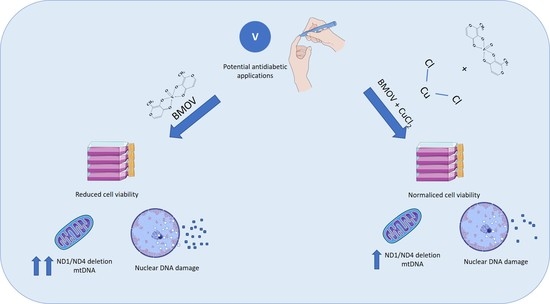
Elucidating the Therapeutic Potential of Bis(Maltolato)OxoVanadium(IV): The Protective Role of Copper in Cellular Metabolism
Abstract
:1. Introduction
2. Results
2.1. Cell Viability (MTT Assay)
2.2. Metal Uptake
2.3. ND1/ND4 mtDNA Deletion
2.4. Comet Assay
3. Discussion
4. Materials and Methods
4.1. Preparation of Metallic Solutions and Exposure to V and Cu
4.2. Cell Conditions
4.3. MTT Assay
4.4. Metal Uptake
4.5. ND1/ND4 mtDNA Deletion
4.6. Comet Aassay
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gummow, B. Vanadium: Environmental Pollution and Health Effects. In Encyclopedia of Environmental Health; Elsevier: Amsterdam, The Netherlands, 2011; pp. 628–636. ISBN 978-0-444-52272-6. [Google Scholar]
- Treviño, S.; Diaz, A. Vanadium and Insulin: Partners in Metabolic Regulation. J. Inorg. Biochem. 2020, 208, 111094. [Google Scholar] [CrossRef]
- Orvig, C.; Caravan, P.; Gelmini, L.; Glover, N.; Herring, F.G.; Li, H.; McNeill, J.H.; Rettig, S.J.; Setyawati, I.A. Reaction Chemistry of BMOV, Bis(Maltolato)Oxovanadium(IV), a Potent Insulin Mimetic Agent. J. Am. Chem. Soc. 1995, 117, 12759–12770. [Google Scholar] [CrossRef]
- Sakurai, H.; Watanabe, H.; Tamura, H.; Yasui, H.; Matsushita, R.; Takada, J. Insulin-Mimetic Vanadyl—Dithiocarbamate Complexes. Inorg. Chim. Acta 1998, 283, 175–183. [Google Scholar] [CrossRef]
- Thompson, K.H.; Lichter, J.; LeBel, C.; Scaife, M.C.; McNeill, J.H.; Orvig, C. Vanadium Treatment of Type 2 Diabetes: A View to the Future. J. Inorg. Biochem. 2009, 103, 554–558. [Google Scholar] [CrossRef]
- Goldfine, A.B.; Patti, M.-E.; Zuberi, L.; Goldstein, B.J.; LeBlanc, R.; Landaker, E.J.; Jiang, Z.Y.; Willsky, G.R.; Kahn, C.R. Metabolic Effects of Vanadyl Sulfate in Humans with Non—Insulin-Dependent Diabetes Mellitus: In Vivo and in Vitro Studies. Metabolism 2000, 49, 400–410. [Google Scholar] [CrossRef]
- Li, S.H.; McNeill, J.H. In Vivo Effects of Vanadium on GLUT4 Translocation in Cardiac Tissue of STZ-Diabetic Rats. Mol. Cell. Biochem. 2001, 217, 121–129. [Google Scholar] [CrossRef]
- Ścibior, A.; Kurus, J. Vanadium and Oxidative Stress Markers—In Vivo Model: A Review. Curr. Med. Chem. 2019, 26, 5456–5500. [Google Scholar] [CrossRef]
- Aureliano, M.; De Sousa-Coelho, A.L.; Dolan, C.C.; Roess, D.A.; Crans, D.C. Biological Consequences of Vanadium Effects on Formation of Reactive Oxygen Species and Lipid Peroxidation. Int. J. Mol. Sci. 2023, 24, 5382. [Google Scholar] [CrossRef]
- He, X.; Jarrell, Z.R.; Liang, Y.; Ryan Smith, M.; Orr, M.L.; Marts, L.; Go, Y.-M.; Jones, D.P. Vanadium Pentoxide Induced Oxidative Stress and Cellular Senescence in Human Lung Fibroblasts. Redox Biol. 2022, 55, 102409. [Google Scholar] [CrossRef]
- Rivas-García, L.; Quiles, J.L.; Varela-López, A.; Arredondo, M.; Lopez, P.; Diéguez, A.R.; Montes-Bayon, M.; Aranda, P.; Llopis, J.; Sánchez-González, C. In Vitro Study of the Protective Effect of Manganese against Vanadium-Mediated Nuclear and Mitochondrial DNA Damage. Food Chem. Toxicol. 2020, 135, 110900. [Google Scholar] [CrossRef]
