Susceptibility of Female Mice to the Dietary Omega-3/Omega-6 Fatty-Acid Ratio: Effects on Adult Hippocampal Neurogenesis and Glia
Abstract
:1. Introduction
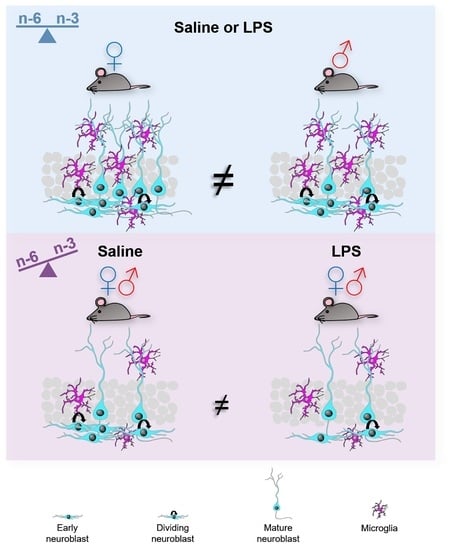
2. Results
2.1. Dietary n-6/n-3 PUFA Content Did Not Affect LPS-Induced Weight Alterations
2.2. Long-Term Effects of Dietary n-6/n-3 PUFA Balance on DG Size
2.3. Effects of Dietary n-6/n-3 PUFA Balance in Adult Hippocampal Neurogenesis
2.4. Effects of Dietary n-6/n-3 PUFA Balance on DG Microglia
2.5. Effects of Dietary n-6/n-3 PUFA Balance in Astrocytes
2.6. Multivariate Analysis of the Data
3. Discussion
4. Materials and Methods
4.1. Mice
4.2. Diets
4.3. LPS and BrdU Administration
4.4. Transcardial Perfusion and Brain Tissue Collection
4.5. Immunofluorescence
4.6. Image Collection
4.7. Cell Quantification
4.8. Analysis of the Extension of Doublecortin Staining, Microglia Surveilled Volume, and Colocalization
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Labrousse, V.F.; Leyrolle, Q.; Amadieu, C.; Aubert, A.; Sere, A.; Coutureau, E.; Grégoire, S.; Bretillon, L.; Pallet, V.; Gressens, P.; et al. Dietary Omega-3 Deficiency Exacerbates Inflammation and Reveals Spatial Memory Deficits in Mice Exposed to Lipopolysaccharide during Gestation. Brain Behav. Immun. 2018, 73, 427–440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madore, C.; Leyrolle, Q.; Morel, L.; Rossitto, M.; Greenhalgh, A.D.; Delpech, J.C.; Martinat, M.; Bosch-Bouju, C.; Bourel, J.; Rani, B.; et al. Essential Omega-3 Fatty Acids Tune Microglial Phagocytosis of Synaptic Elements in the Mouse Developing Brain. Nat. Commun. 2020, 11, 6133. [Google Scholar] [CrossRef] [PubMed]
- Zou, R.; El Marroun, H.; Voortman, T.; Hillegers, M.; White, T.; Tiemeier, H. Maternal Polyunsaturated Fatty Acids during Pregnancy and Offspring Brain Development in Childhood. Am. J. Clin. Nutr. 2021, 114, 124–133. [Google Scholar] [CrossRef]
- Simopoulos, A.P. The Importance of the Ratio of Omega-6/Omega-3 Essential Fatty Acids. Biomed. Pharmacother. 2002, 56, 365–379. [Google Scholar] [CrossRef]
- Baker, E.J.; Miles, E.A.; Burdge, G.C.; Yaqoob, P.; Calder, P.C. Metabolism and Functional Effects of Plant-Derived Omega-3 Fatty Acids in Humans. Prog. Lipid Res. 2016, 64, 30–56. [Google Scholar] [CrossRef]
- Yavin, E.; Himovichi, E.; Eilam, R. Delayed Cell Migration in the Developing Rat Brain Following Maternal Omega 3 Alpha Linolenic Acid Dietary Deficiency. Neuroscience 2009, 162, 1011–1022. [Google Scholar] [CrossRef]
- Rodríguez-Iglesias, N.; Sierra, A.; Valero, J. Rewiring of Memory Circuits: Connecting Adult Newborn Neurons with the Help of Microglia. Front. Cell Dev. Biol. 2019, 7, 24. [Google Scholar] [CrossRef] [Green Version]
- Beltz, B.S.; Tlusty, M.F.; Benton, J.L.; Sandeman, D.C. Omega-3 Fatty Acids Upregulate Adult Neurogenesis. Neurosci. Lett. 2007, 415, 154–158. [Google Scholar] [CrossRef] [Green Version]
- Dyall, S.C.; Michael, G.J.; Michael-Titus, A.T. Omega-3 Fatty Acids Reverse Age-Related Decreases in Nuclear Receptors and Increase Neurogenesis in Old Rats. J. Neurosci. Res. 2010, 88, 2091–2102. [Google Scholar] [CrossRef]
