The Effects of Antioxidants from Natural Products on Obesity, Dyslipidemia, Diabetes and Their Molecular Signaling Mechanism
Abstract
:1. Introduction
2. Method
3. Oxidative Stress and Its Relation to Metabolic Disorders (Obesity, Dyslipidemia and Diabetes)
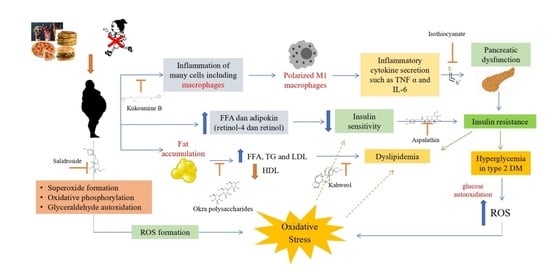
3.1. Obesity
3.2. Dyslipidemia
3.3. Diabetes Mellitus
4. Antioxidant Activities from Natural Products to Treat Obesity, Dyslipidemia and Diabetes Mellitus
4.1. Resveratrol
4.2. Curcumin
4.3. Quercetin
4.4. Anthocyanin
4.5. Other Antioxidants
5. Effect of Antioxidants on Metabolic Disorders of Obesity, Dyslipidemia and Diabetes
5.1. Relationship between Obesity, Dyslipidemia and Diabetes
5.2. Antioxidant Mechanisms Associated with Obesity, Dyslipidemia and Diabetes
6. Antioxidant Compound Signaling Pathways
6.1. The Phosphoinositide 3-kinase/Protein Kinase B (PI3K/AKT)
6.2. The Nuclear Factor Erythroid 2-Related Factor 2 (Nrf2) Signaling
6.3. The Peroxisome Proliferation Activated Receptor γ (PPARγ)
6.4. The Nuclear Factor Kappa-Light-Chain-Enhancer of Activated B Cells (NF-κB) Signaling
6.5. 5′AMP-Activated Protein Kinase (AMPK) Signaling
6.6. AGE/RAGE
6.7. SIRT (Sirtuin)
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Leibowitz, K.L.; Moore, R.H.; Ahima, R.S.; Stunkard, A.J.; Stallings, V.A.; Berkowitz, R.I.; Chittams, J.L.; Faith, M.S.; Stettler, N. Maternal obesity associated with inflammation in their children. World J. Pediatr. 2012, 8, 76–79. [Google Scholar] [CrossRef] [PubMed]
- Manna, P.; Jain, S.K. Obesity, Oxidative Stress, Adipose Tissue Dysfunction, and the Associated Health Risks: Causes and Therapeutic Strategies. Metab. Syndr. Relat. Disord. 2015, 13, 423–444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oyenihi, A.B.; Ayeleso, A.O.; Mukwevho, E.; Masola, B. Antioxidant strategies in the management of diabetic neuropathy. Biomed Res. Int. 2015, 2015, 515042. [Google Scholar] [CrossRef]
- Samavat, H.; Newman, A.R.; Wang, R.; Yuan, J.M.; Wu, A.H.; Kurzer, M.S. Effects of green tea catechin extract on serum lipids in postmenopausal women: A randomized, placebo-controlled clinical trial. Am. J. Clin. Nutr. 2016, 104, 1671–1682. [Google Scholar] [CrossRef] [PubMed]
- Vekic, J.; Zeljkovic, A.; Stefanovic, A.; Jelic-Ivanovic, Z.; Spasojevic-Kalimanovska, V. Obesity and dyslipidemia. Metabolism 2019, 92, 71–81. [Google Scholar] [CrossRef]
- Atlas, D. International Diabetes Federation, 9th ed.; International Diabetes Federation: Brussels, Belgium, 2019. [Google Scholar]
- Mohieldein, A.H.; Hasan, M.; Al-Harbi, K.K.; Alodailah, S.S.; Azahrani, R.M.; Al-Mushawwah, S.A. Dyslipidemia and reduced total antioxidant status in young adult Saudis with prediabetes. Diabetes Metab. Syndr. Clin. Res. Rev. 2015, 9, 287–291. [Google Scholar] [CrossRef]
- Rambhade, S.; Chakraborty, A.K.; Patil, U.K.; Rambhade, A. Diabetes Mellitus-Its complications, factors influencing complications and prevention-An Overview. J. Chem. Pharm. Res. 2010, 2, 7–25. [Google Scholar]
- Kashiyama, K.; Sonoda, S.; Otsuji, Y. Reconsideration of Secondary Risk Management Strategies in Patients with Ischemic Heart Disease. J. UOEH 2017, 39, 11–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vona, R.; Gambardella, L.; Cittadini, C.; Straface, E.; Pietraforte, D. Biomarkers of oxidative stress in metabolic syndrome and associated diseases. Oxid. Med. Cell. Longev. 2019, 2019. [Google Scholar] [CrossRef] [Green Version]
- Pechánová, O.; Varga, Z.V.; Cebová, M.; Giricz, Z.; Pacher, P.; Ferdinandy, P. Cardiac NO signalling in the metabolic syndrome. Br. J. Pharmacol. 2015, 172, 1415–1433. [Google Scholar] [CrossRef] [Green Version]
- Kuk, J.L.; Rotondi, M.; Sui, X.; Blair, S.N.; Ardern, C.I. Individuals with obesity but no other metabolic risk factors are not at significantly elevated all-cause mortality risk in men and women. Clin. Obes. 2018, 8, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Prawitasari, D.S. Diabetes Melitus dan Antioksidan. Keluwih J. Kesehat. Kedokt. 2019, 1, 48–52. [Google Scholar] [CrossRef] [Green Version]
- El-Huneidi, W.; Anjum, S.; Bajbouj, K.; Abu-Gharbieh, E.; Taneera, J. The coffee diterpene, kahweol, ameliorates pancreatic β-cell function in streptozotocin (Stz)-treated rat ins-1 cells through nf-kb and p-akt/bcl-2 pathways. Molecules 2021, 26, 5167. [Google Scholar] [CrossRef] [PubMed]
- Pan, B.; Ge, L.; Xun, Y.Q.; Chen, Y.J.; Gao, C.Y.; Han, X.; Zuo, L.Q.; Shan, H.Q.; Yang, K.H.; Ding, G.W.; et al. Exercise training modalities in patients with type 2 diabetes mellitus: A systematic review and network meta-analysis. Int. J. Behav. Nutr. Phys. Act. 2018, 15, 1–14. [Google Scholar] [CrossRef]
- Yang, D.K.; Kang, H.S. Anti-diabetic effect of cotreatment with quercetin and resveratrol in streptozotocin-induced diabetic rats. Biomol. Ther. 2018, 26, 130–138. [Google Scholar] [CrossRef] [Green Version]
- Dal, S.; Sigrist, S. The Protective Effect of Antioxidants Consumption on Diabetes and Vascular Complications. Diseases 2016, 4, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shabbir, U.; Rubab, M.; Daliri, E.B.; Chelliah, R.; Javed, A.; Oh, D. Curcumin, Quercetin, Catechins and Metabolic Diseases : The Role of Gut Microbiota. Nutrients 2021, 13, 206. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017, 2017. [Google Scholar] [CrossRef]
- Paravicini, T.M.; Touyz, R.M. NADPH oxidases, reactive oxygen species, and hypertension: Clinical implications and therapeutic possibilities. Diabetes Care 2008, 31 (Suppl. 2), S170–S180. [Google Scholar] [CrossRef] [Green Version]