- Ścibior, A.; Adamczyk, A.; Gołębiowska, D.; Niedźwiecka, I.; Fornal, E. The Influence of Combined Magnesium and Vanadate Administration on the Level of Some Elements in Selected Rat Organs: V–Mg Interactions and the Role of Iron-Essential Protein (DMT-1) in the Mechanism Underlying Altered Tissues Iron Level. Metallomics 2014, 6, 907–920. [Google Scholar] [CrossRef]
- Chen, L.; Min, J.; Wang, F. Copper Homeostasis and Cuproptosis in Health and Disease. Signal Transduct. Target. Ther. 2022, 7, 378. [Google Scholar] [CrossRef]
- Ruiz, L.M.; Libedinsky, A.; Elorza, A.A. Role of Copper on Mitochondrial Function and Metabolism. Front. Mol. Biosci. 2021, 8, 711227. [Google Scholar] [CrossRef]
- Plazas Guerrero, C.G.; Acosta Cota, S.D.J.; Castro Sánchez, F.H.; Vergara Jiménez, M.D.J.; Ríos Burgueño, E.R.; Sarmiento Sánchez, J.I.; Picos Corrales, L.A.; Osuna Martínez, U. Evaluation of Sucrose-Enriched Diet Consumption in the Development of Risk Factors Associated to Type 2 Diabetes, Atherosclerosis and Non-Alcoholic Fatty Liver Disease in a Murine Model. Int. J. Environ. Health Res. 2021, 31, 651–669. [Google Scholar] [CrossRef]
- Tsang, T.; Davis, C.I.; Brady, D.C. Copper Biology. Curr. Biol. 2021, 31, R421–R427. [Google Scholar] [CrossRef]
- Rucker, R.B.; Cui, C.T.; Tchaparian, E.H.; Mitchell, A.E.; Clegg, M.; Uriu-Hare, J.Y.; Keen, C.L. Dietary Vanadium, P-ATPase-7A Expression and the Influence on Lysyl Oxidase and Cu Accumulation in Rat Skin and Liver. In Trace Elements in Man and Animals 10; Roussel, A.M., Anderson, R.A., Favier, A.E., Eds.; Springer: New York, NY, USA, 2002; pp. 186–187. ISBN 978-0-306-46378-5. [Google Scholar]
- Ścibior, A.; Gołębiowska, D.; Adamczyk, A.; Kurus, J.; Staniszewska, M.; Sadok, I. Evaluation of Lipid Peroxidation and Antioxidant Defense Mechanisms in the Bone of Rats in Conditions of Separate and Combined Administration of Vanadium (V) and Magnesium (Mg). Chem.-Biol. Interact. 2018, 284, 112–125. [Google Scholar] [CrossRef]
- Treviño, S.; Díaz, A.; Sánchez-Lara, E.; Sanchez-Gaytan, B.L.; Perez-Aguilar, J.M.; González-Vergara, E. Vanadium in Biological Action: Chemical, Pharmacological Aspects, and Metabolic Implications in Diabetes Mellitus. Biol. Trace Elem. Res. 2019, 188, 68–98. [Google Scholar] [CrossRef]
- Arredondo, M.; Muñoz, P.; Mura, C.V.; Núñez, M.T. DMT1, a Physiologically Relevant Apical Cu 1+ Transporter of Intestinal Cells. Am. J. Physiol.-Cell Physiol. 2003, 284, C1525–C1530. [Google Scholar] [CrossRef]
- Cordier, W.; Yousaf, M.; Nell, M.J.; Steenkamp, V. Underlying Mechanisms of Cytotoxicity in HepG2 Hepatocarcinoma Cells Exposed to Arsenic, Cadmium and Mercury Individually and in Combination. Toxicol. Vitr. 2021, 72, 105101. [Google Scholar] [CrossRef]
- Wang, P.; Wu, Q.; Wang, F.; Zhang, Y.; Tong, L.; Jiang, T.; Gu, C.; Huang, S.; Wang, H.; Bu, S.; et al. Evaluating Cellular Uptake of Gold Nanoparticles in HL-7702 and HepG2 Cells for Plasmonic Photothermal Therapy. Nanomedicine 2018, 13, 2245–2259. [Google Scholar] [CrossRef]
