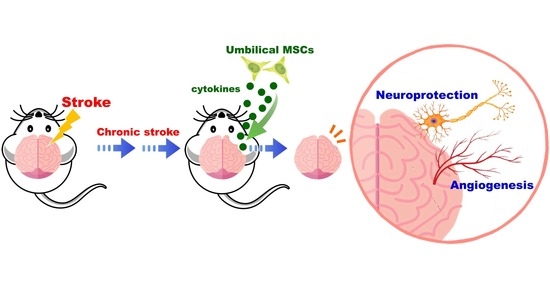
Xenograft of Human Umbilical Mesenchymal Stem Cells Promotes Recovery from Chronic Ischemic Stroke in Rats
Abstract
:1. Introduction
2. Results
2.1. Ischemia–Reperfusion Surgery Causes Cortical Infarction
2.2. HUMSCs Transplantation Improves Motor Function in Rats with Chronic Stroke
2.3. HUMSCs Transplantation Reduces Brain Atrophy in Rats with Chronic Stroke as Assessed by MRI
2.4. HUMSCs Transplantation Preserves Cerebral Cortex in Rats with Chronic Stroke
2.5. HUMSCs Transplantation Helps Neuronal Cell Survival in the Infarcted Brain in Rats with Chronic Stroke
2.6. HUMSCs Transplantation Promotes Angiogenesis in the Infarcted Brain in Rats with Chronic Stroke
2.7. Engrafted HUMSCs Survive and Migrate in the Infarcted Cortex of Rats with Chronic Stroke
2.8. HUMSCs Does Not Differentiate into Neurons and Astrocytes in Rats with Chronic Stroke
3. Discussion
4. Materials and Methods
4.1. Experimental Animals
4.2. Procedures of Middle Cerebral Artery Occlusion (MCAO) and Reperfusion
4.3. Isolation and Culture of Human Umbilical Mesenchymal Stem Cells (HUMSCs)
4.4. Transplantation of HUMSCs
4.5. BisBenzimide-Treated HUMSCs In Vitro
4.6. Experimental Groups
- Normal + Saline group (n = 12): The rats did not receive MCAO and reperfusion, just remove their skulls. On day 14, no treatment was administered except the injection of normal saline into the rat’s cerebral cortex.
- Normal + HUMSCs group (n = 12): The rats did not receive MCAO and reperfusion, just remove their skulls. On day 14, HUMSCs were transplanted into the rat’s cerebral cortex.
- Stroke + Saline group (n = 26): The rats received MCAO and reperfusion. On day 14 post-MCAO, no treatment was administered except the injection of normal saline into the rat’s cerebral cortex.
- Stroke + HUMSCs group (n = 26): The rats received MCAO and reperfusion. HUMSCs were transplanted into the rat’s cerebral cortex on day 14 after MCAO.
4.7. Infarct Cortex Identification
4.8. Magnetic Resonance Imaging (MRI)
4.9. Behavioral Test
4.10. Cylinder Test
4.11. Rotarod Test
4.12. Numbering Brain Cryosections
4.13. Cresyl Violet Staining
4.14. Immunohistochemical (IHC) Staining
4.15. Counting the Number of Neuronal Cells
4.16. Perfusion of the Experimental Animals with Fluorescein Isothiocyanate-Dextran (FITC-dextran)
4.17. Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
| HUMAN RBFOX3: | 310 bp |
| F: 5′-ATCCAGTGGTCGGCGCAGTCTAC-3′ | |
| R: 5′-TACGGGTCGGCAGCTGCGTA-3′ | |
| Human GFAP: | 122 bp |
| F:5′-CTGGAGAGGAAGATTGAGTCGC-3′ | |
| R: 5′-ACGTCAAGCTCCACATGGACCT-3′ | |
| Rat GAPDH: | 160 bp |
| F: 5′-CTCTACCCACGGCAAGTTCAAC-3′ | |
| R: 5′-GGTGAAGACGCCAGTAGACTCCA-3′ | |
| Human GAPDH: | 176 bp |
| F: 5′-TCCTCCACCTTTGACGCT-3′ | |
| R: 5′-CTTCCTCTTGTGCTCTTG-3′ |
4.18. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Benjamin, E.J.; Blaha, M.J.; Chiuve, S.E.; Cushman, M.; Das, S.R.; Deo, R.; De Ferranti, S.D.; Floyd, J.; Fornage, M.; Gillespie, C. Heart disease and stroke statistics-2017 update: A report from the American Heart Association. Circulation 2017, 135, e146–e603. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. The Top 10 Causes of Death. Available online: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed on 9 December 2020).