- Corbi, S.C.T.; Bastos, A.S.; Orrico, S.R.P.; Secolin, R.; Dos Santos, R.A.; Takahashi, C.S.; Scarel-Caminaga, R.M. Elevated micronucleus frequency in patients with type 2 diabetes, dyslipidemia and periodontitis. Mutagenesis 2014, 29, 433–439. [Google Scholar] [CrossRef] [Green Version]
- Ren, W.; Xia, Y.; Chen, S.; Wu, G.; Bazer, F.W.; Zhou, B.; Tan, B.; Zhu, G.; Deng, J.; Yin, Y. Glutamine Metabolism in Macrophages: A Novel Target for Obesity/Type 2 Diabetes. Adv. Nutr. 2019, 10, 221–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, H.; Ballantyne, C.M. Metabolic Inflammation and Insulin Resistance in Obesity. Circ. Res. 2020, 126, 1549–1564. [Google Scholar] [CrossRef]
- Flock, M.R.; Green, M.H.; Kris-Etherton, P.M. Effects of adiposity on plasma lipid response to reductions in dietary saturated fatty acids and cholesterol. Adv. Nutr. 2011, 2, 261–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klop, B.; Elte, J.W.F.; Cabezas, M.C. Dyslipidemia in Obesity: Mechanisms and Potential Targets. Nutrients 2013, 5, 1218–1240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Na, I.J.; Park, J.S.; Park, S.B. Association between abdominal obesity and oxidative stress in Korean adults. Korean J. Fam. Med. 2019, 40, 395–398. [Google Scholar] [CrossRef] [Green Version]
- Luc, K.; Schramm-Luc, A.; Guzik, T.J.; Mikolajczyk, T.P. Oxidative stress and inflammatory markers in prediabetes and diabetes. J. Physiol. Pharmacol. 2019, 70, 809–824. [Google Scholar] [CrossRef]
- Guzik, T.J.; Skiba, D.S.; Touyz, R.M.; Harrison, D.G. The role of infiltrating immune cells in dysfunctional adipose tissue. Cardiovasc. Res. 2017, 113, 1009–1023. [Google Scholar] [CrossRef] [Green Version]
- Niemann, B.; Rohrbach, S.; Miller, M.R.; Newby, D.E.; Fuster, V.; Kovacic, J.C. Oxidative Stress and Cardiovascular Risk: Obesity, Diabetes, Smoking, and Pollution: Part 3 of a 3-Part Series. J. Am. Coll. Cardiol. 2017, 70, 230–251. [Google Scholar] [CrossRef]
- Wang, H.; Peng, D.Q. New insights into the mechanism of low high-density lipoprotein cholesterol in obesity. Lipids Health Dis. 2011, 10, 176. [Google Scholar] [CrossRef] [Green Version]
- Li, K.; Deng, Y.; Deng, G.; Chen, P.; Wang, Y.; Wu, H.; Ji, Z.; Yao, Z.; Zhang, X.; Yu, B.; et al. High cholesterol induces apoptosis and autophagy through the ROS-activated AKT/FOXO1 pathway in tendon-derived stem cells. Stem Cell Res. Ther. 2020, 11, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Pignatelli, P.; Menichelli, D.; Pastori, D.; Violi, F. Oxidative stress and cardiovascular disease: New insights. Kardiol. Pol. 2018, 76, 713–722. [Google Scholar] [CrossRef] [Green Version]
- Czaja, A.J. Autoimmune Hepatitis: Focusing on Treatments other than Steroids. Can. J. Gastroenterol. 2012, 26, 615–620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, R.M.M.; Chua, Z.J.Y.; Tan, J.C.; Yang, Y.; Liao, Z.; Zhao, Y. From pre-diabetes to diab. Medicine 2019, 55, 1–30. [Google Scholar]
- Makki, K.; Froguel, P.; Wolowczuk, I. Adipose Tissue in Obesity-Related Inflammation and Insulin Resistance: Cells, Cytokines, and Chemokines. ISRN Inflamm. 2013, 2013, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, X.; Li, M.; Xiao, M.; Ruan, Q.; Chu, Z.; Ye, Z.; Zhong, L.; Zhang, H.; Huang, X.; Xie, W.; et al. ERβ Accelerates Diabetic Wound Healing by Ameliorating Hyperglycemia-Induced Persistent Oxidative Stress. Front. Endocrinol. 2019, 10, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Sen, S.; Chakraborty, R.; Sridhar, C.; Reddy, Y.S.R.; De, B. Free radicals, antioxidants, diseases and phytomedicines: Current status and future prospect. Int. J. Pharm. Sci. Rev. Res. 2010, 3, 91–100. [Google Scholar]
- Kan, N.W.; Lee, M.C.; Tung, Y.T.; Chiu, C.C.; Huang, C.C.; Huang, W.C. The synergistic effects of resveratrol combined with resistant training on exercise performance and physiological adaption. Nutrients 2018, 10, 1360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Movahed, A.; Raj, P.; Nabipour, I.; Mahmoodi, M.; Ostovar, A. Efficacy and Safety of Resveratrol in Type 1 Diabetes. Nutrients 2020, 12, 161. [Google Scholar] [CrossRef] [Green Version]
- Batista-Jorge, G.C.; Barcala-Jorge, A.S.; Silveira, M.F.; Lelis, D.F.; Andrade, J.M.O.; de Paula, A.M.B.; Guimarães, A.L.S.; Santos, S.H.S. Oral resveratrol supplementation improves Metabolic Syndrome features in obese patients submitted to a lifestyle-changing program. Life Sci. 2020, 256, 117962. [Google Scholar] [CrossRef]
- Rabbani, N.; Xue, M.; Martin, O.W.; Thornalley, P.J. Subjects by trans -Resveratrol and Hesperetin. Nutrients 2021, 13, 2374. [Google Scholar] [CrossRef]
- Zhu, X.; Yang, J.; Zhu, W.; Yin, X.; Yang, B.; Wei, Y.; Guo, X. Combination of berberine with resveratrol improves the lipid-lowering efficacy. Int. J. Mol. Sci. 2018, 19, 3903. [Google Scholar] [CrossRef] [Green Version]
- Campbell, L.; Yu, R.; Li, F.; Zhou, Q.; Chen, D.; Qi, C.; Yin, Y.; Sun, J. Diabetes, Metabolic Syndrome and Obesity: Targets and Therapy Dovepress Modulation of fat metabolism and gut microbiota by resveratrol on high-fat diet-induced obese mice. Diabetes Metab. Syndr. Obes. Targets Ther. 2019, 12, 97–107. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.C.; Lin, K.Y.; Peng, K.Y.; Day, Y.J.; Hung, L.M. Resveratrol exerts anti-obesity effects in high-fat diet obese mice and displays differential dosage effects on cytotoxicity, differentiation, and lipolysis in 3T3-L1 cells. Endocr. J. 2016, 63, 169–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, W.Y.; Choi, J.H. Korean Curcuma longa L. induces lipolysis and regulates leptin in adipocyte cells and rats. Nutr. Res. Pract. 2016, 10, 487–493. [Google Scholar] [CrossRef] [Green Version]
