SMTP-44D Exerts Antioxidant and Anti-Inflammatory Effects through Its Soluble Epoxide Hydrolase Inhibitory Action in Immortalized Mouse Schwann Cells upon High Glucose Treatment
Abstract
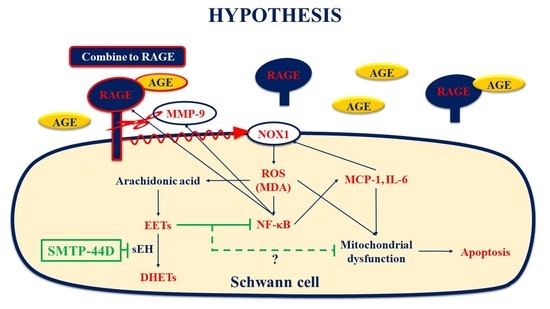
:1. Introduction
2. Results
2.1. Ratio of EETs to DHETs in Response to SMTP-44D in IMS32 under High Glucose Conditions
2.2. Effects of SMTP-44D on NF-κB Nuclear Migration in IMS32 Cells under High Glucose Conditions
2.3. Levels of NOX-1, MDA, IL-6, and MCP-1 in Response to SMTP-44D in IMS32 Cells under High Glucose Conditions
2.4. Effects of SMTP-44D on Apoptosis in IMS32 Cells under High Glucose Conditions
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Cell Culture
4.3. Treatment Protocol
4.4. Cell Sampling
4.5. Measurement of EET and DHET by Liquid Chromatography-Electrospray Ionization Mass Spectrometry (LC-ESI-MS)
4.6. Enzyme-Linked Immunosorbent Assay (ELISA)
4.7. Thiobarbituric Acid Reactive Substances (TBARS) Assay
4.8. TdT-Mediated dUTP Nick-End Labelling (TUNEL) Assay
4.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sobhani, S.; Asayesh, H.; Sharifi, F.; Djalalinia, S.; Baradaran, H.R.; Arzaghi, S.M.; Mansourian, M.; Rezapoor, A.; Ansari, H.; Masoud, M.P.; et al. Prevalence of diabetic peripheral neuropathy in Iran: A systematic review and meta-analysis. J. Diabetes Metab. Disord. 2014, 13, 97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tesfaye, S.; Boulton, A.J.; Dickenson, A.H. Mechanisms and management of diabetic painful distal symmetrical polyneuropathy. Diabetes Care 2013, 36, 2456–2465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brownlee, M. Biochemistry and molecular cell biology of diabetic complications. Nature 2001, 414, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.H.; Lin, J.; Hou, X.H.; Cao, R.; Yu, F.; Liu, H.Q.; Ji, A.L.; Xu, X.N.; Zhang, L.; Wang, F. Effect of docosahexaenoic acid on hippocampal neurons in high-glucose condition: Involvement of PI3K/AKT/nuclear factor-κB-mediated inflammatory pathways. Neuroscience 2014, 274, 218–228. [Google Scholar] [CrossRef]
- Kumar, A.; Negi, G.; Sharma, S.S. JSH-23 targets nuclear factor-kappa B and reverses various deficits in experimental diabetic neuropathy: Effect on neuroinflammation and antioxidant defence. Diabetes Obes. Metab. 2011, 13, 750–758. [Google Scholar] [CrossRef]
- Ranjithkumar, R.; Prathab Balaji, S.; Balaji, B.; Ramesh, R.V.; Ramanathan, M. Standardized Aqueous Tribulus terristris (nerunjil) extract attenuates hyperalgesia in experimentally induced diabetic neuropathic pain model: Role of oxidative stress and inflammatory mediators. Phytother Res. 2013, 27, 1646–1657. [Google Scholar] [CrossRef]
- Hasumi, K.; Yamamichi, S.; Harada, T. Small-molecule modulators of zymogen activation in the fibrinolytic and coagulation systems. FEBS J. 2010, 277, 3675–3687. [Google Scholar] [CrossRef]
- Hasumi, K.; Suzuki, E. Impact of SMTP targeting plasminogen and soluble epoxide hydrolase on thrombolysis, inflammation, and ischemic stroke. Int. J. Mol. Sci. 2021, 22, 954. [Google Scholar] [CrossRef]
- Akamatsu, Y.; Saito, A.; Fujimura, M.; Shimizu, H.; Mekawy, M.; Hasumi, K.; Tominaga, T. Stachybotrys microspora triprenyl phenol-7, a novel fibrinolytic agent, suppresses superoxide production, matrix metalloproteinase-9 expression, and thereby attenuates ischemia/reperfusion injury in rat brain. Neurosci. Lett. 2011, 503, 110–114. [Google Scholar] [CrossRef]