- Kammerer, S.; Küpper, J.-H. Human Hepatocyte Systems for in Vitro Toxicology Analysis. J. Cell. Biotechnol. 2018, 3, 85–93. [Google Scholar] [CrossRef]
- Sánchez-González, C.; Rivas-García, L.; López-Chaves, C.; Rodríguez-Nogales, A.; Algieri, F.; Gálvez, J.; Gómez-Aracena, J.; Vera-Ramírez, L.; Montes-Bayon, M.; Sanz-Medel, A.; et al. Exposure to Bis(Maltolato)Oxovanadium(IV) Increases Levels of Hepcidin MRNA and Impairs the Homeostasis of Iron but Not That of Manganese. Food Chem. Toxicol. 2014, 73, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-González, C.; Rivas-García, L.; Rodríguez-Nogales, A.; Algieri, F.; Gálvez, J.; Aranda, P.; Montes-Bayón, M.; Llopis, J. Vanadium Decreases Hepcidin MRNA Gene Expression in STZ-Induced Diabetic Rats, Improving the Anemic State. Nutrients 2021, 13, 1256. [Google Scholar] [CrossRef]
- Sanchez-Gonzalez, C.; Bermudez-Peña, C.; Guerrero-Romero, F.; Trenzado, C.E.; Montes-Bayon, M.; Sanz-Medel, A.; Llopis, J. Effect of Bis(Maltolato)Oxovanadium (IV) (BMOV) on Selenium Nutritional Status in Diabetic Streptozotocin Rats. Br. J. Nutr. 2012, 108, 893–899. [Google Scholar] [CrossRef]
- Sanchez-Gonzalez, C.; Bermudez-Peña, C.; Trenzado, C.E.; Goenaga-Infante, H.; Montes-Bayon, M.; Sanz-Medel, A.; Llopis, J. Changes in the Antioxidant Defence and in Selenium Concentration in Tissues of Vanadium Exposed Rats. Metallomics 2012, 4, 814–819. [Google Scholar] [CrossRef]
- Samira, M.; Mounira, T.; Kamel, K.; Yacoubi, M.T.; Ben Rhouma, K.; Sakly, M.; Tebourbi, O. Hepatotoxicity of Vanadyl Sulfate in Nondiabetic and Streptozotocin-Induced Diabetic Rats. Can. J. Physiol. Pharmacol. 2018, 96, 1076–1083. [Google Scholar] [CrossRef]
- Sohrabi, M.; Gholami, A.; Azar, M.H.; Yaghoobi, M.; Shahi, M.M.; Shirmardi, S.; Nikkhah, M.; Kohi, Z.; Salehpour, D.; Khoonsari, M.R.; et al. Trace Element and Heavy Metal Levels in Colorectal Cancer: Comparison Between Cancerous and Non-Cancerous Tissues. Biol. Trace Elem. Res. 2018, 183, 1–8. [Google Scholar] [CrossRef]
- Sánchez-González, C.; Moreno, L.; Aranda, P.; Montes-Bayón, M.; Llopis, J.; Rivas-García, L. Effect of Bis(Maltolato)Oxovanadium(IV) on Zinc, Copper, and Manganese Homeostasis and DMT1 MRNA Expression in Streptozotocin-Induced Hyperglycemic Rats. Biology 2022, 11, 814. [Google Scholar] [CrossRef]
- Bonnet, C.; Augustin, S.; Ellouze, S.; Bénit, P.; Bouaita, A.; Rustin, P.; Sahel, J.-A.; Corral-Debrinski, M. The Optimized Allotopic Expression of ND1 or ND4 Genes Restores Respiratory Chain Complex I Activity in Fibroblasts Harboring Mutations in These Genes. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2008, 1783, 1707–1717. [Google Scholar] [CrossRef]
- Danhelovska, T.; Kolarova, H.; Zeman, J.; Hansikova, H.; Vaneckova, M.; Lambert, L.; Kucerova-Vidrova, V.; Berankova, K.; Honzik, T.; Tesarova, M. Multisystem Mitochondrial Diseases Due to Mutations in MtDNA-Encoded Subunits of Complex, I. BMC Pediatr. 2020, 20, 41. [Google Scholar] [CrossRef]