- Sartori, A.C.; Vance, D.E.; Slater, L.Z.; Crowe, M. The Impact of Inflammation on Cognitive Function in Older Adults: Implications for Healthcare Practice and Research. J. Neurosci. Nurs. 2012, 44, 206–217. [Google Scholar] [CrossRef] [Green Version]
- Marsland, A.L.; Gianaros, P.J.; Kuan, D.C.-H.; Sheu, L.K.; Krajina, K.; Manuck, S.B. Brain Morphology Links Systemic Inflammation to Cognitive Function in Midlife Adults. Brain Behav. Immun. 2015, 48, 195–204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feinkohl, I.; Winterer, G.; Spies, C.D.; Pischon, T. Cognitive Reserve and the Risk of Postoperative Cognitive Dysfunction. Dtsch. Ärzteblatt Int. 2017, 114, 110–117. [Google Scholar] [CrossRef] [Green Version]
- Valero, J.; Bernardino, L.; Cardoso, F.; Silva, A.P.; Fontes-Ribeiro, C.; Ambrósio, A.F.; Malva, J.O. Impact of Neuroinflammation on Hippocampal Neurogenesis: Relevance to Aging and Alzheimer’s Disease. J. Alzheimers Dis. 2017, 60, S161–S168. [Google Scholar] [CrossRef] [PubMed]
- Paris, I.; Savage, J.C.; Escobar, L.; Abiega, O.; Gagnon, S.; Hui, C.-W.; Tremblay, M.-È.; Sierra, A.; Valero, J. ProMoIJ: A New Tool for Automatic Three-Dimensional Analysis of Microglial Process Motility. Glia 2018, 66, 828–845. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Aparicio, I.; Paris, I.; Sierra-Torre, V.; Plaza-Zabala, A.; Rodríguez-Iglesias, N.; Márquez-Ropero, M.; Beccari, S.; Huguet, P.; Abiega, O.; Alberdi, E.; et al. Microglia Actively Remodel Adult Hippocampal Neurogenesis through the Phagocytosis Secretome. J. Neurosci. 2020, 40, 1453–1482. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, S.; Svahn, S.L.; Johansson, M.E. Effects of Omega-3 Fatty Acids on Immune Cells. Int. J. Mol. Sci. 2019, 20, 5028. [Google Scholar] [CrossRef] [Green Version]
- Djuricic, I.; Calder, P.C. Beneficial Outcomes of Omega-6 and Omega-3 Polyunsaturated Fatty Acids on Human Health: An Update for 2021. Nutrients 2021, 13, 2421. [Google Scholar] [CrossRef]
- Sears, B.; Saha, A.K. Dietary Control of Inflammation and Resolution. Front. Nutr. 2021, 8, 508. [Google Scholar] [CrossRef]
- Perez, S.E.; Berg, B.M.; Moore, K.A.; He, B.; Counts, S.E.; Fritz, J.J.; Hu, Y.-S.; Lazarov, O.; Lah, J.J.; Mufson, E.J. DHA Diet Reduces AD Pathology in Young APPswe/PS1ΔE9 Transgenic Mice: Possible Gender Effects. J. Neurosci. Res. 2010, 88, 1026–1040. [Google Scholar] [CrossRef] [Green Version]
- da Costa, A.E.M.; Gomes, N.S.; Gadelha Filho, C.V.J.; Linhares, M.G.O.E.S.; da Costa, R.O.; Chaves Filho, A.J.M.; Cordeiro, R.C.; Vasconcelos, G.S.; da Silva, F.E.R.; da Araujo, T.S.; et al. Sex Influences in the Preventive Effects of Peripubertal Supplementation with N-3 Polyunsaturated Fatty Acids in Mice Exposed to the Two-Hit Model of Schizophrenia. Eur. J. Pharmacol. 2021, 897, 173949. [Google Scholar] [CrossRef]
