Early Effects of Extracellular Vesicles Secreted by Adipose Tissue Mesenchymal Cells in Renal Ischemia Followed by Reperfusion: Mechanisms Rely on a Decrease in Mitochondrial Anion Superoxide Production
Abstract
:1. Introduction
2. Results
2.1. Characterization of Extracellular Vesicles Secreted by Adipose Tissue Mesenchymal Stromal Cells
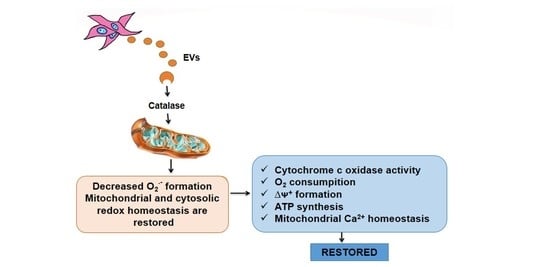
2.2. Mitochondria Subjected to Hypoxic Damage Are Key Targets for the Extracellular Vesicles Secreted by Mesenchymal Cells
2.3. Subcapsular Administration of Extracellular Vesicles Secreted by Adipose Tissue Mesenchymal Cells Restores Normal Values of Anion Superoxide Formation by Renal Mitochondria after Ischemia/Reperfusion
2.4. Twenty-Four Hours after Ischemia Followed by Reperfusion, the Acute Renal Lesions Still Persist
2.5. The Early Anti-Oxidative Responses after EVs Administration Co-Exist with Unmodified Transcriptional Activation of Key Enzymes Involved in Redox Regulation
2.6. Apoptotic Processes Are Partially Reversed When Renal Proximal Tubule Cells Are Co-Cultured with Extracellular Vesicles after Hypoxia
3. Discussion
4. Materials and Methods
4.1. Ethical Statement
4.2. Isolation and Characterization of Extracellular Vesicles Secreted by Adipose Mesenchymal Stromal Cells
4.3. Animals and Treatments
4.4. Renal Cells Cultures and Hypoxia/Reoxygenation Protocol
4.5. Flow Cytometry
4.6. Analysis of Mitochondrial Morphology and Qualitative Evaluation of the Electrical Potential Difference across the Inner Mitochondrial Membrane from Renal Cells in Co-Culture with Extracellular Vesicles
4.7. Time Course of Reactive O2 Species Formation
4.8. Oxygen Consumption by Isolated Mitochondria from Kidney Proximal Tubule Cells
4.9. RNA Isolation, Reverse Transcription and Real Time Quantitative Polymerase Chain Reaction (qRT-PCR)
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pieretti, J.C.; Junho, C.V.C.; Carneiro-Ramos, M.S.; Seabra, A.B. H2S- and NO-releasing gasotransmitter platform: A crosstalk signaling pathway in the treatment of acute kidney injury. Pharmacol. Res. 2020, 161, 105121. [Google Scholar] [CrossRef]
- Neyra, J.A.; Leaf, D.E. Risk prediction models for acute kidney injury in critically Ill patients: Opus in progressu. Nephron 2018, 140, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Silver, S.A.; Chertow, G.M. The economic consequences of acute kidney injury. Nephron 2017, 137, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.Y.; Bellomo, R. Renal replacement therapy in the ICU: Intermittent hemodialysis, sustained low-efficiency dialysis or continuous renal replacement therapy? Curr. Opin. Crit. Care 2018, 24, 437–442. [Google Scholar] [CrossRef]
- Iacovella, G.M.; Kumar, N. Controversies surrounding renal replacement therapy in the critically Ill patient. Semin. Respir. Crit. Care Med. 2019, 40, 662–672. [Google Scholar] [CrossRef]
- Oliver, J.A. Adult renal stem cells and renal repair. Curr. Opin. Nephrol. Hypertens. 2004, 13, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Bussolati, B.; Bruno, S.; Grange, C.; Buttiglieri, S.; Deregibus, M.C.; Cantino, D.; Camussi, G. Isolation of renal progenitor cells from adult human kidney. Am. J. Pathol. 2005, 166, 545–555. [Google Scholar] [CrossRef] [Green Version]
