Supramolecular Hydrogen Bonding Assembly from Non-Coplanar Aromatic Tetra-1H-Pyrazoles with Crystallization-Induced Emission (CIE)
Abstract
:1. Introduction
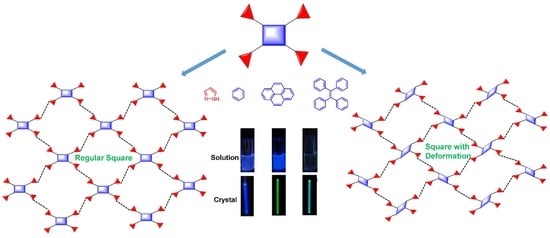
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, J.Y.; Wang, J.K.; Hou, B.H.; Huang, X.; Wang, T.; Bao, Y.; Hao, H.X. Porous hydrogen-bonded organic frameworks (HOFs): From design to potential applications. Chem. Eng. J. 2020, 399, 125873. [Google Scholar] [CrossRef]
- Hisaki, I.; Xin, C.; Takahashi, K.; Nakamura, T. Designing Hydrogen-Bonded Organic Frameworks (HOFs) with Permanent Porosity. Angew. Chem. Int. Ed. 2019, 58, 11160–11170. [Google Scholar] [CrossRef] [PubMed]
- Adachi, T.; Ward, M.D. Versatile and Resilient Hydrogen-Bonded Host Frameworks. Acc. Chem. Res. 2016, 49, 2669–2679. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.B.; He, Y.; Li, P.; Wang, H.; Zhou, W.B. Multifunctional porous hydrogen-bonded organic framework materials. Chem. Soc. Rev. 2019, 48, 1362–1389. [Google Scholar] [CrossRef]
- Nunzio, M.R.; Suzuki, Y.; Hisaki, I.; Douhal, A. HOFs Built from Hexatopic Carboxylic Acids: Structure, Porosity, Stability, and Photophysics. Int. J. Mol. Sci. 2022, 23, 1929. [Google Scholar] [CrossRef]
- Wang, B.; Lin, R.B.; Zhang, Z.J.; Xiang, S.C.; Chen. B.L. Hydrogen-Bonded Organic Frameworks as a Tunable Platform for Functional Materials. J. Am. Chem. Soc. 2020, 142, 14399–14416. [Google Scholar] [CrossRef]
- Li, P.H.; Ryder, M.R.; Stoddart, J.F. Hydrogen-Bonded Organic Frameworks: A Rising Class of Porous Molecular Materials. Acc. Mater. Res. 2020, 1, 77–87. [Google Scholar] [CrossRef]
- Peng, H.Q.; Liu, B.; Liu, J.; Wei, P.; Zhang, H.; Han, T.; Qi, J.; Lam, J.W.Y.; Zhang, W.; Tang, B.Z. “Seeing” and Controlling Photoisomerization by (Z)-/(E)-Isomers with Aggregation-Induced Emission Characteristics. ACS Nano 2019, 13, 12120–12126. [Google Scholar] [CrossRef]
- Wang, B.; He, R.; Xie, L.H.; Lin, Z.J.; Zhang, X.; Wang, J.; Huang, H.L.; Zhang, Z.J.; Schanze, K.S.; Zhang, J.; et al. Microporous Hydrogen-Bonded Organic Framework for Highly Efficient Turn-Up Fluorescent Sensing of Aniline. J. Am. Chem. Soc. 2020, 142, 12478–12485. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, J.X.; Li, L.B.; Pei, J.; Krishna, R.; Wu, H.; Zhou, W.; Qian, G.D.; Chen, B.L.; Li, B. A Rod-Packing Hydrogen-Bonded Organic Framework with Suitable Pore Confinement for Benchmark Ethane/Ethylene Separation. Angew. Chem. Int. Ed. 2021, 60, 10304–10310. [Google Scholar] [CrossRef]
- Yang, Y.S.; Li, L.B.; Lin, R.B.; Ye, Y.X.; Yao, Z.Z.; Yang, L.; Xiang, F.H.; Chen, S.M.; Zhang, Z.J.; Xiang, S.C.; et al. Ethylene/ethane separation in a stable hydrogen-bonded organic framework through a gating mechanism. Nat. Chem. 2021, 13, 933–939. [Google Scholar] [CrossRef]