- Valero, J.; Mastrella, G.; Neiva, I.; Sánchez, S.; Malva, J.O. Long-Term Effects of an Acute and Systemic Administration of LPS on Adult Neurogenesis and Spatial Memory. Front. Neurosci. 2014, 8, 83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dantzer, R.; O’Connor, J.C.; Freund, G.G.; Johnson, R.W.; Kelley, K.W. From Inflammation to Sickness and Depression: When the Immune System Subjugates the Brain. Nat. Rev. Neurosci. 2008, 9, 46–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ninkovic, J.; Mori, T.; Götz, M. Distinct Modes of Neuron Addition in Adult Mouse Neurogenesis. J. Neurosci. 2007, 27, 10906–10911. [Google Scholar] [CrossRef] [Green Version]
- Imayoshi, I.; Sakamoto, M.; Ohtsuka, T.; Takao, K.; Miyakawa, T.; Yamaguchi, M.; Mori, K.; Ikeda, T.; Itohara, S.; Kageyama, R. Roles of Continuous Neurogenesis in the Structural and Functional Integrity of the Adult Forebrain. Nat. Neurosci. 2008, 11, 1153–1161. [Google Scholar] [CrossRef]
- Encinas, J.M.; Enikolopov, G. Identifying and Quantitating Neural Stem and Progenitor Cells in the Adult Brain. In Methods in Cell Biology; Elsevier: Amsterdam, The Netherlands, 2008; Volume 85, pp. 243–272. ISBN 978-0-12-372558-5. [Google Scholar]
- Plümpe, T.; Ehninger, D.; Steiner, B.; Klempin, F.; Jessberger, S.; Brandt, M.; Römer, B.; Rodriguez, G.; Kronenberg, G.; Kempermann, G. Variability of Doublecortin-Associated Dendrite Maturation in Adult Hippocampal Neurogenesis Is Independent of the Regulation of Precursor Cell Proliferation. BMC Neurosci. 2006, 7, 77. [Google Scholar] [CrossRef] [Green Version]
- Brown, J.P.; Couillard-Després, S.; Cooper-Kuhn, C.M.; Winkler, J.; Aigner, L.; Kuhn, H.G. Transient Expression of Doublecortin during Adult Neurogenesis. J. Comp. Neurol. 2003, 467, 1–10. [Google Scholar] [CrossRef]
- Kempermann, G.; Jessberger, S.; Steiner, B.; Kronenberg, G. Milestones of Neuronal Development in the Adult Hippocampus. Trends Neurosci. 2004, 27, 447–452. [Google Scholar] [CrossRef]
- Lemaire, V.; Tronel, S.; Montaron, M.-F.; Fabre, A.; Dugast, E.; Abrous, D.N. Long-Lasting Plasticity of Hippocampal Adult-Born Neurons. J. Neurosci. 2012, 32, 3101–3108. [Google Scholar] [CrossRef] [Green Version]
- Cole, J.D.; Espinueva, D.F.; Seib, D.R.; Ash, A.M.; Cooke, M.B.; Cahill, S.P.; O’Leary, T.P.; Kwan, S.S.; Snyder, J.S. Adult-Born Hippocampal Neurons Undergo Extended Development and Are Morphologically Distinct from Neonatally-Born Neurons. J. Neurosci. 2020, 40, 5740–5756. [Google Scholar] [CrossRef]
- Sierra, A.; Abiega, O.; Shahraz, A.; Neumann, H. Janus-Faced Microglia: Beneficial and Detrimental Consequences of Microglial Phagocytosis. Front. Cell. Neurosci. 2013, 7, 6. [Google Scholar] [CrossRef] [Green Version]
- Chugh, D.; Ekdahl, C.T. Interactions Between Microglia and Newly Formed Hippocampal Neurons in Physiological and Seizure-Induced Inflammatory Environment. Brain Plast. 2016, 1, 215–221. [Google Scholar] [CrossRef] [Green Version]
- Giovannoni, F.; Quintana, F.J. The Role of Astrocytes in CNS Inflammation. Trends Immunol. 2020, 41, 805–819. [Google Scholar] [CrossRef]
- Beccari, S.; Valero, J.; Maletic-Savatic, M.; Sierra, A. A Simulation Model of Neuroprogenitor Proliferation Dynamics Predicts Age-Related Loss of Hippocampal Neurogenesis but Not Astrogenesis. Sci. Rep. 2017, 7, 16528. [Google Scholar] [CrossRef] [Green Version]