- Members, W.G.; Lloyd-Jones, D.; Adams, R.J.; Brown, T.M.; Carnethon, M.; Dai, S.; De Simone, G.; Ferguson, T.B.; Ford, E.; Furie, K. Heart disease and stroke statistics—2010 update: A report from the American Heart Association. Circulation 2010, 121, e46–e215. [Google Scholar]
- Kowalska, K.; Krzywoszański, Ł.; Droś, J.; Pasińska, P.; Wilk, A.; Klimkowicz-Mrowiec, A. Early Depression Independently of Other Neuropsychiatric Conditions, Influences Disability and Mortality after Stroke (Research Study-Part of PROPOLIS Study). Biomedicines 2020, 8, 509. [Google Scholar] [CrossRef] [PubMed]
- Carrillo-Mora, P.; Pérez-De la Cruz, V.; Estrada-Cortés, B.; Toussaint-González, P.; Martínez-Cortéz, J.A.; Rodríguez-Barragán, M.; Quinzaños-Fresnedo, J.; Rangel-Caballero, F.; Gamboa-Coria, G.; Sánchez-Vázquez, I.; et al. Serum Kynurenines Correlate with Depressive Symptoms and Disability in Poststroke Patients: A Cross-sectional Study. Neurorehabilt. Neural Repair 2020, 34, 936–944. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Vécsei, L. Editorial of Special Issue “Crosstalk between Depression, Anxiety, and Dementia: Comorbidity in Behavioral Neurology and Neuropsychiatry”. Biomedicines 2021, 9, 517. [Google Scholar] [CrossRef]
- Nishimura, K.; Takata, K. Combination of Drugs and Cell Transplantation: More Beneficial Stem Cell-Based Regenerative Therapies Targeting Neurological Disorders. Int. J. Mol. Sci. 2021, 22, 9047. [Google Scholar] [CrossRef]
- Suda, S.; Nito, C.; Yokobori, S.; Sakamoto, Y.; Nakajima, M.; Sowa, K.; Obinata, H.; Sasaki, K.; Savitz, S.I.; Kimura, K. Recent advances in cell-based therapies for ischemic stroke. Int. J. Mol. Sci. 2020, 21, 6718. [Google Scholar] [CrossRef]
- Fu, Y.S.; Cheng, Y.C.; Lin, M.Y.A.; Cheng, H.; Chu, P.M.; Chou, S.C.; Shih, Y.H.; Ko, M.H.; Sung, M.S. Conversion of human umbilical cord mesenchymal stem cells in Wharton’s jelly to dopaminergic neurons in vitro: Potential therapeutic application for Parkinsonism. Stem Cells 2006, 24, 115–124. [Google Scholar] [CrossRef]
- Ko, T.L.; Fu, Y.Y.; Shin, Y.H.; Lin, Y.H.; Ko, M.H.; Fu, T.W.; Lin, T.Y.; Hsiao, H.S.; Chu, P.M.; Fu, Y.S. A high-efficiency induction of dopaminergic cells from human umbilical mesenchymal stem cells for the treatment of hemiparkinsonian rats. Cell Transplant. 2015, 24, 2251–2262. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.C.; Shih, Y.H.; Ko, M.H.; Hsu, S.Y.; Cheng, H.; Fu, Y.S. Transplantation of human umbilical mesenchymal stem cells from Wharton’s jelly after complete transection of the rat spinal cord. PLoS ONE 2008, 3, e3336. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.C.; Ko, T.L.; Shih, Y.H.; Lin, M.Y.A.; Fu, T.W.; Hsiao, H.S.; Hsu, J.Y.C.; Fu, Y.S. Human umbilical mesenchymal stem cells promote recovery after ischemic stroke. Stroke 2011, 42, 2045–2053. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, P.Y.; Shih, Y.H.; Tseng, Y.J.; Ko, T.L.; Fu, Y.S.; Lin, Y.Y. Xenograft of human umbilical mesenchymal stem cells from Wharton’s jelly as a potential therapy for rat pilocarpine-induced epilepsy. Brain Behav. Immun. 2016, 54, 45–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsai, P.J.; Yeh, C.C.; Huang, W.J.; Min, M.Y.; Huang, T.H.; Ko, T.L.; Huang, P.Y.; Chen, T.H.; Hsu, S.P.; Soong, B.W. Xenografting of human umbilical mesenchymal stem cells from Wharton’s jelly ameliorates mouse spinocerebellar ataxia type 1. Transl. Neurodegener. 2019, 8, 29. [Google Scholar] [CrossRef] [PubMed]
- Tsai, P.C.; Fu, T.W.; Chen, Y.M.A.; Ko, T.L.; Chen, T.H.; Shih, Y.H.; Hung, S.C.; Fu, Y.S. The therapeutic potential of human umbilical mesenchymal stem cells from Wharton’s jelly in the treatment of rat liver fibrosis. Liver Transplant. 2009, 15, 484–495. [Google Scholar] [CrossRef]
- Fan, Y.P.; Hsia, C.C.; Tseng, K.W.; Liao, C.K.; Fu, T.W.; Ko, T.L.; Chiu, M.M.; Shih, Y.H.; Huang, P.Y.; Chiang, Y.C. The Therapeutic Potential of Human Umbilical Mesenchymal Stem Cells from Wharton’s Jelly in the Treatment of Rat Peritoneal Dialysis-Induced Fibrosis. Stem Cells Transl. Med. 2016, 5, 235–247. [Google Scholar] [CrossRef]
- Chu, K.A.; Wang, S.Y.; Yeh, C.C.; Fu, T.W.; Fu, Y.Y.; Ko, T.L.; Chiu, M.M.; Chen, T.H.; Tsai, P.J.; Fu, Y.S. Reversal of bleomycin-induced rat pulmonary fibrosis by a xenograft of human umbilical mesenchymal stem cells from Wharton’s jelly. Theranostics 2019, 9, 6646–6664. [Google Scholar] [CrossRef]
- Chu, K.A.; Yeh, C.C.; Kuo, F.H.; Lin, W.R.; Hsu, C.W.; Chen, T.H.; Fu, Y.S. Comparison of reversal of rat pulmonary fibrosis of nintedanib, pirfenidone, and human umbilical mesenchymal stem cells from Wharton’s jelly. Stem Cell Res. Ther. 2020, 11, 513. [Google Scholar] [CrossRef]
- Chao, K.C.; Chao, K.F.; Fu, Y.S.; Liu, S.H. Islet-like clusters derived from mesenchymal stem cells in Wharton’s Jelly of the human umbilical cord for transplantation to control type 1 diabetes. PLoS ONE 2008, 3, e1451. [Google Scholar] [CrossRef] [Green Version]
- Fu, Y.S.; Lu, C.H.; Chu, K.A.; Yeh, C.C.; Chiang, T.L.; Ko, T.L.; Chiu, M.M.; Chen, C.F. Xenograft of human umbilical mesenchymal stem cells from Wharton’s jelly differentiating into osteocytes and reducing osteoclast activity reverses osteoporosis in ovariectomized rats. Cell Transplant. 2018, 27, 194–208. [Google Scholar] [CrossRef]
- Chen, C.F.; Chen, Y.C.; Fu, Y.S.; Tsai, S.W.; Wu, P.K.; Chen, C.M.; Chang, M.C.; Chen, W.M. Characterization of Osteogenesis and Chondrogenesis of Human Decellularized Allogeneic Bone with Mesenchymal Stem Cells Derived from Bone Marrow, Adipose Tissue, and Wharton’s Jelly. Int. J. Mol. Sci. 2021, 22, 8987. [Google Scholar] [CrossRef]