- Ferguson, B.S.; Nam, H.; Morrison, R.F. Curcumin inhibits 3T3-L1 preadipocyte proliferation by mechanisms involving post-transcriptional p27 regulation. Biochem. Biophys. Rep. 2016, 5, 16–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, D.; Pan, Y.; Yu, N.; Bai, Y.; Ma, R.; Mo, F.; Zuo, J.; Chen, B.; Jia, Q.; Zhang, D.; et al. Curcumin improves adipocytes browning and mitochondrial function in 3T3-L1 cells and obese rodent model. R. Soc. Open Sci. 2021, 8, 200974. [Google Scholar] [CrossRef] [PubMed]
- Labban, R.S.M.; Alfawaz, H.A.; Almnaizel, A.T.; Al-Muammar, M.N.; Bhat, R.S.; El-Ansary, A. Garcinia mangostana extract and curcumin ameliorate oxidative stress, dyslipidemia, and hyperglycemia in high fat diet-induced obese Wistar albino rats. Sci. Rep. 2021, 11, 1–11. [Google Scholar] [CrossRef]
- Thota, R.N.; Acharya, S.H.; Garg, M.L. Curcumin and/or omega-3 polyunsaturated fatty acids supplementation reduces insulin resistance and blood lipids in individuals with high risk of type 2 diabetes: A randomised controlled trial. Lipids Health Dis. 2019, 18, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thota, R.N.; Rosato, J.I.; Dias, C.B.; Burrows, T.L.; Martins, R.N.; Garg, M.L. Dietary supplementation with curcumin reduce circulating levels of glycogen synthase kinase-3Β and islet amyloid polypeptide in adults with high risk of type 2 diabetes and Alzheimer’s disease. Nutrients 2020, 12, 1032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shamsi-Goushki, A.; Mortazavi, Z.; Mirshekar, M.A.; Mohammadi, M.; Moradi-Kor, N.; Jafari-Maskouni, S.; Shahraki, M. Comparative effects of curcumin versus nano-curcumin on insulin resistance, serum levels of apelin and lipid profile in type 2 diabetic rats. Diabetes Metab. Syndr. Obes. Targets Ther. 2020, 13, 2337–2346. [Google Scholar] [CrossRef] [PubMed]
- Roxo, D.F.; Arcaro, C.A.; Gutierres, V.O.; Costa, M.C.; Oliveira, J.O.; Lima, T.F.O.; Assis, R.P.; Brunetti, I.L.; Baviera, A.M. Curcumin combined with metformin decreases glycemia and dyslipidemia, and increases paraoxonase activity in diabetic rats. Diabetol. Metab. Syndr. 2019, 11, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Xie, T.; Chen, X.; Chen, W.; Huang, S.; Peng, X.; Tian, L.; Wu, X.; Huang, Y. Curcumin is a Potential Adjuvant to Alleviates Diabetic Retinal Injury via Reducing Oxidative Stress and Maintaining Nrf2 Pathway Homeostasis. Front. Pharmacol. 2021, 12, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Zhai, M.; Jiang, L.; Song, F.; Zhang, B.; Li, J.; Li, H.; Li, B.; Xia, L.; Xu, L.; et al. Tetrahydrocurcumin ameliorates diabetic cardiomyopathy by attenuating high glucose-induced oxidative stress and fibrosis via activating the SIRT1 pathway. Oxid. Med. Cell. Longev. 2019, 2019, 6746907. [Google Scholar] [CrossRef] [Green Version]
- Lima, T.F.O.; Costa, M.C.; Figueiredo, I.D.; Inácio, M.D.; Rodrigues, M.R.; Assis, R.P.; Baviera, A.M.; Brunetti, I.L. Curcumin, Alone or in Combination with Aminoguanidine, Increases Antioxidant Defenses and Glycation Product Detoxification in Streptozotocin-Diabetic Rats: A Therapeutic Strategy to Mitigate Glycoxidative Stress. Oxid. Med. Cell. Longev. 2020, 2020, 1036360. [Google Scholar] [CrossRef]
- Li, J.; Wu, N.; Chen, X.; Chen, H.; Yang, X.; Liu, C. Curcumin protects islet cells from glucolipotoxicity by inhibiting oxidative stress and NADPH oxidase activity both in vitro and in vivo. Islets 2019, 11, 152–164. [Google Scholar] [CrossRef] [PubMed]
- Weisberg, S.; Leibel, L.; Tortoriello, D. Proteasome inhibitors, including curcumin, improve pancreatic β-cell function and insulin sensitivity in diabetic mice. Nutr. Diabetes 2016, 48, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmad, M.; Sultana, M.; Raina, R.; Pankaj, N.K.; Verma, P.K.; Prawez, S. Hypoglycemic, Hypolipidemic, and Wound Healing Potential of Quercetin in Streptozotocin-Induced Diabetic Rats. Pharmacogn. Mag. 2017, 13, 633–639. [Google Scholar] [CrossRef]
- Han, Y.; Wu, J.Z.; Shen, J.Z.; Chen, L.; He, T.; Jin, M.W.; Liu, H. Pentamethylquercetin induces adipose browning and exerts beneficial effects in 3T3-L1 adipocytes and high-fat diet-fed mice. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Srinivasan, P.; Vijayakumar, S.; Kothandaraman, S.; Palani, M. Anti-diabetic activity of quercetin extracted from Phyllanthus emblica L. fruit: In silico and in vivo approaches. J. Pharm. Anal. 2018, 8, 109–118. [Google Scholar] [CrossRef]
- Zhuang, M.; Qiu, H.; Li, P.; Hu, L.; Wang, Y.; Rao, L. Islet protection and amelioration of type 2 diabetes mellitus by treatment with quercetin from the flowers of Edgeworthia gardneri. Drug Des. Devel. Ther. 2018, 12, 955–966. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.S.; Cha, Y.J.; Lee, K.H.; Yim, J.E. Onion peel extract reduces the percentage of body fat in overweight and obese subjects: A 12-week, randomized, double-blind, placebo-controlled study. Nutr. Res. Pract. 2016, 10, 175–181. [Google Scholar] [CrossRef] [Green Version]
- Wu, T.; Gao, Y.; Guo, X.; Zhang, M.; Gong, L. Blackberry and blueberry anthocyanin supplementation counteract high-fat-diet-induced obesity by alleviating oxidative stress and inflammation and accelerating energy expenditure. Oxid. Med. Cell. Longev. 2018, 2018, 4051232. [Google Scholar] [CrossRef]