- Antonietta Ajmone-Cat, M.; Lavinia Salvatori, M.; De Simone, R.; Mancini, M.; Biagioni, S.; Bernardo, A.; Cacci, E.; Minghetti, L. Docosahexaenoic Acid Modulates Inflammatory and Antineurogenic Functions of Activated Microglial Cells. J. Neurosci. Res. 2012, 90, 575–587. [Google Scholar] [CrossRef]
- Dinel, A.L.; Rey, C.; Baudry, C.; Fressange-Mazda, C.; Le Ruyet, P.; Nadjar, A.; Pallet, P.; Joffre, C.; Layé, S. Enriched Dairy Fat Matrix Diet Prevents Early Life Lipopolysaccharide-Induced Spatial Memory Impairment at Adulthood. Prostaglandins Leukot. Essent. Fat. Acids 2016, 113, 9–18. [Google Scholar] [CrossRef]
- Tyrtyshnaia, A.; Bondar, A.; Konovalova, S.; Sultanov, R.; Manzhulo, I. N-Docosahexanoylethanolamine Reduces Microglial Activation and Improves Hippocampal Plasticity in a Murine Model of Neuroinflammation. Int. J. Mol. Sci. 2020, 21, 9703. [Google Scholar] [CrossRef]
- Lo Van, A.; Hachem, M.; Lagarde, M.; Bernoud-Hubac, N. Omega-3 Docosahexaenoic Acid Is a Mediator of Fate-Decision of Adult Neural Stem Cells. Int. J. Mol. Sci. 2019, 20, 4240. [Google Scholar] [CrossRef] [Green Version]
- Ma, D.; Zhang, M.; Larsen, C.P.; Xu, F.; Hua, W.; Yamashima, T.; Mao, Y.; Zhou, L. DHA Promotes the Neuronal Differentiation of Rat Neural Stem Cells Transfected with GPR40 Gene. Brain Res. 2010, 1330, 1–8. [Google Scholar] [CrossRef]
- Sakayori, N.; Maekawa, M.; Numayama-Tsuruta, K.; Katura, T.; Moriya, T.; Osumi, N. Distinctive Effects of Arachidonic Acid and Docosahexaenoic Acid on Neural Stem /Progenitor Cells. Genes Cells 2011, 16, 778–790. [Google Scholar] [CrossRef]
- Katakura, M.; Hashimoto, M.; Okui, T.; Shahdat, H.M.; Matsuzaki, K.; Shido, O. Omega-3 Polyunsaturated Fatty Acids Enhance Neuronal Differentiation in Cultured Rat Neural Stem Cells. Stem Cells Int. 2013, 2013, e490476. [Google Scholar] [CrossRef] [Green Version]
- Rashid, M.A.; Katakura, M.; Kharebava, G.; Kevala, K.; Kim, H.-Y. N-Docosahexaenoylethanolamine Is a Potent Neurogenic Factor for Neural Stem Cell Differentiation. J. Neurochem. 2013, 125, 869–884. [Google Scholar] [CrossRef] [Green Version]
- Grundy, T.; Toben, C.; Jaehne, E.J.; Corrigan, F.; Baune, B.T. Long-Term Omega-3 Supplementation Modulates Behavior, Hippocampal Fatty Acid Concentration, Neuronal Progenitor Proliferation and Central TNF-α Expression in 7 Month Old Unchallenged Mice. Front. Cell. Neurosci. 2014, 8, 399. [Google Scholar] [CrossRef] [Green Version]
- Varela-Nallar, L.; Inestrosa, N.C. Wnt Signaling in the Regulation of Adult Hippocampal Neurogenesis. Front. Cell. Neurosci. 2013, 7, 100. [Google Scholar] [CrossRef] [Green Version]
- Zhao, W.-N.; Hylton, N.K.; Wang, J.; Chindavong, P.S.; Alural, B.; Kurtser, I.; Subramanian, A.; Mazitschek, R.; Perlis, R.H.; Haggarty, S.J. Activation of WNT and CREB Signaling Pathways in Human Neuronal Cells in Response to the Omega-3 Fatty Acid Docosahexaenoic Acid (DHA). Mol. Cell. Neurosci. 2019, 99, 103386. [Google Scholar] [CrossRef]