- Bhatti, J.S.; Bhatti, G.K.; Reddy, P.H. Mitochondrial Dysfunction and Oxidative Stress in Metabolic Disorders—A Step towards Mitochondria Based Therapeutic Strategies. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2017, 1863, 1066–1077. [Google Scholar] [CrossRef]
- Cobine, P.A.; Moore, S.A.; Leary, S.C. Getting out What You Put in: Copper in Mitochondria and Its Impacts on Human Disease. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2021, 1868, 118867. [Google Scholar] [CrossRef] [PubMed]
- Peters, K.G.; Davis, M.G.; Howard, B.W.; Pokross, M.; Rastogi, V.; Diven, C.; Greis, K.D.; Eby-Wilkens, E.; Maier, M.; Evdokimov, A.; et al. Mechanism of Insulin Sensitization by BMOV (Bis Maltolato Oxo Vanadium); Unliganded Vanadium (VO4) as the Active Component. J. Inorg. Biochem. 2003, 96, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Rivas-García, L.; Quiles, J.L.; Varela-López, A.; Giampieri, F.; Battino, M.; Bettmer, J.; Montes-Bayón, M.; Llopis, J.; Sánchez-González, C. Ultra-Small Iron Nanoparticles Target Mitochondria Inducing Autophagy, Acting on Mitochondrial DNA and Reducing Respiration. Pharmaceutics 2021, 13, 90. [Google Scholar] [CrossRef] [PubMed]




| V (ng/106 Cells) | Cu (ng/106 Cells) | |
|---|---|---|
| Control | 4.8 ± 0.6 | 307 ± 110 |
| 3 mg/L V | 50 ± 0.4 a | 503 ± 110 a |
| 3 mg/L Cu | 4.84 ± 0.4 b | 5340 ± 1551 a,b |
| 3 mg/L Cu- 3 mg/L V | 42 ± 0.2 a,c | 2255 ± 707 a,b,c |
| Tail Moment Value | |
|---|---|
| Control | 0 |
| 3 mg/L V | 0 |
| 3 mg/L Cu | 35.6 a |
| 3 mg/L Cu–3 mg/L V | 10.3 a,b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rivas-García, L.; López-Varela, A.; Quiles, J.L.; Montes-Bayón, M.; Aranda, P.; Llopis, J.; Sánchez-González, C. Elucidating the Therapeutic Potential of Bis(Maltolato)OxoVanadium(IV): The Protective Role of Copper in Cellular Metabolism. Int. J. Mol. Sci. 2023, 24, 9367. https://doi.org/10.3390/ijms24119367
Rivas-García L, López-Varela A, Quiles JL, Montes-Bayón M, Aranda P, Llopis J, Sánchez-González C. Elucidating the Therapeutic Potential of Bis(Maltolato)OxoVanadium(IV): The Protective Role of Copper in Cellular Metabolism. International Journal of Molecular Sciences. 2023; 24(11):9367. https://doi.org/10.3390/ijms24119367
Chicago/Turabian StyleRivas-García, Lorenzo, Alfonso López-Varela, José L. Quiles, María Montes-Bayón, Pilar Aranda, Juan Llopis, and Cristina Sánchez-González. 2023. "Elucidating the Therapeutic Potential of Bis(Maltolato)OxoVanadium(IV): The Protective Role of Copper in Cellular Metabolism" International Journal of Molecular Sciences 24, no. 11: 9367. https://doi.org/10.3390/ijms24119367
APA StyleRivas-García, L., López-Varela, A., Quiles, J. L., Montes-Bayón, M., Aranda, P., Llopis, J., & Sánchez-González, C. (2023). Elucidating the Therapeutic Potential of Bis(Maltolato)OxoVanadium(IV): The Protective Role of Copper in Cellular Metabolism. International Journal of Molecular Sciences, 24(11), 9367. https://doi.org/10.3390/ijms24119367