- Humphreys, B.D.; Valerius, M.T.; Kobayashi, A.; Mugford, J.W.; Soeung, S.; Duffield, J.S.; McMahon, A.P.; Bonventre, J.V. Intrinsic epithelial cells repair the kidney after injury. Cell Stem Cell 2008, 2, 284–291. [Google Scholar] [CrossRef] [Green Version]
- Kusaba, T.; Lalli, M.; Kramann, R.; Kobayashi, A.; Humphreys, B.D. Differentiated kidney epithelial cells repair injured proximal tubule. Proc. Natl. Acad. Sci. USA 2014, 111, 1527–1532. [Google Scholar] [CrossRef] [Green Version]
- Collino, F.; Lopes, J.A.; Corrêa, S.; Abdelhay, E.; Takiya, C.M.; Wendt, C.H.C.; de Miranda, K.R.; Vieyra, A.; Lindoso, R.S. Adipose-derived mesenchymal stromal cells under hypoxia: Changes in extracellular vesicles secretion and improvement of renal recovery after ischemic injury. Cell. Physiol. Biochem. 2019, 52, 1463–1483. [Google Scholar] [CrossRef]
- Han, S.J.; Lee, H.T. Mechanisms and therapeutic targets of ischemic acute kidney injury. Kidney Res. Clin. Pract. 2019, 38, 427–440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baud, L.; Ardaillou, R. Involvement of reactive oxygen species in kidney damage. Br. Med. Bull. 1993, 49, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Mulay, S.R.; Holderied, A.; Kumar, S.V.; Anders, H.J. Targeting inflammation in so-called acute kidney injury. Semin. Nephrol. 2016, 36, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Đurašević, S.; Stojković, M.; Bogdanović, L.; Pavlović, S.; Borković-Mitić, S.; Grigorov, I.; Bogojević, D.; Jasnić, N.; Tosti, T.; Đurović, S.; et al. The effects of meldonium on the renal acute ischemia/reperfusion injury in rats. J. Mol. Sci. 2019, 20, 5747. [Google Scholar] [CrossRef] [Green Version]
- Fortrie, G.; de Geus, H.R.H.; Betjes, M.G.H. The aftermath of acute kidney injury: A narrative review of long-term mortality and renal function. Crit. Care. 2019, 24, 24. [Google Scholar] [CrossRef] [Green Version]
- Bhatraju, P.K.; Zelnick, L.R.; Chinchilli, V.M.; Moledina, D.G.; Coca, S.G.; Parikh, C.R.; Garg, A.X.; Hsu, C.Y.; Go, A.S.; Liu, K.D.; et al. Association between early recovery of kidney function after acute kidney injury and long-term clinical outcomes. JAMA Netw. Open 2020, 3, e202682. [Google Scholar] [CrossRef]
- Willms, E.; Johansson, H.J.; Mäger, I.; Lee, Y.; Blomberg, K.E.; Sadik, M.; Alaarg, A.; Smith, C.I.; Lehtiö, J.; El Andaloussi, S.; et al. Cells release subpopulations of exosomes with distinct molecular and biological properties. Sci. Rep. 2016, 6, 22519. [Google Scholar] [CrossRef]
- Knox, F.G.; Fleming, J.S.; Rennie, D.W. Effects of osmotic diuresis on sodium reabsorption and oxygen consumption of kidney. Am. J. Physiol. 1966, 210, 751–759. [Google Scholar] [CrossRef] [Green Version]
- Nanayakkara, G.K.; Wang, H.; Yang, X. Proton leak regulates mitochondrial reactive oxygen species generation in endothelial cell activation and inflammation—A novel concept. Arch. Biochem. Biophys. 2019, 662, 68–74. [Google Scholar] [CrossRef]
- Nicholls, D.G. The influence of respiration and ATP hydrolysis on the proton-electrochemical gradient across the inner membrane of rat-liver mitochondria as determined by ion distribution. Eur. J. Biochem. 1974, 50, 305–315. [Google Scholar] [CrossRef]