- Hashimoto, T.; Shibata, K.; Nobe, K.; Hasumi, K.; Honda, K. A novel embolic model of cerebral infarction and evaluation of Stachybotrys microspora triprenyl phenol-7 (SMTP-7), a novel fungal triprenyl phenol metabolite. J. Pharmacol. Sci. 2010, 114, 41–49. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Ohta, Y.; Shang, J.; Morihara, R.; Nakano, Y.; Fukui, Y.; Liu, X.; Shi, X.; Feng, T.; Yamashita, T.; et al. Antineuroinflammatory Effect of SMTP-7 in Ischemic Mice. J. Stroke Cerebrovasc. Dis. 2018, 27, 3084–3094. [Google Scholar] [CrossRef] [PubMed]
- Sawada, H.; Nishimura, N.; Suzuki, E.; Zhuang, J.; Hasegawa, K.; Takamatsu, H.; Honda, K.; Hasumi, K. SMTP-7, a novel small-molecule thrombolytic for ischemic stroke: A study in rodents and primates. J. Cereb. Blood Flow Metab. 2014, 34, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Shibata, K.; Hashimoto, T.; Nobe, K.; Hasumi, K.; Honda, K. A novel finding of a low-molecular-weight compound, SMTP-7, having thrombolytic and anti-inflammatory effects in cerebral infarction of mice. Naunyn Schmiedebergs Arch. Pharmacol. 2010, 382, 245–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, E.; Nishimura, N.; Yoshikawa, T.; Kunikiyo, Y.; Hasegawa, K.; Hasumi, K. Efficacy of SMTP-7, a small-molecule anti-inflammatory thrombolytic, in embolic stroke in monkeys. Pharmacol. Res. Perspect. 2018, 6, e00448. [Google Scholar] [CrossRef] [Green Version]
- Shibata, K.; Hashimoto, T.; Hasumi, K.; Nobe, K. Potent efficacy of Stachybotrys microspora triprenyl phenol-7, a small molecule having anti-inflammatory and antioxidant activities, in a mouse model of acute kidney injury. Eur. J. Pharmacol. 2021, 910, 174496. [Google Scholar] [CrossRef]
- Koide, H.; Narasaki, R.; Hasegawa, K.; Nishimura, N.; Hasumi, K. A new series of the SMTP plasminogen modulator with a phenylglycine-based side-chain. J. Antibiot. 2012, 65, 91–93. [Google Scholar] [CrossRef]
- Matsumoto, N.; Suzuki, E.; Ishikawa, M.; Shirafuji, T.; Hasumi, K. Soluble epoxide hydrolase as an anti-inflammatory target of the thrombolytic stroke drug SMTP-7. J. Biol. Chem. 2014, 289, 35826–35838. [Google Scholar] [CrossRef] [Green Version]
- Shibata, K.; Hashimoto, T.; Hasumi, K.; Honda, K.; Nobe, K. Evaluation of the effects of a new series of SMTPs in the acetic acid-induced embolic cerebral infarct mouse model. Eur. J. Pharmacol. 2018, 818, 221–227. [Google Scholar] [CrossRef]
- Shi, X.; Ohta, Y.; Shang, J.; Morihara, R.; Nakano, Y.; Fukui, Y.; Liu, X.; Feng, T.; Huang, Y.; Sato, K.; et al. Neuroprotective effects of SMTP-44D in mice stroke model in relation to neurovascular unit and trophic coupling. J. Neurosci. Res. 2018, 96, 1887–1899. [Google Scholar] [CrossRef]
- Shinouchi, R.; Shibata, K.; Hashimoto, T.; Jono, S.; Hasumi, K.; Nobe, K. SMTP-44D improves diabetic neuropathy symptoms in mice through its antioxidant and anti-inflammatory activities. Pharmacol. Res. Perspect. 2020, 8, e00648. [Google Scholar] [CrossRef]
- Pillarisetti, S.; Khanna, I. A multimodal disease modifying approach to treat neuropathic pain--inhibition of soluble epoxide hydrolase (sEH). Drug Discov. Today 2015, 20, 1382–1390. [Google Scholar] [CrossRef] [PubMed]
- Michaelis, U.R.; Fleming, I. From endothelium-derived hyperpolarizing factor (EDHF) to angiogenesis: Epoxyeicosatrienoic acids (EETs) and cell signaling. Pharmacol. Ther. 2006, 111, 584–595. [Google Scholar] [CrossRef] [PubMed]
- Spector, A.A.; Norris, A.W. Action of epoxyeicosatrienoic acids on cellular function. Am. J. Physiol. Cell Physiol. 2007, 292, C996–C1012. [Google Scholar] [CrossRef] [PubMed]
