great scallop Pecten maximus. Mar. Genom. 2014, 15, 3–4. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Widdows, J. Metabolic responses of the common mussel Mytilus edulis to hypoxia and anoxia. Mar. Ecol. Prog. Ser. 1993, 95, 205–214. [Google Scholar] [CrossRef]
- Pörtner, H.O.; Lannig, G. Chapter 4 Oxygen and Capacity Limited Thermal Tolerance. In Fish Physiology; Academic Press: Cambridge, MA, USA, 2009; Volume 27, pp. 143–191. [Google Scholar]
- Li, Q.; Zhang, F.; Wang, M.; Li, M.; Sun, S. Effects of hypoxia on survival, behavior, and metabolism of Zhikong scallop Chlamys farreri Jones et Preston 1904. J. Oceanol. Limnol. 2020, 38, 351–363. [Google Scholar] [CrossRef]
- Too, C.K.L.; Croll, R.P. Detection of FMRFamide-like immunoreactivities in the sea scallop Placopecten magellanicus by immunohistochemistry and Western blot analysis. Cell Tissue Res. 1995, 281, 295–304. [Google Scholar] [CrossRef]
- Henry, M.; Benlinmame, N.; Belhsen, O.; Jule, Y.; Mathieu, M. Immunohistochemical localization of FMRFamide-containing neurons and nerve fibers in the ganglia and the gonad wall of the scallop, Pecten maximus (L). Neuropeptides 1995, 28, 79–84. [Google Scholar] [CrossRef]
- Price, D.; Greenberg, M. Structure of a molluscan cardioexcitatory neuropeptide. Science 1977, 197, 670–671. [Google Scholar] [CrossRef]
- Kingston, A.C.N.; Chappell, D.R.; Miller, H.V.; Lee, S.J.; Speiser, D.I. Expression of G proteins in the eyes and parietovisceral ganglion of the bay scallop argopecten irradians. Biol. Bull. 2017, 233, 83–95. [Google Scholar] [CrossRef]
- López-Vera, E.; Aguilar, M.B.; Heimer de la Cotera, E.P. FMRFamide and related peptides in the phylum mollusca. Peptides 2008, 29, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Chin, G.J.; Payza, K.; Price, D.A.; Greenberg, M.J.; Doble, K.E. Characterization and Solubilization of the FMRFamide Receptor of Squid. Biol. Bull. 1994, 187, 185–199. [Google Scholar] [CrossRef] [PubMed]
- Di Cosmo, A.; Di Cristo, C. Neuropeptidergic control of the optic gland of Octopus vulgaris: FMRF- amide and GnRH immunoreactivity. J. Comp. Neurol. 1998, 398, 1–12. [Google Scholar] [CrossRef]
- Jacklet, J.W.; Klose, M.; Goldberg, M. FMRF-amide-like immunoreactive efferent fibers and FMRF-amide suppression of pacemaker neurons in eyes of Bulla. J. Neurobiol. 1987, 18, 433–449. [Google Scholar] [CrossRef]
- Painter, S.D.; Greenberg, M.J. A survey of the responses of bivalves hearts to the molluscan neuropeptide FMRFamide and to 5-hydroxytryptamine. Biol. Bull. 1982, 162, 311–332. [Google Scholar] [CrossRef]
- Greenberg, M.J.; Price, D.A. Cardioregulatory peptides in molluscs. Soc. Gen. Physiol. Ser. 1980, 35, 107–126. [Google Scholar]
- Nagle, G.T. The molluscan neuropeptide FMRfamide: Calcium-dependent release and blood levels in Macrocallista (bivalvia). Life Sci. 1982, 30, 803–807. [Google Scholar] [CrossRef]
- Price, D.A.; Greenberg, M.J. Purification and Characterization of A Cardioexcitatory Neuropeptide from the Central Ganglia of a Bivalve Mollusc. Prep. Biochem. 1977, 7, 261–281. [Google Scholar] [CrossRef]
- Deaton, L.E. Potentiation of Hypoosmotic Cellular Volume Regulation in the Quahog, Mercenaria mercenaria, by 5-hydroxytryptamine, FMRFamide, and Phorbol Esters. Biol. Bull. 1990, 178, 260–266. [Google Scholar] [CrossRef]
- Gruhl, A. Serotonergic and FMRFamidergic nervous systems in gymnolaemate bryozoan larvae. Zoomorphology 2009, 128, 135–156. [Google Scholar] [CrossRef]
- Gruhl, A. Neuromuscular system of the larva of Fredericella sultana (Bryozoa: Phylactolaemata). Zool. Anz. 2010, 249, 139–149. [Google Scholar] [CrossRef]
- Croll, R.P. Gastropod chemoreception. Biol. Rev. 1983, 58, 293–319. [Google Scholar] [CrossRef]
- Croll, R.P. Sensory Control of Respiratory Pumping in Aplysia Californica. J. Exp. Biol. 1985, 117, 15–27. [Google Scholar] [CrossRef]
- Madrid, K.P.; Price, D.A.; Greenberg, M.J.; Khan, H.R.; Saleuddin, A.S.M. FMRFamide-related peptides from the kidney of the snail, Helisoma trivolvis. Peptides 1994, 15, 31–36. [Google Scholar] [CrossRef]