- Khan, M.I.; Shin, J.H.; Shin, T.S.; Kim, M.Y.; Cho, N.J.; Kim, J.D. Anthocyanins from Cornus kousa ethanolic extract attenuate obesity in association with anti-angiogenic activities in 3T3-L1 cells by down-regulating adipogeneses and lipogenesis. PLoS ONE 2018, 13, e0208556. [Google Scholar] [CrossRef] [PubMed]
- Han, M.H.; Kim, H.J.; Jeong, J.W.; Park, C.; Kim, B.W.; Choi, Y.H. Inhibition of adipocyte differentiation by anthocyanins isolated from the fruit of Vitis coignetiae Pulliat is associated with the activation of AMPK signaling pathway. Toxicol. Res. 2018, 34, 13–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, W.; Yan, Z.; Li, D.; Ma, Y.; Zhou, J.; Sui, Z. Antioxidant and anti-inflammatory effects of blueberry anthocyanins on high glucose-induced human retinal capillary endothelial cells. Oxid. Med. Cell. Longev. 2018, 2018, 1862462. [Google Scholar] [CrossRef] [PubMed]
- Suantawee, T.; Elazab, S.T.; Hsu, W.H.; Yao, S.; Cheng, H.; Adisakwattana, S. Cyanidin stimulates insulin secretion and pancreatic β-cell gene expression through activation of L-type voltage-dependent ca2+ channels. Nutrients 2017, 9, 814. [Google Scholar] [CrossRef] [PubMed]
- Kongthitilerd, P.; Thilavech, T.; Marnpae, M.; Rong, W.; Yao, S.; Adisakwattana, S.; Cheng, H.; Suantawee, T. Cyanidin-3-rutinoside stimulated insulin secretion through activation of L-type voltage-dependent Ca2+ channels and the PLC-IP3 pathway in pancreatic β-cells. Biomed. Pharmacother. 2022, 146, 112494. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, Z.; Zhao, H.; Wang, X.; Pang, J.; Li, Q.; Yang, Y.; Ling, W. Anthocyanin supplementation improves anti-oxidative and anti-inflammatory capacity in a dose–response manner in subjects with dyslipidemia. Redox Biol. 2020, 32, 101474. [Google Scholar] [CrossRef] [PubMed]
- Rowicka, G.; Dyląg, H.; Ambroszkiewicz, J.; Riahi, A.; Weker, H.; Chełchowska, M. Total Oxidant and Antioxidant Status in Prepubertal Children with Obesity. Oxid. Med. Cell. Longev. 2017, 2017, 5621989. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.C.; Huang, Y.W.; Hou, C.Y.; Chen, Y.T.; Di Dong, C.; Chen, C.W.; Singhania, R.R.; Leang, J.Y.; Hsieh, S.L. The anti-obesity effects of lemon fermented products in 3t3-l1 preadipocytes and in a rat model with high-calorie diet-induced obesity. Nutrients 2021, 13, 2809. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.; Zhang, J.; Liu, B.; Yan, T.; Xu, F.; Xiao, F. Polysaccharide from Okra (Abelmoschus esculentus). Molecules 2019, 24, 1906. [Google Scholar] [CrossRef] [Green Version]
- Yoshitomi, R.; Yamamoto, M.; Kumazoe, M.; Fujimura, Y.; Yonekura, M.; Shimamoto, Y.; Nakasone, A.; Kondo, S.; Hattori, H.; Haseda, A.; et al. The combined effect of green tea and α-glucosyl hesperidin in preventing obesity: A randomized placebo-controlled clinical trial. Sci. Rep. 2021, 11, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Fotschki, B.; Jurgo, A.; Kosmala, M.; Majewski, M.; Ognik, K. Extract against Pro-Oxidative and Pro-Inflammatory Rat Model. Molecules 2020, 25, 5874. [Google Scholar] [CrossRef]
- de Sousa, G.M.; Cazarin, C.B.B.; Maróstica Junior, M.R.; Lamas, C.d.A.; Quitete, V.H.A.C.; Pastore, G.M.; Bicas, J.L. The effect of α-terpineol enantiomers on biomarkers of rats fed a high-fat diet. Heliyon 2020, 6, e03752. [Google Scholar] [CrossRef] [PubMed]
- Hey-Mogensen, M.; Højlund, K.; Vind, B.F.; Wang, L.; Dela, F.; Beck-Nielsen, H.; Fernström, M.; Sahlin, K. Effect of physical training on mitochondrial respiration and reactive oxygen species release in skeletal muscle in patients with obesity and type 2 diabetes. Diabetologia 2010, 53, 1976–1985. [Google Scholar] [CrossRef] [Green Version]
- Anderson, J.C.; Clarke, E.J.; Arkin, A.P.; Voigt, C.A. Environmentally controlled invasion of cancer cells by engineered bacteria. J. Mol. Biol. 2006, 355, 619–627. [Google Scholar] [CrossRef]
- Li, Y.P.; Reid, M.B. NF-κB mediates the protein loss induced by TNF-α in differentiated skeletal muscle myotubes. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2000, 279, 1165–1170. [Google Scholar] [CrossRef]
- Heymsfield, S.B.; Wadden, T.A. Mechanisms, Pathophysiology, and Management of Obesity. N. Engl. J. Med. 2017, 376, 254–266. [Google Scholar] [CrossRef]
- Jung, U.J.; Choi, M.S. Obesity and its metabolic complications: The role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. Int. J. Mol. Sci. 2014, 15, 6184–6223. [Google Scholar] [CrossRef] [Green Version]
- Tchkonia, T.; Thomou, T.; Zhu, Y.I.; Karagiannides, I.; Pothoulakis, C.; Jensen, M.D.; Kirkland, J.L. Mechanisms and metabolic implications of regional differences among fat depots. Cell Metab. 2013, 17, 644–656. [Google Scholar] [CrossRef] [Green Version]
- Heymsfield, S.B.; Hu, H.H.; Shen, W.; Carmichael, O. Emerging Technologies and Their Applications in Lipid Compartment Measurement Lipid Compartment Measurement Advances HHS Public Access. Trends Endocrinol Metab 2015, 26, 688–698. [Google Scholar] [CrossRef] [Green Version]
- McCullough, A.J. The clinical features, diagnosis and natural history of nonalcoholic fatty liver disease. Clin. Liver Dis. 2004, 8, 521–533. [Google Scholar] [CrossRef]