- Nicholls, D.G. Mitochondrial membrane potential and aging. Aging Cell 2004, 3, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Brand, M.D.; Nicholls, D.G. Assessing mitochondrial dysfunction in cells. Biochem. J. 2011, 435, 297–312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonventre, J.V. Kidney Injury Molecule-1 (KIM-1): A specific and sensitive biomarker of kidney injury. Scand. J. Clin. Lab. Investig. 2008, 241, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Devarajan, P. Neutrophil gelatinase-associated lipocalin (NGAL): A new marker of kidney disease. Scand. J. Clin. Lab. Investig. 2008, 241, 89–94. [Google Scholar] [CrossRef] [Green Version]
- Glorieux, C.; Zamocky, M.; Sandoval, J.M.; Verrax, J.; Calderon, P.B. Regulation of catalase expression in healthy and cancerous cells. Free Radic. Biol. Med. 2015, 87, 84–97. [Google Scholar] [CrossRef]
- Lever, J.M.; Boddu, R.; George, J.F.; Agarwal, A. Heme oxygenase-1 in kidney health and disease. Antioxid. Redox Signal. 2016, 25, 165–183. [Google Scholar] [CrossRef] [Green Version]
- Loboda, A.; Damulewicz, M.; Pyza, E.; Jozkowicz, A.; Dulak, J. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: An evolutionarily conserved mechanism. Cell. Mol. Life Sci. 2016, 73, 3221–3247. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez-Vicente, A.; Hong, N.; Garvin, J.L. Effects of reactive oxygen species on renal tubular transport. Am. J. Physiol. Renal. Physiol. 2019, 317, F444–F455. [Google Scholar] [CrossRef]
- Jastroch, M.; Divakaruni, A.S.; Mookerjee, S.; Treberg, J.R.; Brand, M.D. Mitochondrial proton and electron leaks. Essays Biochem. 2010, 47, 53–67. [Google Scholar] [CrossRef] [Green Version]
- Brand, M.D.; Buckingham, J.A.; Esteves, T.C.; Green, K.; Lambert, A.J.; Miwa, S.; Murphy, M.P.; Pakay, J.L.; Talbot, D.A.; Echtay, K.S. Mitochondrial superoxide and aging: Uncoupling-protein activity and superoxide production. Biochem. Soc. Symp. 2004, 71203–71213. [Google Scholar] [CrossRef]
- Murphy, M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009, 417, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Lustgarten, M.S.; Bhattacharya, A.; Muller, F.L.; Jang, Y.C.; Shimizu, T.; Shirasawa, T.; Richardson, A.; van Remmen, H. Complex I generated, mitochondrial matrix-directed superoxide is released from the mitochondria through voltage dependent anion channels. Biochem. Biophys. Res. Commun. 2012, 422, 515–521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dröse, S. Differential effects of complex II on mitochondrial ROS production and their relation to cardioprotective pre- and postconditioning. Biochim. Biophys. Acta 2013, 1827, 578–587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hekimi, S.; Wang, Y.; Noë, A. Mitochondrial ROS and the effectors of the intrinsic apoptotic pathway in aging cells: The discerning killers! Front. Genet. 2016, 7, 161. [Google Scholar] [CrossRef] [Green Version]
- Robb, E.L.; Hall, A.R.; Prime, T.A.; Eaton, S.; Szibor, M.; Viscomi, C.; James, A.M.; Murphy, M.P. Control of mitochondrial superoxide production by reverse electron transport at complex I. J. Biol. Chem. 2018, 293, 9869–9879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, J.; Vinothkumar, K.R.; Hirst, J. Structure of mammalian respiratory complex I. Nature 2016, 536, 354–358. [Google Scholar] [CrossRef] [Green Version]