- Ortega-Martínez, S. A New Perspective on the Role of the CREB Family of Transcription Factors in Memory Consolidation via Adult Hippocampal Neurogenesis. Front. Mol. Neurosci. 2015, 8, 46. [Google Scholar] [CrossRef] [Green Version]
- Elinder, F.; Liin, S.I. Actions and Mechanisms of Polyunsaturated Fatty Acids on Voltage-Gated Ion Channels. Front. Physiol. 2017, 8, 43. [Google Scholar] [CrossRef] [Green Version]
- Di Miceli, M.; Bosch-Bouju, C.; Layé, S. PUFA and Their Derivatives in Neurotransmission and Synapses: A New Hallmark of Synaptopathies. Proc. Nutr. Soc. 2020, 79, 388–403. [Google Scholar] [CrossRef]
- Aryal, S.; Hussain, S.; Drevon, C.A.; Nagelhus, E.; Hvalby, Ø.; Jensen, V.; Walaas, S.I.; Davanger, S. Omega-3 Fatty Acids Regulate Plasticity in Distinct Hippocampal Glutamatergic Synapses. Eur. J. Neurosci. 2019, 49, 40–50. [Google Scholar] [CrossRef]
- Rey, C.; Nadjar, A.; Joffre, F.; Amadieu, C.; Aubert, A.; Vaysse, C.; Pallet, V.; Layé, S.; Joffre, C. Maternal N-3 Polyunsaturated Fatty Acid Dietary Supply Modulates Microglia Lipid Content in the Offspring. Prostaglandins Leukot. Essent. Fatty Acids 2018, 133, 1–7. [Google Scholar] [CrossRef]
- Serhan, C.N.; Chiang, N.; Dalli, J. New Pro-Resolving n-3 Mediators Bridge Resolution of Infectious Inflammation to Tissue Regeneration. Mol. Asp. Med. 2018, 64, 1–17. [Google Scholar] [CrossRef]
- Medeiros, R.; Kitazawa, M.; Passos, G.F.; Baglietto-Vargas, D.; Cheng, D.; Cribbs, D.H.; LaFerla, F.M. Aspirin-Triggered Lipoxin A4 Stimulates Alternative Activation of Microglia and Reduces Alzheimer Disease–Like Pathology in Mice. Am. J. Pathol. 2013, 182, 1780–1789. [Google Scholar] [CrossRef] [Green Version]
- Dunn, H.C.; Ager, R.R.; Baglietto-Vargas, D.; Cheng, D.; Kitazawa, M.; Cribbs, D.H.; Medeiros, R. Restoration of Lipoxin A4 Signaling Reduces Alzheimer’s Disease-Like Pathology in the 3xTg-AD Mouse Model. J. Alzheimers Dis. 2015, 43, 893–903. [Google Scholar] [CrossRef] [Green Version]
- Martini, A.C.; Berta, T.; Forner, S.; Chen, G.; Bento, A.F.; Ji, R.-R.; Rae, G.A. Lipoxin A4 Inhibits Microglial Activation and Reduces Neuroinflammation and Neuropathic Pain after Spinal Cord Hemisection. J. Neuroinflamm. 2016, 13, 75. [Google Scholar] [CrossRef] [Green Version]
- Layé, S.; Nadjar, A.; Joffre, C.; Bazinet, R.P. Anti-Inflammatory Effects of Omega-3 Fatty Acids in the Brain: Physiological Mechanisms and Relevance to Pharmacology. Pharmacol. Rev. 2018, 70, 12–38. [Google Scholar] [CrossRef]
- Madore, C.; Nadjar, A.; Delpech, J.-C.; Sere, A.; Aubert, A.; Portal, C.; Joffre, C.; Layé, S. Nutritional N-3 PUFAs Deficiency during Perinatal Periods Alters Brain Innate Immune System and Neuronal Plasticity-Associated Genes. Brain Behav. Immun. 2014, 41, 22–31. [Google Scholar] [CrossRef]
- Childs, C.E. Sex Hormones and N-3 Fatty Acid Metabolism. Proc. Nutr. Soc. 2020, 79, 219–224. [Google Scholar] [CrossRef]
- Yagi, S.; Galea, L.A.M. Sex Differences in Hippocampal Cognition and Neurogenesis. Neuropsychopharmacology 2019, 44, 200–213. [Google Scholar] [CrossRef] [Green Version]