- Calle, E.E.; Thun, M.J. Obesity and cancer. Oncogene 2004, 23, 6365–6378. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, S.; Fujita, T.; Shimabukuro, M.; Iwaki, M.; Yamada, Y.; Nakajima, Y.; Nakayama, O.; Makishima, M.; Matsuda, M.; Shimomura, I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Invest. 2004, 114, 1752–1761. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Ma, P.; Chen, J.; Xie, W. Ficus carica leaves extract inhibited pancreatic β-cell apoptosis by inhibiting AMPK/JNK/caspase-3 signaling pathway and antioxidation. Biomed. Pharmacother. 2020, 122, 109689. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.J.; McAllister, M.J.; Slusher, A.L.; Webb, H.E.; Mock, J.T.; Acevedo, E.O. Obesity-Related Oxidative Stress: The Impact of Physical Activity and Diet Manipulation. Sport. Med.-Open 2015, 1, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tangvarasittichai, S. Oxidative stress, insulin resistance, dyslipidemia and type 2 diabetes mellitus. World J. Diabetes 2015, 6, 456. [Google Scholar] [CrossRef] [PubMed]
- Drago, D.; Manea, M.M.; Timofte, D.; Ionescu, D. Mechanisms of Herbal Nephroprotection in diabetes mellitus. J. Diabetes Res. 2020, 2020. [Google Scholar] [CrossRef]
- Zhao, Q.; Li, L.; Zhu, Y.; Hou, D.; Li, Y.; Guo, X.; Wang, Y.; Olatunji, O.J.; Wan, P.; Gong, K. Kukoamine B ameliorate insulin resistance, oxidative stress, inflammation and other metabolic abnormalities in high-fat/high-fructose-fed rats. Diabetes Metab. Syndr. Obes. Targets Ther. 2020, 13, 1843–1853. [Google Scholar] [CrossRef]
- Chen, L.; Liu, P.; Feng, X.; Ma, C. Salidroside suppressing LPS-induced myocardial injury by inhibiting ROS-mediated PI3K/Akt/mTOR pathway in vitro and in vivo. J. Cell. Mol. Med. 2017, 21, 3178–3189. [Google Scholar] [CrossRef] [Green Version]
- Zheng, T.; Yang, X.; Li, W.; Wang, Q.; Chen, L.; Wu, D.; Bian, F.; Xing, S.; Jin, S. Salidroside attenuates high-fat diet-induced nonalcoholic fatty liver disease via AMPK-dependent TXNIP/NLRP3 pathway. Oxid. Med. Cell. Longev. 2018, 2018, 8597897. [Google Scholar] [CrossRef]
- Qi, Z.L.; Liu, Y.H.; Qi, S.M.; Ling, L.F.; Feng, Z.Y.; Li, Q. Salidroside protects PC12 cells from H2O2-induced apoptosis via suppressing NOX2-ROS-MAPKs signaling pathway. Nan Fang Yi Ke Da Xue Xue Bao 2016, 37, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Baek, J.H.; Kim, N.J.; Song, J.K.; Chun, K.H. Kahweol inhibits lipid accumulation and induces Glucose-uptake through activation of AMP-activated protein kinase (AMPK). BMB Rep. 2017, 50, 566–571. [Google Scholar] [CrossRef] [PubMed]
- Mazibuko-Mbeje, S.E.; Dludla, P.V.; Johnson, R.; Joubert, E.; Louw, J.; Ziqubu, K.; Tiano, L.; Silvestri, S.; Orlando, P.; Opoku, A.R.; et al. Aspalathin, a natural product with the potential to reverse hepatic insulin resistance by improving energy metabolism and mitochondrial respiration. PLoS ONE 2019, 14, e0216172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazibuko-Mbeje, S.E.; Dludla, P.V.; Roux, C.; Johnson, R.; Ghoor, S.; Joubert, E.; Louw, J.; Opoku, A.R.; Muller, C.J.F. Aspalathin-enriched green rooibos extract reduces hepatic insulin resistance by modulating PI3K/AKT and AMPK pathways. Int. J. Mol. Sci. 2019, 20, 633. [Google Scholar] [CrossRef] [Green Version]
- Johnson, R.; Dludla, P.V.; Muller, C.J.F.; Huisamen, B.; Essop, M.F.; Louw, J. The Transcription Profile Unveils the Cardioprotective Effect of Aspalathin against Lipid Toxicity in an in Vitro H9c2 Model. Molecules 2017, 22, 219. [Google Scholar] [CrossRef] [Green Version]
- Li, C.X.; Gao, J.G.; Wan, X.Y.; Chen, Y.; Xu, C.F.; Feng, Z.M.; Zeng, H.; Lin, Y.M.; Ma, H.; Xu, P.; et al. Allyl isothiocyanate ameliorates lipid accumulation and inflammation in nonalcoholic fatty liver disease via the Sirt1/AMPK and NF-κB signaling pathways. World J. Gastroenterol. 2019, 25, 5120–5133. [Google Scholar] [CrossRef]
- Cheng, D.; Gao, L.; Su, S.; Sargsyan, D.; Wu, R.; Raskin, I.; Kong, A.N. Moringa Isothiocyanate Activates Nrf2: Potential Role in Diabetic Nephropathy. AAPS J. 2019, 21, 31. [Google Scholar] [CrossRef] [PubMed]
- Khutami, C.; Sumiwi, S.A.; Ikram, N.K.K.; Muchtaridi, M. The Effect of Antioxidants from Natural Products on Obesity, Dyslipidemia and Diabetes and Their Molecular Signaling Mechanism; Universitas Padjadjaran: Bandung, Indonesia, 2021. [Google Scholar]
- Herrera-Balandrano, D.D.; Chai, Z.; Hutabarat, R.P.; Beta, T.; Feng, J.; Ma, K.; Li, D.; Huang, W. Hypoglycemic and hypolipidemic effects of blueberry anthocyanins by AMPK activation: In vitro and in vivo studies. Redox Biol. 2021, 46, 102100. [Google Scholar] [CrossRef]
- Rao, Y.; Yu, H.; Gao, L.; Lu, Y.T.; Xu, Z.; Liu, H.; Gu, L.Q.; Ye, J.M.; Huang, Z.S. Natural alkaloid bouchardatine ameliorates metabolic disorders in high-fat diet-fed mice by stimulating the sirtuin 1/liver kinase B-1/AMPK axis. Br. J. Pharmacol. 2017, 174, 2457–2470. [Google Scholar] [CrossRef]
- Xu, M.; Sun, B.; Li, D.; Mao, R.; Li, H.; Li, Y.; Wang, J. Beneficial effects of small molecule oligopeptides isolated from Panax ginseng meyer on pancreatic beta-cell dysfunction and death in diabetic rats. Nutrients 2017, 9, 1061. [Google Scholar] [CrossRef] [Green Version]