- Wang, Y.J.; Zhang, M.H.; Yang, Q.Q.; Yin, J.B.; Liu, D.; Shang, Y.X.; Kang, Z.X.; Wang, R.M.; Sun, D.F.; Jiang, J.Z. Single-crystal-to-single-crystal transformation and proton conductivity of three hydrogen-bonded organic frameworks. Chem. Commun. 2020, 56, 15529–15532. [Google Scholar] [CrossRef]
- Han, B.; Wang, H.L.; Wang, C.M.; Wu, H.; Zhou, W.; Chen, B.L.; Jiang, J.Z. Jumping Crystal of a Hydrogen-Bonded Organic Framework Induced by the Collective Molecular Motion of a Twisted π System. Angew. Chem. Int. Ed. 2019, 58, 10345–10352. [Google Scholar]
- Suzuki, Y.; Gutiérrez, M.; Tanaka, S.; Gomez, E.; Tohnai, N.; Yasuda, N.; Matubayasi, N.; Douhal, A.; Hisaki, I. Construction of isostructural hydrogen-bonded organic frameworks: Limitations and possibilities of pore expansion. Chem. Sci. 2021, 12, 9607–9618. [Google Scholar] [CrossRef]
- Yang, W.; Wang, J.X.; Yu, B.Q.; Li, B.; Wang, H.L.; Jiang, J.Z. A Robust Hydrogen-Bonded Organic Framework with 7-Fold Interpenetration Nets and High Permanent Microporosity. Cryst. Growth Des. 2022, 22, 1817–1823. [Google Scholar] [CrossRef]
- Wen, P.; Gao, Z.; Zhang, R.; Li, A.; Zhang, F.; Li, J.; Xie, J.; Wu, Y.; Wu, M.; Guo, K. A–π–D–π–A carbazole derivatives with remarkable solvatochromism and mechanoresponsive luminescence turn-on. J. Mater. Chem. C 2017, 5, 6136–6143. [Google Scholar] [CrossRef]
- Peng, Y.X.; Gan, Y.T.; Shi, R.G.; Huang, W.; Tao, T.J. Construction of a Layered Hydrogen-Bonded Organic Framework Showing High-Contrast Mechanoresponsive Luminescence Turn-On. Phys. Chem. C 2018, 122, 29488–29497. [Google Scholar] [CrossRef]
- Huang, Q.Y.; Li, W.L.; Yang, Z.; Zhao, J.; Li, Y.; Mao, Z.; Yang, Z.Y.; Liu, S.W.; Zhang, Y.; Chi, Z.G. Achieving Bright Mechanoluminescence in a Hydrogen-Bonded Organic Framework by Polar Molecular Rotor Incorporation. CCS Chem. 2021, 3, 1499–1509. [Google Scholar] [CrossRef]
- Cai, S.; Shi, H.; Zhang, Z.; Wang, X.; Ma, H.; Gan, N.; Wu, Q.; Cheng, Z.; Ling, K.; Gu, M.; et al. Hydrogen-Bonded Organic Aromatic Frameworks for Ultralong Phosphorescence by Intralayer π–π Interactions. Angew. Chem. Int. Ed. 2018, 57, 4005–4009. [Google Scholar] [CrossRef]
- Jindal, S.; Maka, V.K.; Anjum, G.; Moorthy, J.N. Anthracene-Bisimidazole Tetraacid Linker-Based Metal–Organic Nanosheets for Turn-On Fluorescence Sensing of Nerve Agent Mimics. ACS Appl. Nano Mater. 2021, 4, 449–458. [Google Scholar] [CrossRef]
- Hisaki, I.J. Hydrogen-bonded porous frameworks constructed by rigid π-conjugated molecules with carboxy groups. Incl. Phenom. Macro. 2020, 96, 215–223. [Google Scholar] [CrossRef] [Green Version]