- Wirth, C.; Brandt, U.; Hunte, C.; Zickermann, V. Structure and function of mitochondrial complex I. Biochim. Biophys. Acta 2016, 1857, 902–914. [Google Scholar] [CrossRef]
- Iwata, S.; Lee, J.W.; Okada, K.; Lee, J.K.; Iwata, M.; Rasmussen, B.; Link, T.A.; Ramaswamy, S.; Jap, B.K. Complete structure of the 11-subunit bovine mitochondrial cytochrome bc1 complex. Science 1998, 281, 64–71. [Google Scholar] [CrossRef]
- Brookes, P.S.; Yoon, Y.; Robotham, J.L.; Anders, M.W.; Sheu, S.S. Calcium, ATP, and ROS: A mitochondrial love-hate triangle. Am. J. Physiol. Physiol. Cell Physiol. 2004, 287, C817–C833. [Google Scholar] [CrossRef]
- Lenaz, G.; Genova, M.L. Structure and organization of mitochondrial respiratory complexes: A new understanding of an old subject. Antioxid. Redox Signal. 2010, 12, 961–1008. [Google Scholar] [CrossRef]
- Lapuente-Brun, E.; Moreno-Loshuertos, R.; Acín-Pérez, R.; Latorre-Pellicer, A.; Colás, C.; Balsa, E.; Perales-Clemente, E.; Quirós, P.M.; Calvo, E.; Rodríguez-Hernández, M.A.; et al. Supercomplex assembly determines electron flux in the mitochondrial electron transport chain. Science 2013, 340, 1567–1570. [Google Scholar] [CrossRef] [PubMed]
- Milenkovic, D.; Blaza, J.N.; Larsson, N.G.; Hirst, J. The enigma of the respiratory chain supercomplex. Cell Metab. 2017, 25, 765–776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, X.; O’Neill, K.L.; Huang, K. The third model of Bax/Bak activation: A Bcl-2 family feud finally resolved? F1000Research 2020, 9, 935. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Li, Y.; Wen, D.; Liu, M.; Ma, Y.; Cong, B. NGAL protects against endotoxin-induced renal tubular damage by suppressing apoptosis. BMC Nephrol. 2018, 19, 168. [Google Scholar] [CrossRef]
- Faubel, S.; Lewis, E.C.; Reznikov, L.; Ljubanovic, D.; Hoke, T.S.; Somerset, H.; Oh, D.J.; Lu, L.; Klein, C.L.; Dinarello, C.A.; et al. Cisplatin-induced acute renal failure is associated with an increase in the cytokines interleukin (IL)-1beta, IL-18, IL-6, and neutrophil infiltration in the kidney. J. Pharmacol. Exp. Ther. 2007, 322, 8–15. [Google Scholar] [CrossRef]
- Zhang, M.Z.; Yao, B.; Yang, S.; Jiang, L.; Wang, S.; Fan, X.; Yin, H.; Wong, K.; Miyazawa, T.; Jianchun, C.; et al. CSF-1 signaling mediates recovery from acute kidney injury. J. Clin. Investig. 2012, 122, 4519–4532. [Google Scholar] [CrossRef] [Green Version]
- Ramseyer, V.D.; Garvin, J.L. Tumor necrosis factor-α: Regulation of renal function and blood pressure. Am. J. Physiol. Renal. Physiol. 2013, 304, F1231–F1242. [Google Scholar] [CrossRef] [Green Version]
- Shalaby, M.R.; Waage, A.; Espevik, T. Cytokine regulation of interleukin 6 production by human endothelial cells. Cell. Immunol. 1989, 121, 372–382. [Google Scholar] [CrossRef]
- Nechemia-Arbely, Y.; Barkan, D.; Pizov, G.; Shriki, A.; Rose-John, S.; Galun, E.; Axelrod, J.H. IL-6/IL-6R axis plays a critical role in acute kidney injury. J. Am. Soc. Nephrol. 2008, 19, 1106–1115. [Google Scholar] [CrossRef]
- Su, H.; Lei, C.T.; Zhang, C. Interleukin-6 signaling pathway and its role in kidney disease: An update. Front. Immunol. 2017, 8, 405. [Google Scholar] [CrossRef] [Green Version]