- Alam, M.B.; An, H.; Ra, J.S.; Lim, J.Y.; Lee, S.H.; Yoo, C.Y.; Lee, S.H. Gossypol from cottonseeds ameliorates glucose uptake by mimicking insulin signaling and improves glucose homeostasis in mice with streptozotocin-induced diabetes. Oxid. Med. Cell. Longev. 2018, 2018, 5796102. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, M.; Dong, H.; Yu, X.; Zhang, J. Anti-hypoglycemic and hepatocyte-protective effects of hyperoside from Zanthoxylum bungeanum leaves in mice with high-carbohydrate/high-fat diet and alloxan-induced diabetes. Int. J. Mol. Med. 2018, 41, 77–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.Y.; Stewart, D.A.; Ye, X.M.; Yin, L.H.; Pathmasiri, W.W.; McRitchie, S.L.; Fennell, T.R.; Cheung, H.Y.; Sumner, S.J. A metabolomics approach to investigate kukoamine B—A potent natural product with anti-diabetic properties. Front. Pharmacol. 2019, 9, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Hu, X.; Kou, L.; Zhang, B.; Zhang, C. Lycium barbarum Polysaccharide Mediated the Antidiabetic and Antinephritic Effects in Diet-Streptozotocin-Induced Diabetic Sprague Dawley Rats via Regulation of NF- B. Biomed Res. Int. 2016, 2016, 3140290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suchal, K.; Malik, S.; Khan, S.I.; Malhotra, R.K.; Goyal, S.N.; Bhatia, J.; Kumari, S.; Ojha, S.; Arya, D.S. Protective effect of mangiferin on myocardial ischemia-reperfusion injury in streptozotocin-induced diabetic rats: Role of AGE-RAGE/MAPK pathways. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Gao, J.; Liu, P.; Shen, Z.; Xu, K.; Wu, C.; Tian, F.; Chen, M.; Wang, L.; Li, P. Morroniside Promotes PGC-1 α -Mediated Cholesterol Efflux in Sodium Palmitate or High Glucose-Induced Mouse Renal Tubular Epithelial Cells. Biomed Res. Int. 2021, 2021, 9942152. [Google Scholar] [CrossRef]
- Jin, B.R.; Lee, M.; An, H.J. Nodakenin represses obesity and its complications via the inhibition of the VLDLR signalling pathway in vivo and in vitro. Cell Prolif. 2021, 54, 1–15. [Google Scholar] [CrossRef]
- El Khatib, N.; Morel, S.; Hugon, G.; Rapior, S.; Carnac, G.; Saint, N. Identification of a sesquiterpene lactone from arctium lappa leaves with antioxidant activity in primary human muscle cells. Molecules 2021, 26, 1328. [Google Scholar] [CrossRef]
- Li, X.; Gong, H.; Yang, S.; Yang, L.; Fan, Y.; Zhou, Y. Pectic Bee Pollen Polysaccharide from Rosa rugosa Alleviates Diet-Induced Hepatic Steatosis and Insulin Resistance via Induction of AMPK/mTOR-Mediated Autophagy. Molecules 2017, 22, 699. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Huang, S.; Wu, X.; Feng, Y.; Shen, Y.; Zhao, Q.S.; Leng, Y. Activation of SIK1 by phanginin A inhibits hepatic gluconeogenesis by increasing PDE4 activity and suppressing the cAMP signaling pathway. Mol. Metab. 2020, 41, 101045. [Google Scholar] [CrossRef]
- Torabi, S.; DiMarco, N.M. Original Research: Polyphenols extracted from grape powder induce lipogenesis and glucose uptake during differentiation of murine preadipocytes. Exp. Biol. Med. 2016, 241, 1776–1785. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Cao, P.; Wang, H.; Tang, Z.; Wang, N.; Wang, J.; Zhang, Y. Chronic administration of Angelica sinensis polysaccharide effectively improves fatty liver and glucose homeostasis in high-fat diet-fed mice. Sci. Rep. 2016, 6, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, X.; Lu, K.; Wang, L.; Lv, M.; Fu, W. Astraglaus polysaccharide protects diabetic cardiomyopathy by activating NRG1/ErbB pathway. Biosci. Trends 2018, 12, 149–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, M.; Zhu, T.; Tong, J.; Caidan, R.; Wang, K.; Kai, G.; Zhang, W.; Ru, L.; Pengcuo, J.; Tong, L. Screening active components from Rubus amabilis for pancreatic β-cells protection. Pharm. Biol. 2020, 58, 674–685. [Google Scholar] [CrossRef]
- Zhang, D.; Li, M. Puerarin prevents cataract development and progression in diabetic rats through Nrf2/HO-1 signaling. Mol. Med. Rep. 2019, 20, 1017–1024. [Google Scholar] [CrossRef]
- Choi, J.; Oh, S.; Son, M.; Byun, K. Pyrogallol-phloroglucinol-6,6-bieckol alleviates obesity and systemic inflammation in a mouse model by reducing expression of RAGE and RAGE ligands. Mar. Drugs 2019, 17, 612. [Google Scholar] [CrossRef] [Green Version]
- Fourny, N.; Lan, C.; Sérée, E.; Bernard, M.; Desrois, M. Protective effect of resveratrol against ischemia-reperfusion injury via enhanced high energy compounds and eNOS-SIRT1 expression in type 2 diabetic female rat heart. Nutrients 2019, 11, 105. [Google Scholar] [CrossRef] [Green Version]
- Ju, L.; Wen, X.; Wang, C.; Wei, Y.; Peng, Y.; Ding, Y.; Feng, L.; Shu, L. Salidroside, a natural antioxidant, improves β-cell survival and function via activating AMPK pathway. Front. Pharmacol. 2017, 8, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Wu, X.; Shi, F.; Liu, Y. Comparison of antidiabetic effects of saponins and polysaccharides from Momordica charantia L. in STZ-induced type 2 diabetic mice. Biomed. Pharmacother. 2019, 109, 744–750. [Google Scholar] [CrossRef]
- Belhadj, S.; Hentati, O.; Hamdaoui, G.; Fakhreddine, K.; Maillard, E.; Dal, S.; Sigrist, S. Beneficial effect of Jojoba seed extracts on hyperglycemia-induced oxidative stress in RINm5f beta cells. Nutrients 2018, 10, 384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, T.X.; Cheng, X.Y.; Wang, Y.; Yin, W. Toosendanin inhibits adipogenesis by activating Wnt/β-catenin signaling. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Yamamoto, M. Molecular basis of the Keap1–Nrf2 system. Free Radic. Biol. Med. 2015, 88, 93–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wardyn, J.D.; Ponsford, A.H.; Sanderson, C.M. Dissecting molecular cross-talk between Nrf2 and NF-κB response pathways. Biochem. Soc. Trans. 2015, 43, 621–626. [Google Scholar] [CrossRef] [Green Version]
- Albrahim, T.; Alonazi, M.A. Lycopene corrects metabolic syndrome and liver injury induced by high fat diet in obese rats through antioxidant, anti-inflammatory, antifibrotic pathways. Biomed. Pharmacother. 2021, 141, 111831. [Google Scholar] [CrossRef]
- Chen, H.; Jiang, Y.; Yang, Z.; Hu, W.; Xiong, L.; Wang, N.; Liu, X.; Zheng, G.; Ouyang, K.; Wang, W. Effects of Chimonanthus nitens Oliv. Leaf Extract on Glycolipid Metabolism and Antioxidant Capacity in Diabetic Model Mice. Oxid. Med. Cell. Longev. 2017, 2017, 7648505. [Google Scholar] [CrossRef] [PubMed]