- Chen, T.H.; Popov, I.; Kaveevivitchai, W.; Chuang, Y.C.; Chen, Y.-S.; Daugulis, O.; Jacobson, A.J.; Miljanić, O.Š. Thermally robust and porous noncovalent organic framework with high affinity for fluorocarbons and CFCs. Nat. Commun. 2014, 5, 5131–5139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hashim, M.I.; Le, H.T.M.; Chen, T.H.; Chen, Y.-S.; Daugulis, O.; Liang, X.; Makarenko, T.; Wu, C.H.; Wu, J.I. Dissecting Porosity in Molecular Crystals: Influence of Geometry, Hydrogen Bonding, and [π···π] Stacking on the Solid-State Packing of Fluorinated Aromatics. J. Am. Chem. Soc. 2018, 140, 6014–6026. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.L.; Hashim, M.I.; Miljanić, O. Aggregation-induced emission in precursors to porous molecular crystals. Chem. Commun. 2017, 53, 10022–10025. [Google Scholar] [CrossRef]
- Zhang, Z.L.; Lieu, T.; Wu, C.H.; Wang, X.Q.; Wu, J.I.; Daugulis, O.; Miljanić, O. Solvation-dependent switching of solid-state luminescence of a fluorinated aromatic tetrapyrazole. Chem. Commun. 2019, 55, 9387–9390. [Google Scholar] [CrossRef]
- Steiner, T.; Desiraju, G.R. The Weak Hydrogen Bond in Structural Chemistry and Biology; OUP: Oxford, UK, 1999; pp. 108–113. [Google Scholar]
- Sheldrick, G.M. SHELXS-97. Acta Crystallogr. 1990, A64, 112–122, (Univ. Göttingen, 1990). Available online: https://www.scirp.org/(S(vtj3fa45qm1ean45vvffcz55))/reference/ReferencesPapers.aspx?ReferenceID=849699 (accessed on 8 March 2022).
- Sheldrick, G.M. SHELXL-97. Acta Crystallogr. 1997, A64, 112–122, (Univ. Göttingen, 1997). Available online: https://www.scirp.org/(S(i43dyn45teexjx455qlt3d2q))/reference/ReferencesPapers.aspx?ReferenceID=768629 (accessed on 8 March 2022).






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Zhao, L.-R.; Tong, J.; Yu, Y.-M.; Wang, X.-Y.; Yu, S.-Y. Supramolecular Hydrogen Bonding Assembly from Non-Coplanar Aromatic Tetra-1H-Pyrazoles with Crystallization-Induced Emission (CIE). Int. J. Mol. Sci. 2022, 23, 4206. https://doi.org/10.3390/ijms23084206
Wang J, Zhao L-R, Tong J, Yu Y-M, Wang X-Y, Yu S-Y. Supramolecular Hydrogen Bonding Assembly from Non-Coplanar Aromatic Tetra-1H-Pyrazoles with Crystallization-Induced Emission (CIE). International Journal of Molecular Sciences. 2022; 23(8):4206. https://doi.org/10.3390/ijms23084206
Chicago/Turabian StyleWang, Ji, Li-Rong Zhao, Jin Tong, Yan-Min Yu, Xia-Yan Wang, and Shu-Yan Yu. 2022. "Supramolecular Hydrogen Bonding Assembly from Non-Coplanar Aromatic Tetra-1H-Pyrazoles with Crystallization-Induced Emission (CIE)" International Journal of Molecular Sciences 23, no. 8: 4206. https://doi.org/10.3390/ijms23084206
APA StyleWang, J., Zhao, L. -R., Tong, J., Yu, Y. -M., Wang, X. -Y., & Yu, S. -Y. (2022). Supramolecular Hydrogen Bonding Assembly from Non-Coplanar Aromatic Tetra-1H-Pyrazoles with Crystallization-Induced Emission (CIE). International Journal of Molecular Sciences, 23(8), 4206. https://doi.org/10.3390/ijms23084206