- Paller, M.S.; Hoidal, J.R.; Ferris, T.F. Oxygen free radicals in ischemic acute renal failure in the rat. J. Clin. Investig. 1984, 74, 1156–1164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baliga, R.; Ueda, N.; Walker, P.D.; Shah, S.V. Oxidant mechanisms in toxic acute renal failure. Am. J. Kidney Dis. 1997, 29, 465–477. [Google Scholar] [CrossRef]
- Gyurászová, M.; Gurecká, R.; Bábíčková, J.; Tóthová, Ľ. Oxidative stress in the pathophysiology of kidney disease: Implications for noninvasive monitoring and identification of biomarkers. Oxid. Med. Cell. Longev. 2020, 2020, 5478708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cowan, D.B.; Weisel, R.D.; Williams, W.G.; Mickle, D.A. The regulation of glutathione peroxidase gene expression by oxygen tension in cultured human cardiomyocytes. J. Mol. Cell. Cardiol. 1992, 24, 423–433. [Google Scholar] [CrossRef]
- Bryan, H.K.; Olayanju, A.; Goldring, C.E.; Park, B.K. The Nrf2 cell defence pathway: Keap1-dependent and -independent mechanisms of regulation. Biochem. Pharmacol. 2013, 85, 705–717. [Google Scholar] [CrossRef] [Green Version]
- Wagner, E.F.; Nebreda, A.R. Signal integration by JNK and p38 MAPK pathways in cancer development. Nat. Rev. Cancer 2009, 9, 537–549. [Google Scholar] [CrossRef]
- Vigolo, E.; Markó, L.; Hinze, C.; Müller, D.N.; Schmidt-Ullrich, R.; Schmidt-Ott, K.M. Canonical BMP signaling in tubular cells mediates recovery after acute kidney injury. Kidney Int. 2019, 95, 108–122. [Google Scholar] [CrossRef]
- De Godoy, M.A.; Saraiva, L.M.; de Carvalho, L.R.P.; Vasconcelos-Dos-Santos, A.; Beiral, H.J.V.; Ramos, A.B.; Silva, L.R.P.; Leal, R.B.; Monteiro, V.H.S.; Braga, C.V.; et al. Mesenchymal stem cells and cell-derived extracellular vesicles protect hippocampal neurons from oxidative stress and synapse damage induced by amyloid-β oligomers. J. Biol. Chem. 2018, 293, 1957–1975. [Google Scholar] [CrossRef] [Green Version]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. J. Physiol. 2020, 598, 3793–3801. [Google Scholar] [CrossRef]
- Collino, F.; Lopes, J.A.; Tapparo, M.; Tortelote, G.G.; Kasai-Brunswick, T.H.; Lopes, G.M.C.; Almeida, D.B.; Skovronova, R.; Wendt, C.H.C.; Miranda, K.R.; et al. Extracellular vesicles derived from induced pluripotent stem cells promote renoprotection in acute kidney injury model. Cells 2020, 9, 453. [Google Scholar] [CrossRef] [Green Version]
- Beiral, H.J.; Rodrigues-Ferreira, C.; Fernandes, A.M.; Gonsalez, S.R.; Mortari, N.C.; Takiya, C.M.; Sorenson, M.M.; Figueiredo-Freitas, C.; Galina, A.; Vieyra, A. The impact of stem cells on electron fluxes, proton translocation, and ATP synthesis in kidney mitochondria after ischemia/reperfusion. Cell Transplant. 2014, 23, 207–220. [Google Scholar] [CrossRef] [PubMed]
- Whittembury, G.; Proverbio, F. Two modes of Na extrusion in cells from guinea pig kidney cortex slices. Pflugers Arch. 1970, 316, 1–25. [Google Scholar] [CrossRef]
- Bianco, M.; Lopes, J.A.; Beiral, H.J.V.; Filho, J.D.D.; Frankenfeld, S.P.; Fortunato, R.S.; Gattass, C.R.; Vieyra, A.; Takiya, C.M. The contralateral kidney presents with impaired mitochondrial functions and disrupted redox homeostasis after 14 days of unilateral ureteral obstruction in mice. PLoS ONE 2019, 14, e0218986. [Google Scholar] [CrossRef] [PubMed]