- Lagace, D.C.; Fischer, S.J.; Eisch, A.J. Gender and Endogenous Levels of Estradiol Do Not Influence Adult Hippocampal Neurogenesis in Mice. Hippocampus 2007, 17, 175–180. [Google Scholar] [CrossRef]
- Sasmono, R.T.; Oceandy, D.; Pollard, J.W.; Tong, W.; Pavli, P.; Wainwright, B.J.; Ostrowski, M.C.; Himes, S.R.; Hume, D.A. A Macrophage Colony-Stimulating Factor Receptor–Green Fluorescent Protein Transgene Is Expressed throughout the Mononuclear Phagocyte System of the Mouse. Blood 2003, 101, 1155–1163. [Google Scholar] [CrossRef] [Green Version]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An Open-Source Platform for Biological-Image Analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [Green Version]
- Demšar, J.; Curk, T.; Erjavec, A.; Gorup, C.; Hocevar, T.; Milutinovic, M.; Možina, M.; Polajnar, M.; Toplak, M.; Staric, A.; et al. Orange: Data Mining Toolbox in Python. J. Mach. Learn. Res. 2013, 14, 2349–2353. [Google Scholar]
- Quinlan, J.R. Induction of Decision Trees. Mach. Learn. 1986, 1, 81–106. [Google Scholar] [CrossRef] [Green Version]










| Ingredients | Amount |
|---|---|
| Casein | 180 |
| Cornstarch | 460 |
| Sucrose | 230 |
| Cellulose | 20 |
| Fat (1) | 50 |
| Mineral mix (2) | 50 |
| Vitamin mix (3) | 10 |
| DIET | Ω6/Ω3 Balanced | Ω6/Ω3 Deficient |
|---|---|---|
| 16:0 | 22.6 | 6.2 |
| 18:0 | 3.3 | 4.4 |
| Other saturated FAs | 1.8 | 1.6 |
| Total saturated FAs | 27.7 | 12.2 |
| 16:1 Ω7 | 0.2 | 0.1 |
| 18:1 Ω9 | 57.9 | 26.0 |
| 18:1 Ω7 | 1.5 | 0.9 |
| Other monounsaturated FAs | 0.4 | 0.2 |
| Total monounsaturated FAs | 60.0 | 27.2 |
| 18:2 Ω6 (LA) | 10.6 | 60.5 |
| 20:4 Ω6 (AA) | ND | ND |
| Total Ω6 PUFAs | 10.7 | 60.5 |
| 18:3 Ω3 (ALA) | 1.6 | 0.1 |
| 18:4 Ω3 | ND | ND |
| 20:5 Ω3 | ND | ND |
| 22:5 Ω3 | ND | ND |
| 22:6 Ω3 (DHA) | ND | ND |
| Total Ω3 PUFAs | 1.6 | 0.1 |
| Total PUFAs | 12.3 | 60.6 |
| Ω6/Ω3 ratio | 6.7 | >500 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Iglesias, N.; Nadjar, A.; Sierra, A.; Valero, J. Susceptibility of Female Mice to the Dietary Omega-3/Omega-6 Fatty-Acid Ratio: Effects on Adult Hippocampal Neurogenesis and Glia. Int. J. Mol. Sci. 2022, 23, 3399. https://doi.org/10.3390/ijms23063399
Rodríguez-Iglesias N, Nadjar A, Sierra A, Valero J. Susceptibility of Female Mice to the Dietary Omega-3/Omega-6 Fatty-Acid Ratio: Effects on Adult Hippocampal Neurogenesis and Glia. International Journal of Molecular Sciences. 2022; 23(6):3399. https://doi.org/10.3390/ijms23063399
Chicago/Turabian StyleRodríguez-Iglesias, Noelia, Agnes Nadjar, Amanda Sierra, and Jorge Valero. 2022. "Susceptibility of Female Mice to the Dietary Omega-3/Omega-6 Fatty-Acid Ratio: Effects on Adult Hippocampal Neurogenesis and Glia" International Journal of Molecular Sciences 23, no. 6: 3399. https://doi.org/10.3390/ijms23063399
APA StyleRodríguez-Iglesias, N., Nadjar, A., Sierra, A., & Valero, J. (2022). Susceptibility of Female Mice to the Dietary Omega-3/Omega-6 Fatty-Acid Ratio: Effects on Adult Hippocampal Neurogenesis and Glia. International Journal of Molecular Sciences, 23(6), 3399. https://doi.org/10.3390/ijms23063399