- Brasier, A.R. The NF-κB regulatory network. Cardiovasc. Toxicol. 2006, 6, 111–130. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.L.; Zhao, K.C.; Yuan, W.; Zhou, F.; Song, H.Y.; Liu, G.L.; Huang, J.; Zou, J.J.; Zhao, B.; Xie, S.P. MicroRNA-31-5p Exacerbates Lipopolysaccharide-Induced Acute Lung Injury via Inactivating Cab39/AMPK α Pathway. Oxid. Med. Cell. Longev. 2020, 2020, 8822361. [Google Scholar] [CrossRef] [PubMed]
- Tayanloo-Beik, A.; Roudsari, P.P.; Rezaei-Tavirani, M.; Biglar, M.; Tabatabaei-Malazy, O.; Arjmand, B.; Larijani, B. Diabetes and Heart Failure: Multi-Omics Approaches. Front. Physiol. 2021, 12, 1–12. [Google Scholar] [CrossRef]
- Kay, A.M.; Simpson, C.L.; Stewart, J.A. The Role of AGE/RAGE Signaling in Diabetes-Mediated Vascular Calcification. J. Diabetes Res. 2016, 2016, 6809703. [Google Scholar] [CrossRef] [Green Version]




| Compounds | Sources | Experimental Models | Mechanisms | Ref. |
|---|---|---|---|---|
| Anthocyanin (100 and 400 mg/kg for 5 weeks) | Vaccinium corymbosum | Streptozotocin-induced diabetic rats and HepG2 cells. | Hyperglycemia and hyperlipidemia are inhibited by reducing the expression of enzymes involved in gluconeogenesis, lipogenesis, and lipolysis via the adenosine monophosphate (AMPK)-activated kinase signaling pathway in HepG2 cells. | [101] |
| Aspalathin (10 g/mL for 3 h) | Aspalathus linearis (Green rooibos) | C2C12 skeletal muscle cells and 3T3-L1 fat cells induced with palmitate. | Aspalathin modulates the major insulin signaling PI3K/AKT and AMPK effectors to ameliorate insulin resistance by increasing glucose transporter expression. | [96] |
| Bouchardatine (50 mg/kg/days) | Bouchardatia neurococca | Male C57BL/6J mice induced with HFD. | Bou may have therapeutic potential for obesity-related metabolic diseases by increasing the capacity of energy expenditure in adipose tissues and liver through a mechanism involving the SIRT1–LKB1–AMPK axis. | [102] |
| Ginseng oligopeptides (GOPs) (0.125, 0.5 and 2.0 g/kg bw for 7, 24 and 52 weeks) | Panax ginseng | Mice that were induced with a high-fat diet for 4 weeks. | Oligopeptides increase the normal content of insulin and protect pancreatic cells from apoptosis associated with type 2 diabetes mellitus by inhibiting NF-κB activity to protect against inflammation due to diabetes. | [103] |
| Gossypol in vivo (1 and 2.5 mg/kg at 0, 30, 60, 90, 120, 150 and 180 min on glucose tolerance test) and in vitro (25, 50 and 75 μg/mL for 24 h) | Gossypium sp. | Mouse myoblast cells (C2C12) and streptozotocin-induced (STZ) mouse myoblasts. | Gossypol (GSP) can activate the insulin receptor substrate 1 (IRS-1)/protein kinase B (Akt) signaling pathway and can translocate glucose transporter 4 (GLUT 4) into the plasma membrane at C2C12 myotube, thereby increasing glucose uptake. | [104] |
| Hyperoside (200, 100 and 50 mg/kg for 4 weeks) | Zanthoxylum bungeanum | Mice that were induced with alloxan and a high-fat diet. | Hyperoside inhibits the phosphorylation of p65/NF-κB, MAPK (including p38, JNK and ERK1/2). | [105] |
| Isothiocyanate (Moringa isothiocyanate/MIC-1) (5 μM for 24 h) | Moringa oleifera | HK-2 cells were given high glucose to induce oxidative stress. | Nrf2-ARE is activated by MIC1 to suppress inflammation and reduce oxidative stress. | [99] |
| Kahweol (2.5 and 5 µM for 24 h) | Coffea sp. | INS-1 cells tonal clonal induced with streptozotocin (STZ). | Kahweol downregulates NF-κB, antioxidant proteins, inhibitors of DNA binding and cell differentiation. | [14] |
| Kukoamine B (50mg/kg/day for 9 weeks) | Lycium chinense | Diabetic mouse model (dB/dB) using metabolomics approach (Biocrates p180) | Kukoamine B regulates the NF-κB/PPAR transcriptional pathway to reduce inflammation in diabetes. | [106] |
| Lycium barbarum Polysaccharide (LBPS) (100, 250, and 500mg/kg for 4 weeks) | Lycium barbarum | HFD and streptozotocin-induced mice. | It inhibits serum levels of inflammatory factors (IL-2, IL-6, TNF-α, and IFN-α), protects kidney damage and inhibits NF-κB expression. | [107] |
| Mangiferin (40 mg/kg for 28 days) | Mangifera indica | Research on myocardial ischemia-reperfusion (IR) in diabetic rats. | Mangiferin can reduce IR injury in diabetic rats through inhibition of the AGE-RAGE/MAPK pathway thereby preventing oxidative stress, apoptosis and inflammation. | [108] |
| Morroniside (6.25, 12.5, 25, 50 and 100 μmol/L for 24 h) | Cornus officinalis Sieb. | In vitro study using rat renal tubular epithelial cells (mRETCs) induced with palmitate and glucose. | Morroniside increases cholesterol reduction via the PGC1a/LXR pathway and and it also downregulates RAGE, p38MAPK and NF-κB expression via the AGEs/RAGE signaling pathway. | [109] |
| Nodakenin (NK) (10 and 20 mg/kg for 5 weeks) | Angelicae gigas | Male C57BL/6N mice with a high-fat diet. | Administration of NK can improve the phosphorylation level of AMPK, indicating that NK exerts anti-adipogenic and antioxidant effects. | [110] |
| Onopordopicrin (0.125, 0.25 and 0.5 µg/mL for 24 h) | Arctium lappa | A model of human muscle cells exposed to H2O2 oxidative stress. | Onopordopicrin has antioxidant activity by limiting the production of free radicals and DNA damage and through activation of the Nrf2/HO-1 signaling pathway in muscle cells. | [111] |