- Sahajpal, V.; Ashton, N. Increased glomerular angiotensin II binding in rats exposed to a maternal low protein diet in utero. J. Physiol. 2005, 563, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Silva, P.A.; Muzi-Filho, H.; Pereira-Acácio, A.; Dias, J.; Martins, J.F.; Landim-Vieira, M.; Verdoorn, K.S.; Lara, L.S.; Vieira-Filho, L.D.; Cabral, E.V.; et al. Altered signaling pathways linked to angiotensin II underpin the upregulation of renal Na+-ATPase in chronically undernourished rats. Biochim. Biophys. Acta 2014, 1842, 2357–2366. [Google Scholar] [CrossRef] [Green Version]









| mRNA | Sequence |
|---|---|
| rSOD1 F1 | AAGAGAGGCATGTTGGAGACC |
| rSOD1 R1 | ACGGCCAATGATGGAATGCT |
| rCAT F1 | CAGCTCCGCAATCCTACACC |
| rCAT R1 | GGACATCGGGTTTCTGAGGG |
| rIL6 F1 | AAGCCAGAGTCATTCAGAGC |
| rIL6 R1 | GTCCTTAGCCACTCCTTCTG |
| rTNFA F1 | CTTCTCATTCCTGCTCGTGG |
| rTNFA R1 | TGATCTGAGTGTGAGGGTCTG |
| rKIM1 F1 | ACCTGATCAGACAGAGTGTGC |
| rKIM1 R1 | ATCTACAGAGCCTGGAAGAAGCA |
| rHO-1 F1 | AGGTGCACATCCGTGCAGAG |
| rHO-1 R1 | CTTCCAGGGCCGTATAGATATGGTA |
| rNGAL F1 | GGGCTGTCCGATGAACTGAA |
| rNGAL R1 | CATTGGTCGGTGGGAACAGA |
| rGAPDH F1 | GCCAAAAGGGTCATCATCTC |
| rGAPDH R1 | GGCCATCCACAGTCTTCT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopes, J.A.; Collino, F.; Rodrigues-Ferreira, C.; Sampaio, L.d.S.; Costa-Sarmento, G.; Wendt, C.H.C.; Almeida, F.P.; Miranda, K.R.; Kasai-Brunswick, T.H.; Lindoso, R.S.; et al. Early Effects of Extracellular Vesicles Secreted by Adipose Tissue Mesenchymal Cells in Renal Ischemia Followed by Reperfusion: Mechanisms Rely on a Decrease in Mitochondrial Anion Superoxide Production. Int. J. Mol. Sci. 2022, 23, 2906. https://doi.org/10.3390/ijms23062906
Lopes JA, Collino F, Rodrigues-Ferreira C, Sampaio LdS, Costa-Sarmento G, Wendt CHC, Almeida FP, Miranda KR, Kasai-Brunswick TH, Lindoso RS, et al. Early Effects of Extracellular Vesicles Secreted by Adipose Tissue Mesenchymal Cells in Renal Ischemia Followed by Reperfusion: Mechanisms Rely on a Decrease in Mitochondrial Anion Superoxide Production. International Journal of Molecular Sciences. 2022; 23(6):2906. https://doi.org/10.3390/ijms23062906
Chicago/Turabian StyleLopes, Jarlene A., Federica Collino, Clara Rodrigues-Ferreira, Luzia da Silva Sampaio, Glória Costa-Sarmento, Camila H. C. Wendt, Fernando P. Almeida, Kildare R. Miranda, Tais H. Kasai-Brunswick, Rafael S. Lindoso, and et al. 2022. "Early Effects of Extracellular Vesicles Secreted by Adipose Tissue Mesenchymal Cells in Renal Ischemia Followed by Reperfusion: Mechanisms Rely on a Decrease in Mitochondrial Anion Superoxide Production" International Journal of Molecular Sciences 23, no. 6: 2906. https://doi.org/10.3390/ijms23062906
APA StyleLopes, J. A., Collino, F., Rodrigues-Ferreira, C., Sampaio, L. d. S., Costa-Sarmento, G., Wendt, C. H. C., Almeida, F. P., Miranda, K. R., Kasai-Brunswick, T. H., Lindoso, R. S., & Vieyra, A. (2022). Early Effects of Extracellular Vesicles Secreted by Adipose Tissue Mesenchymal Cells in Renal Ischemia Followed by Reperfusion: Mechanisms Rely on a Decrease in Mitochondrial Anion Superoxide Production. International Journal of Molecular Sciences, 23(6), 2906. https://doi.org/10.3390/ijms23062906