- Dos Santos, A.d.V.; da Costa Reis, J.; Paredes, B.D.; Moraes, L.; Giraldi-Guimarães, A.; Mendez-Otero, R. Therapeutic window for treatment of cortical ischemia with bone marrow-derived cells in rats. Brain Res. 2010, 1306, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Darsalia, V.; Allison, S.J.; Cusulin, C.; Monni, E.; Kuzdas, D.; Kallur, T.; Lindvall, O.; Kokaia, Z. Cell number and timing of transplantation determine survival of human neural stem cell grafts in stroke-damaged rat brain. J. Cereb. Blood Flow Metab. 2011, 31, 235–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishizaka, S.; Horie, N.; Satoh, K.; Fukuda, Y.; Nishida, N.; Nagata, I. Intra-arterial cell transplantation provides timing-dependent cell distribution and functional recovery after stroke. Stroke 2013, 44, 720–726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janowski, M.; Lyczek, A.; Engels, C.; Xu, J.; Lukomska, B.; Bulte, J.W.; Walczak, P. Cell size and velocity of injection are major determinants of the safety of intracarotid stem cell transplantation. J. Cereb. Blood Flow Metab. 2013, 33, 921–927. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Frutos, B.; Otero-Ortega, L.; Gutiérrez-Fernández, M.; Fuentes, B.; Ramos-Cejudo, J.; Díez-Tejedor, E. Stem Cell Therapy and Administration Routes After Stroke. Transl. Stroke Res. 2016, 7, 378–387. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Y.; Zhang, C.; Chopp, M.; Gosiewska, A.; Hong, K. Delayed administration of human umbilical tissue-derived cells improved neurological functional recovery in a rodent model of focal ischemia. Stroke 2011, 42, 1437–1444. [Google Scholar] [CrossRef] [Green Version]
- Hamblin, M.H.; Lee, J.P. Neural Stem Cells for Early Ischemic Stroke. Int. J. Mol. Sci. 2021, 22, 7703. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, G.; Gu, Y.; Guo, X. Meta-Analysis and Systematic Review of Neural Stem Cells therapy for experimental ischemia stroke in preclinical studies. Sci. Rep. 2016, 6, 32291. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Z.; Wang, L.; Qu, M.; Liang, H.; Li, W.; Li, Y.; Deng, L.; Zhang, Z.; Yang, G.Y. Mesenchymal stem cells attenuate blood-brain barrier leakage after cerebral ischemia in mice. J. Neuroinflamm. 2018, 15, 135. [Google Scholar] [CrossRef] [Green Version]
- Yanar, K.; Molbay, M.; Özaydın-Goksu, E.; Unek, G.; Cetindağ, E.; Unal, A.; Korgun, E.T. Contribution of Human Trophoblast Progenitor Cells to Neurogenesis in Rat Focal Cerebral Ischemia Model. Brain Inj. 2021, 35, 850–862. [Google Scholar] [CrossRef]
- Qin, M.; Chen, R.; Li, H.; Liang, H.; Xue, Q.; Li, F.; Chen, Y.; Zhang, X. Direct Reprogramming of Human Amniotic Fluid Stem Cells by OCT4 and Application in Repairing of Cerebral Ischemia Damage. Int. J. Biol. Sci. 2016, 12, 558–568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.C.; Liu, B.S.; Shen, C.C.; Lin, C.H.; Chiao, M.T.; Cheng, H.C. Transplantation of adipose tissue-derived stem cells for treatment of focal cerebral ischemia. Curr. Neurovasc. Res. 2011, 8, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Grandvuillemin, I.; Garrigue, P.; Ramdani, A.; Boubred, F.; Simeoni, U.; Dignat-George, F.; Sabatier, F.; Guillet, B. Long-Term Recovery After Endothelial Colony-Forming Cells or Human Umbilical Cord Blood Cells Administration in a Rat Model of Neonatal Hypoxic-Ischemic Encephalopathy. Stem Cells Transl. Med. 2017, 6, 1987–1996. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Tang, H.; Zhu, J.; Zhang, J.H. Transplanting Mesenchymal Stem Cells for Treatment of Ischemic Stroke. Cell Transplant. 2018, 27, 1825–1834. [Google Scholar] [CrossRef] [PubMed]