| Pectic bee pollen polysaccharide (RBPP-P) in vitro (0.1 mg/mL for 24 h and in vivo (20 mg/kg for 8 weeks) | Rosa rugosa | HepG2 cells treated with high-glucose and high-fatty acids and obese mice with a high-fat diet (HFD) inducer. | This polysaccharide is able to decrease hepatic steatosis and insulin resistance by promoting autophagy through AMPK/mTOR-mediated signaling pathways. | [112] |
| Phanginin A (250 mg/kg for 26 days) | Caesalpinia sappan | Male ob/ob mice. | Phanginin A activates SIK1 and causes inhibition of gluconeogenesis with increased PDE4 and inhibition of the cAMP/PKA/CREB pathway in the liver. | [113] |
| Polyphenol (125–500 mg GP/mL for 8 days) | Vitis vinivera | Preadiposit 3T3-F442A cells. | It induces adiposity differentiation through upregulation of GLUT-4, PI3K and adipogenic genes. | [114] |
| Polysaccharide (200 and 400 mg/kg bw for 8 weeks) | Okra (Abelmoschus esculentus (L.) Moench). | Rats that were given a high-fat diet (HFD) combined with injection of 100 mg/kg streptozotocin (STZ) intraperitoneally (ip). | Okra polysaccharide (OP) exert their type 2 antidiabetic effects in part by modulating oxidative stress via Nrf2 transport in the PI3K/AKT/GSK3β pathway. | [72] |
| Polysaccharide (80, 160 and 320 mg/kg/day for 4 weeks) | Angelica sinensis | BALB/C mice induced with a high-fat diet were used. | Angelica sinensis polysaccharide (ASP) is reported to lower blood glucose and improve insulin resistance through regulation of metabolic enzymes and activation of the PI3K/Akt pathway in HFD mice. It can also decrease lipid accumulation and fatty liver by increasing PPARγ expression and activation of the adiponectin signaling pathway SIRT1 and AMPK. | [115] |
| Polysaccharide (0.1, 1.0, 10 and 100 μg/mL for 0, 12, 24, 48 and 72 h) | Astragalus mongholicus | AGE-induced DCM cell model. | Astragalus polysaccharides can decrease intracellular ROS levels, increase SOD activity and GSH-Px and lower MDA and NO levels. | [116] |
| Procyanidin (25, 50 and 75 μg/mL for 24 h) | Rubus amabilis | MIN6 cells were given 0.5 mM palmitate (PA) for 24 h to induce cell apoptosis. | Procyanidin can activate the PI3K/Akt/FoxO1 signal to protect MIN6 cells from apoptosis induced by palmitate induction. | [117] |
| Puerarin (25, 50 and 100 mg/kg for 12 weeks) | Pueraria lobata | Mice induced with streptozotocin. | Puerarin significantly lowers blood sugar levels and prevents cataracts as well as lowers the level of expression of retinal vascular endothelial growth factor and interleukin-1β and increases the expression of Nrf2 and Ho-1 mRNA so that it can reduce oxidative stress in diabetic rats. | [118] |
| Pyrogallol-phloroglucinol-6,6-bieckol (PPB) (2 mg/kg for 4 weeks) | Ecklonia cava | C57BL/6N mice induced with HFD for 8 weeks. | It inhibits RAGE ligands, reduces RAGE expression and binding of RAGE and RAGE ligands and reduces proinflammatory cytokines that cause obesity. | [119] |
| Resveratrol (1 mg/kg/day for 8 weeks) | Polygonum cuspidatum | Goto-Kakizaki (GK) type 2 diabetic female rats. | Resveratrol increases adenine nucleotide and citrate synthase activity by increasing the expression of eNOS-SIRT1 and P-AKT. | [120] |
| Salidroside (100 mg/kg/day for 5 weeks) | Rhodiola rosea | Mice induced by high-fat diet (HFD). | Salidroside suppresses ROS production and inhibits the JNK-caspase apoptotic cascade, inhibiting FOXO-1 by activating AMPK-AKT. | [121] |
| Saponins (40 mg/kg) | Momordica carantia L. | Mice that were induced with a high-fat diet and streptozotocin. | Saponins exhibit hypoglycemic activity possibly via the AMPK/NF-κB signaling pathway by activating AMPK phosphorylation and energy metabolism of the body. | [122] |
| Simmondsin (10, 20, 40, 80 and 150 µg/mL for simmondsin for 24 h) | Simmondsia Chinensis | Fructose-induced oxidative stress in RIN5f beta cells. | Simmondsin is reported to reduce ROS by 69%, activate caspase-3, increase antioxidant defense, inhibit p22phox and increase Nrf2 factor. | [123] |
| Toosendanin (TSN) in vitro (12.5 nM, 25 and 50 nM for 6 days) in vivo (0.1 mg/kg/day for one month) | Melia toosendan | 3T3L1 preadipocytes and mice induced with a high-fat diet. | TSN can inhibit adipocyte differentiation and lipid accumulation by activating Wnt/β-catenin signaling, inhibiting mRNA and protein levels of PPAR-γ and C/EBP-α, which proves that TSN can inhibit adipogenesis via its mechanism in inhibiting transcription factor cascades. | [124] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khutami, C.; Sumiwi, S.A.; Khairul Ikram, N.K.; Muchtaridi, M. The Effects of Antioxidants from Natural Products on Obesity, Dyslipidemia, Diabetes and Their Molecular Signaling Mechanism. Int. J. Mol. Sci. 2022, 23, 2056. https://doi.org/10.3390/ijms23042056
Khutami C, Sumiwi SA, Khairul Ikram NK, Muchtaridi M. The Effects of Antioxidants from Natural Products on Obesity, Dyslipidemia, Diabetes and Their Molecular Signaling Mechanism. International Journal of Molecular Sciences. 2022; 23(4):2056. https://doi.org/10.3390/ijms23042056
Chicago/Turabian StyleKhutami, Chindiana, Sri Adi Sumiwi, Nur Kusaira Khairul Ikram, and Muchtaridi Muchtaridi. 2022. "The Effects of Antioxidants from Natural Products on Obesity, Dyslipidemia, Diabetes and Their Molecular Signaling Mechanism" International Journal of Molecular Sciences 23, no. 4: 2056. https://doi.org/10.3390/ijms23042056
APA StyleKhutami, C., Sumiwi, S. A., Khairul Ikram, N. K., & Muchtaridi, M. (2022). The Effects of Antioxidants from Natural Products on Obesity, Dyslipidemia, Diabetes and Their Molecular Signaling Mechanism. International Journal of Molecular Sciences, 23(4), 2056. https://doi.org/10.3390/ijms23042056