- Cinci, L.; Corti, F.; Di Cesare Mannelli, L.; Micheli, L.; Zanardelli, M.; Ghelardini, C. Oxidative, metabolic, and apoptotic responses of Schwann cells to high glucose levels. J. Biochem. Mol. Toxicol. 2015, 29, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, N.P.; Vægter, C.B.; Andersen, H.; Østergaard, L.; Calcutt, N.A.; Jensen, T.S. Schwann cell interactions with axons and microvessels in diabetic neuropathy. Nat. Rev. Neurol. 2017, 13, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Viader, A.; Golden, J.P.; Baloh, R.H.; Schmidt, R.E.; Hunter, D.A.; Milbrandt, J. Schwann cell mitochondrial metabolism supports long-term axonal survival and peripheral nerve function. J. Neurosci. 2011, 31, 10128–10140. [Google Scholar] [CrossRef] [PubMed]
- Tatsumi, Y.; Kato, A.; Sango, K.; Himeno, T.; Kondo, M.; Kato, Y.; Kamiya, H.; Nakamura, J.; Kato, K. Omega-3 polyunsaturated fatty acids exert anti-oxidant effects through the nuclear factor (erythroid-derived 2)-related factor 2 pathway in immortalized mouse Schwann cells. J. Diabetes Investig. 2019, 10, 602–612. [Google Scholar] [CrossRef]
- Dai, M.; Wu, L.; He, Z.; Zhang, S.; Chen, C.; Xu, X.; Wang, P.; Gruzdev, A.; Zeldin, D.C.; Wang, D.W. Epoxyeicosatrienoic acids regulate macrophage polarization and prevent LPS-induced cardiac dysfunction. J. Cell. Physiol. 2015, 230, 2108–2119. [Google Scholar] [CrossRef] [Green Version]
- Luo, J.; Hu, S.; Fu, M.; Luo, L.; Li, Y.; Li, W.; Cai, Y.; Dong, R.; Yang, Y.; Tu, L.; et al. Inhibition of soluble epoxide hydrolase alleviates insulin resistance and hypertension via downregulation of SGLT2 in the mouse kidney. J. Biol. Chem. 2021, 296, 100667. [Google Scholar] [CrossRef]
- Node, K.; Huo, Y.; Ruan, X.; Yang, B.; Spiecker, M.; Ley, K.; Zeldin, D.C.; Liao, J.K. Anti-inflammatory properties of cytochrome P450 epoxygenase-derived eicosanoids. Science 1999, 285, 1276–1279. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Chen, M.; Yuan, L.; Xiang, Y.; Zheng, R.; Zhu, S. 14,15-EET promotes mitochondrial biogenesis and protects cortical neurons against oxygen/glucose deprivation-induced apoptosis. Biochem. Biophys. Res. Commun. 2014, 450, 604–609. [Google Scholar] [CrossRef] [PubMed]
- Kang, R.; Tang, D.; Livesey, K.M.; Schapiro, N.E.; Lotze, M.T.; Zeh, H.J., 3rd. The Receptor for Advanced Glycation End-products (RAGE) protects pancreatic tumor cells against oxidative injury. Antioxid. Redox Signal. 2011, 15, 2175–2184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suryavanshi, S.V.; Kulkarni, Y.A. NF-κβ: A Potential Target in the Management of Vascular Complications of Diabetes. Front. Pharmacol. 2017, 8, 798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grund, E.M.; Kagan, D.; Tran, C.A.; Zeitvogel, A.; Starzinski-Powitz, A.; Nataraja, S.; Palmer, S.S. Tumor necrosis factor-alpha regulates inflammatory and mesenchymal responses via mitogen-activated protein kinase kinase, p38, and nuclear factor kappaB in human endometriotic epithelial cells. Mol. Pharmacol. 2008, 73, 1394–1404. [Google Scholar] [CrossRef]
- Zhang, H.; Mao, Y.F.; Zhao, Y.; Xu, D.F.; Wang, Y.; Xu, C.F.; Dong, W.W.; Zhu, X.Y.; Ding, N.; Jiang, L.; et al. Upregulation of Matrix Metalloproteinase-9 Protects against Sepsis-Induced Acute Lung Injury via Promoting the Release of Soluble Receptor for Advanced Glycation End Products. Oxid. Med. Cell. Longev. 2021, 2021, 8889313. [Google Scholar] [CrossRef]