- Arvidsson, A.; Collin, T.; Kirik, D.; Kokaia, Z.; Lindvall, O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat. Med. 2002, 8, 963–970. [Google Scholar] [CrossRef] [Green Version]
- Boese, A.C.; Le, Q.S.E.; Pham, D.; Hamblin, M.H.; Lee, J.P. Neural stem cell therapy for subacute and chronic ischemic stroke. Stem Cell Res. Ther. 2018, 9, 154. [Google Scholar] [CrossRef]
- Parent, J.M.; Vexler, Z.S.; Gong, C.; Derugin, N.; Ferriero, D.M. Rat forebrain neurogenesis and striatal neuron replacement after focal stroke. Ann. Neurol. 2002, 52, 802–813. [Google Scholar] [CrossRef]
- Gu, N.; Rao, C.; Tian, Y.; Di, Z.; Liu, Z.; Chang, M.; Lei, H. Anti-inflammatory and antiapoptotic effects of mesenchymal stem cells transplantation in rat brain with cerebral ischemia. J. Stroke Cerebrovasc. Dis. 2014, 23, 2598–2606. [Google Scholar] [CrossRef]
- Tian, L.; Zhu, W.; Liu, Y.; Gong, Y.; Lv, A.; Wang, Z.; Ding, X.; Li, S.; Fu, Y.; Lin, Y.; et al. Neural Stem Cells Transfected with Leukemia Inhibitory Factor Promote Neuroprotection in a Rat Model of Cerebral Ischemia. Neurosci. Bull. 2019, 35, 901–908. [Google Scholar] [CrossRef]
- Zhou, L.; Lin, Q.; Wang, P.; Yao, L.; Leong, K.; Tan, Z.; Huang, Z. Enhanced neuroprotective efficacy of bone marrow mesenchymal stem cells co-overexpressing BDNF and VEGF in a rat model of cardiac arrest-induced global cerebral ischemia. Cell Death Dis. 2017, 8, e2774. [Google Scholar] [CrossRef]
- Asgari Taei, A.; Nasoohi, S.; Hassanzadeh, G.; Kadivar, M.; Dargahi, L.; Farahmandfar, M. Enhancement of angiogenesis and neurogenesis by intracerebroventricular injection of secretome from human embryonic stem cell-derived mesenchymal stem cells in ischemic stroke model. Biomed. Pharmacother. 2021, 140, 111709. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Chu, L.; Ren, C.; Wang, J.; Sun, S.; Li, T.; Yin, Y. Enhanced Migration of Bone Marrow-Derived Mesenchymal Stem Cells with Tetramethylpyrazine and Its Synergistic Effect on Angiogenesis and Neurogenesis After Cerebral Ischemia in Rats. Stem Cells Dev. 2019, 28, 871–881. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.; Chang, M.S.; Koh, S.H.; Choi, Y.K. Repair Mechanisms of the Neurovascular Unit after Ischemic Stroke with a Focus on VEGF. Int. J. Mol. Sci. 2021, 22, 8543. [Google Scholar] [CrossRef] [PubMed]
- Neal, E.G.; Acosta, S.A.; Kaneko, Y.; Ji, X.; Borlongan, C.V. Regulatory T-cells within bone marrow-derived stem cells actively confer immunomodulatory and neuroprotective effects against stroke. J. Cereb. Blood Flow Metab. 2019, 39, 1750–1758. [Google Scholar] [CrossRef]