- Khan, H.R.; Price, D.A.; Doble, K.E.; Greenberg, M.J.; Saleuddin, A.S.M. Osmoregulation and FMRFamide-related peptides in the salt marsh snail Melampus bidentatus (Say) (Mollusca: Pulmonata). Biol. Bull. 1999, 196, 153–162. [Google Scholar] [CrossRef]
- Eertman, R.H.M.; Wagenvoort, A.J.; Hummel, H.; Smaal, A.C. “Survival in air” of the blue mussel Mytilus edulis L. as a sensitive response to pollution-induced environmental stress. J. Exp. Mar. Biol. Ecol. 1993, 170, 179–195. [Google Scholar] [CrossRef]
- Giannetto, A.; Maisano, M.; Cappello, T.; Oliva, S.; Parrino, V.; Natalotto, A.; De Marco, G.; Barberi, C.; Romeo, O.; Mauceri, A.; et al. Hypoxia-Inducible Factor α and Hif-prolyl Hydroxylase Characterization and Gene Expression in Short-Time Air-Exposed Mytilus galloprovincialis. Mar. Biotechnol. 2015, 17, 768–781. [Google Scholar] [CrossRef]
- Kawabe, S.; Yokoyama, Y. Role of hypoxia-inducible factor α in response to hypoxia and heat shock in the Pacific oyster Crassostrea gigas. Mar. Biotechnol. 2012, 14, 106–119. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Dyachuk, V.; Odintsova, N. Development of the larval muscle system in the mussel Mytilus trossulus (Mollusca, Bivalvia). Dev. Growth Differ. 2009, 51, 69–79. [Google Scholar] [CrossRef]
- Dyachuk, V.A.; Maiorova, M.A.; Odintsova, N.A. Identification of β integrin-like- and fibronectin-like proteins in the bivalve mollusk Mytilus trossulus. Dev. Growth Differ. 2015, 57, 515–528. [Google Scholar] [CrossRef] [PubMed]
- Yurchenko, O.V.; Savelieva, A.V.; Kolotuchina, N.K.; Dyachuk, V.A. Peripheral sensory neurons govern development of the nervous system in bivalve larvae. EvoDevo 2019, 10, 22. [Google Scholar] [CrossRef] [PubMed]
- Kotsyuba, E.; Kalachev, A.; Kameneva, P.; Dyachuk, V. Distribution of Molecules Related to Neurotransmission in the Nervous System of the Mussel Crenomytilus grayanus. Front. Neuroanat. 2020, 14, 35. [Google Scholar] [CrossRef] [PubMed]
- Wanninger, A. Evolutionary Developmental Biology of Invertebrates 2; Wanninger, A., Ed.; Springer: Vienna, Austria, 2015. [Google Scholar] [CrossRef]
- Villamil, L.; Gómez-León, J.; Gómez-Chiarri, M. Role of nitric oxide in the defenses of Crassostrea virginica to experimental infection with the protozoan parasite Perkinsus marinus. Dev. Comp. Immunol. 2007, 31, 968–977. [Google Scholar] [CrossRef] [PubMed]
- Pechenik, J.A.; Cochrane, D.E.; Li, W.; West, E.T.; Pires, A.; Leppo, M. Nitric oxide inhibits metamorphosis in larvae of Crepidula fornicata, the slippershell snail. Biol. Bull. 2007, 213, 160–171. [Google Scholar] [CrossRef] [Green Version]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [Green Version]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Otsu, N. A threshold selection method from gray-level histograms. IEEE Trans. Syst. Man Cybern. 1979, 9, 62–66. [Google Scholar] [CrossRef] [Green Version]










Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kotsyuba, E.; Dyachuk, V. Effect of Air Exposure-Induced Hypoxia on Neurotransmitters and Neurotransmission Enzymes in Ganglia of the Scallop Azumapecten farreri. Int. J. Mol. Sci. 2022, 23, 2027. https://doi.org/10.3390/ijms23042027
Kotsyuba E, Dyachuk V. Effect of Air Exposure-Induced Hypoxia on Neurotransmitters and Neurotransmission Enzymes in Ganglia of the Scallop Azumapecten farreri. International Journal of Molecular Sciences. 2022; 23(4):2027. https://doi.org/10.3390/ijms23042027
Chicago/Turabian StyleKotsyuba, Elena, and Vyacheslav Dyachuk. 2022. "Effect of Air Exposure-Induced Hypoxia on Neurotransmitters and Neurotransmission Enzymes in Ganglia of the Scallop Azumapecten farreri" International Journal of Molecular Sciences 23, no. 4: 2027. https://doi.org/10.3390/ijms23042027
APA StyleKotsyuba, E., & Dyachuk, V. (2022). Effect of Air Exposure-Induced Hypoxia on Neurotransmitters and Neurotransmission Enzymes in Ganglia of the Scallop Azumapecten farreri. International Journal of Molecular Sciences, 23(4), 2027. https://doi.org/10.3390/ijms23042027