- Zhang, L.; Bukulin, M.; Kojro, E.; Roth, A.; Metz, V.V.; Fahrenholz, F.; Nawroth, P.P.; Bierhaus, A.; Postina, R. Receptor for advanced glycation end products is subjected to protein ectodomain shedding by metalloproteinases. J. Biol. Chem. 2008, 283, 35507–35516. [Google Scholar] [CrossRef] [Green Version]
- Dwir, D.; Giangreco, B.; Xin, L.; Tenenbaum, L.; Cabungcal, J.H.; Steullet, P.; Goupil, A.; Cleusix, M.; Jenni, R.; Chtarto, A.; et al. MMP9/RAGE pathway overactivation mediates redox dysregulation and neuroinflammation, leading to inhibitory/excitatory imbalance: A reverse translation study in schizophrenia patients. Mol. Psychiatry 2020, 25, 2889–2904. [Google Scholar] [CrossRef] [Green Version]
- Delaney, C.L.; Russell, J.W.; Cheng, H.L.; Feldman, E.L. Insulin-like growth factor-I and over-expression of Bcl-xL prevent glucose-mediated apoptosis in Schwann cells. J. Neuropathol. Exp. Neurol. 2001, 60, 147–160. [Google Scholar] [CrossRef] [Green Version]
- Sekido, H.; Suzuki, T.; Jomori, T.; Takeuchi, M.; Yabe-Nishimura, C.; Yagihashi, S. Reduced cell replication and induction of apoptosis by advanced glycation end products in rat Schwann cells. Biochem. Biophys. Res. Commun. 2004, 320, 241–248. [Google Scholar] [CrossRef]
- Matsumoto, N.; Suzuki, E.; Tsujihara, K.; Nishimura, Y.; Hasumi, K. Structure-activity relationships of the plasminogen modulator SMTP with respect to the inhibition of soluble epoxide hydrolase. J. Antibiot. 2015, 68, 685–690. [Google Scholar] [CrossRef] [Green Version]
- Sasaki, Y.; Kamiyama, S.; Kamiyama, A.; Matsumoto, K.; Akatsu, M.; Nakatani, Y.; Kuwata, H.; Ishikawa, Y.; Ishii, T.; Yokoyama, C.; et al. Genetic-deletion of Cyclooxygenase-2 Downstream Prostacyclin Synthase Suppresses Inflammatory Reactions but Facilitates Carcinogenesis, unlike Deletion of Microsomal Prostaglandin E Synthase-1. Sci. Rep. 2015, 5, 17376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ochiai, T.; Sasaki, Y.; Kuwata, H.; Nakatani, Y.; Yokoyama, C.; Hara, S. Coordinated action of microsomal prostaglandin E synthase-1 and prostacyclin synthase on contact hypersensitivity. Biochem. Biophys. Res. Commun. 2021, 546, 124–129. [Google Scholar] [CrossRef] [PubMed]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shinouchi, R.; Shibata, K.; Jono, S.; Hasumi, K.; Nobe, K. SMTP-44D Exerts Antioxidant and Anti-Inflammatory Effects through Its Soluble Epoxide Hydrolase Inhibitory Action in Immortalized Mouse Schwann Cells upon High Glucose Treatment. Int. J. Mol. Sci. 2022, 23, 5187. https://doi.org/10.3390/ijms23095187
Shinouchi R, Shibata K, Jono S, Hasumi K, Nobe K. SMTP-44D Exerts Antioxidant and Anti-Inflammatory Effects through Its Soluble Epoxide Hydrolase Inhibitory Action in Immortalized Mouse Schwann Cells upon High Glucose Treatment. International Journal of Molecular Sciences. 2022; 23(9):5187. https://doi.org/10.3390/ijms23095187
Chicago/Turabian StyleShinouchi, Ryosuke, Keita Shibata, Shiori Jono, Keiji Hasumi, and Koji Nobe. 2022. "SMTP-44D Exerts Antioxidant and Anti-Inflammatory Effects through Its Soluble Epoxide Hydrolase Inhibitory Action in Immortalized Mouse Schwann Cells upon High Glucose Treatment" International Journal of Molecular Sciences 23, no. 9: 5187. https://doi.org/10.3390/ijms23095187
APA StyleShinouchi, R., Shibata, K., Jono, S., Hasumi, K., & Nobe, K. (2022). SMTP-44D Exerts Antioxidant and Anti-Inflammatory Effects through Its Soluble Epoxide Hydrolase Inhibitory Action in Immortalized Mouse Schwann Cells upon High Glucose Treatment. International Journal of Molecular Sciences, 23(9), 5187. https://doi.org/10.3390/ijms23095187