- Shi, K.; Tian, D.C.; Li, Z.G.; Ducruet, A.F.; Lawton, M.T.; Shi, F.D. Global brain inflammation in stroke. Lancet Neurol. 2019, 18, 1058–1066. [Google Scholar] [CrossRef]
- Xia, Y.; Hu, G.; Chen, Y.; Yuan, J.; Zhang, J.; Wang, S.; Li, Q.; Wang, Y.; Deng, Z. Embryonic Stem Cell Derived Small Extracellular Vesicles Modulate Regulatory T Cells to Protect against Ischemic Stroke. ACS Nano 2021, 15, 7370–7385. [Google Scholar] [CrossRef]
- Yu, Z.; Wenyan, T.; Xuewen, S.; Baixiang, D.; Qian, W.; Zhaoyan, W.; Yinxiang, Y.; Suqing, Q.; Zuo, L. Immunological effects of the intraparenchymal administration of allogeneic and autologous adipose-derived mesenchymal stem cells after the acute phase of middle cerebral artery occlusion in rats. J. Transl. Med. 2018, 16, 339. [Google Scholar] [CrossRef]
- Gutiérrez-Fernández, M.; Rodríguez-Frutos, B.; Ramos-Cejudo, J.; Otero-Ortega, L.; Fuentes, B.; Vallejo-Cremades, M.T.; Sanz-Cuesta, B.E.; Díez-Tejedor, E. Comparison between xenogeneic and allogeneic adipose mesenchymal stem cells in the treatment of acute cerebral infarct: Proof of concept in rats. J. Transl. Med. 2015, 13, 46. [Google Scholar] [CrossRef] [Green Version]
- Jansen of Lorkeers, S.J.; Eding, J.E.; Vesterinen, H.M.; van der Spoel, T.I.; Sena, E.S.; Duckers, H.J.; Doevendans, P.A.; Macleod, M.R.; Chamuleau, S.A. Similar effect of autologous and allogeneic cell therapy for ischemic heart disease: Systematic review and meta-analysis of large animal studies. Circ. Res. 2015, 116, 80–86. [Google Scholar] [CrossRef]

 indicates the first injection site.
indicates the first injection site.
 indicates the first injection site.
indicates the first injection site.




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, Y.-S.; Yeh, C.-C.; Chu, P.-M.; Chang, W.-H.; Lin, M.-Y.A.; Lin, Y.-Y. Xenograft of Human Umbilical Mesenchymal Stem Cells Promotes Recovery from Chronic Ischemic Stroke in Rats. Int. J. Mol. Sci. 2022, 23, 3149. https://doi.org/10.3390/ijms23063149
Fu Y-S, Yeh C-C, Chu P-M, Chang W-H, Lin M-YA, Lin Y-Y. Xenograft of Human Umbilical Mesenchymal Stem Cells Promotes Recovery from Chronic Ischemic Stroke in Rats. International Journal of Molecular Sciences. 2022; 23(6):3149. https://doi.org/10.3390/ijms23063149
Chicago/Turabian StyleFu, Yu-Show, Chang-Ching Yeh, Pei-Ming Chu, Wen-Hsing Chang, Maan-Yuh Anya Lin, and Yung-Yang Lin. 2022. "Xenograft of Human Umbilical Mesenchymal Stem Cells Promotes Recovery from Chronic Ischemic Stroke in Rats" International Journal of Molecular Sciences 23, no. 6: 3149. https://doi.org/10.3390/ijms23063149
APA StyleFu, Y. -S., Yeh, C. -C., Chu, P. -M., Chang, W. -H., Lin, M. -Y. A., & Lin, Y. -Y. (2022). Xenograft of Human Umbilical Mesenchymal Stem Cells Promotes Recovery from Chronic Ischemic Stroke in Rats. International Journal of Molecular Sciences, 23(6), 3149. https://doi.org/10.3390/ijms23063149