Vitamin D Receptor Deficiency Upregulates Pulmonary Artery Kv7 Channel Activity
Abstract
:1. Introduction
2. Results
2.1. Effects of Vdr Ablation on RV Weight and Endothelial Function
2.2. Kv7 Activity in Vdr−/− Pulmonary Vasculature
2.3. Lung Histology
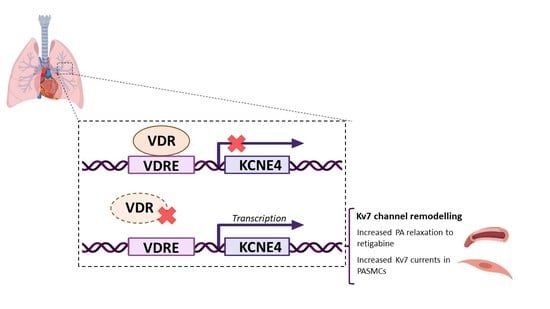
2.4. VDRE in the KCNE4 Gene
2.5. Regulation of Kv7 Expression by Vdr
3. Discussion
4. Materials and Methods
4.1. Ethical Approval
4.2. Animal Model
4.3. RV Hypertrophy and Lung Histology
4.4. Arterial Reactivity
4.5. Electrophysiological Studies
4.6. Identification of VDREs in the KCNE4 Gene by In Silico Analysis
4.7. Western Blot
4.8. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Simonneau, G.; Montani, D.; Celermajer, D.S.; Denton, C.P.; Gatzoulis, M.A.; Krowka, M.; Williams, P.G.; Souza, R. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur. Respir. J. 2019, 53, 1801913. [Google Scholar] [CrossRef]
- Prins, K.W.; Thenappan, T. World Health Organization Group I Pulmonary Hypertension: Epidemiology and Pathophysiology. Cardiol. Clin. 2016, 34, 363–374. [Google Scholar] [CrossRef] [Green Version]
- Antigny, F.; Hautefort, A.; Meloche, J.; Belacel-Ouari, M.; Manoury, B.; Rucker-Martin, C.; Pechoux, C.; Potus, F.; Nadeau, V.; Tremblay, E.; et al. Potassium Channel Subfamily K Member 3 (KCNK3) Contributes to the Development of Pulmonary Arterial Hypertension. Circulation 2016, 133, 1371–1385. [Google Scholar] [CrossRef] [Green Version]
- Gurney, A.M.; Osipenko, O.N.; MacMillan, D.; McFarlane, K.M.; Tate, R.J.; Kempsill, F.E. Two-pore domain K channel, TASK-1, in pulmonary artery smooth muscle cells. Circ. Res. 2003, 93, 957–964. [Google Scholar] [CrossRef] [PubMed]
- Lambert, M.; Boet, A.; Rucker-Martin, C.; Mendes-Ferreira, P.; Capuano, V.; Hatem, S.; Adao, R.; Bras-Silva, C.; Hautefort, A.; Michel, J.B.; et al. Loss of KCNK3 is a hallmark of RV hypertrophy/dysfunction associated with pulmonary hypertension. Cardiovasc. Res. 2018, 114, 880–893. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Juhaszova, M.; Rubin, L.J.; Yuan, X.J. Hypoxia inhibits gene expression of voltage-gated K+ channel alpha subunits in pulmonary artery smooth muscle cells. J. Clin. Investig. 1997, 100, 2347–2353. [Google Scholar] [CrossRef] [Green Version]
- Yuan, X.J.; Wang, J.; Juhaszova, M.; Gaine, S.P.; Rubin, L.J. Attenuated K+ channel gene transcription in primary pulmonary hypertension. Lancet 1998, 351, 726–727. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.J.; Wang, J.; Juhaszova, M.; Golovina, V.A.; Rubin, L.J. Molecular basis and function of voltage-gated K+ channels in pulmonary arterial smooth muscle cells. Am. J. Physiol. 1998, 274, L621–L635. [Google Scholar] [CrossRef] [PubMed]
- Mondejar-Parreno, G.; Perez-Vizcaino, F.; Cogolludo, A. Kv7 Channels in Lung Diseases. Front. Physiol. 2020, 11, 634. [Google Scholar] [CrossRef]
- Barrese, V.; Stott, J.B.; Greenwood, I.A. KCNQ-Encoded Potassium Channels as Therapeutic Targets. Annu. Rev. Pharmacol. Toxicol. 2018, 58, 625–648. [Google Scholar] [CrossRef]
- Mackie, A.R.; Byron, K.L. Cardiovascular KCNQ (Kv7) potassium channels: Physiological regulators and new targets for therapeutic intervention. Mol. Pharmacol. 2008, 74, 1171–1179. [Google Scholar] [CrossRef] [Green Version]
- Robbins, J. KCNQ potassium channels: Physiology, pathophysiology, and pharmacology. Pharmacol. Ther. 2001, 90, 1–19. [Google Scholar] [CrossRef]
- de Jong, I.E.M.; Jepps, T.A. Impaired Kv7 channel function in cerebral arteries of a tauopathy mouse model (rTg4510). Physiol. Rep. 2018, 6, e13920. [Google Scholar] [CrossRef] [Green Version]
- Mondejar-Parreno, G.; Barreira, B.; Callejo, M.; Morales-Cano, D.; Barrese, V.; Esquivel-Ruiz, S.; Olivencia, M.A.; Macias, M.; Moreno, L.; Greenwood, I.A.; et al. Uncovered Contribution of Kv7 Channels to Pulmonary Vascular Tone in Pulmonary Arterial Hypertension. Hypertension 2020, 76, 1134–1146. [Google Scholar] [CrossRef]
- Strutz-Seebohm, N.; Seebohm, G.; Fedorenko, O.; Baltaev, R.; Engel, J.; Knirsch, M.; Lang, F. Functional coassembly of KCNQ4 with KCNE-beta- subunits in Xenopus oocytes. Cell. Physiol. Biochem. 2006, 18, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Jepps, T.A.; Carr, G.; Lundegaard, P.R.; Olesen, S.P.; Greenwood, I.A. Fundamental role for the KCNE4 ancillary subunit in Kv7.4 regulation of arterial tone. J. Physiol. 2015, 593, 5325–5340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roth, D.E.; Abrams, S.A.; Aloia, J.; Bergeron, G.; Bourassa, M.W.; Brown, K.H.; Calvo, M.S.; Cashman, K.D.; Combs, G.; De-Regil, L.M.; et al. Global prevalence and disease burden of vitamin D deficiency: A roadmap for action in low- and middle-income countries. Ann. N. Y. Acad. Sci. 2018, 1430, 44–79. [Google Scholar] [CrossRef]
- Bikle, D.; Christakos, S. New aspects of vitamin D metabolism and action—Addressing the skin as source and target. Nat. Rev. Endocrinol. 2020, 16, 234–252. [Google Scholar] [CrossRef]
- Christakos, S.; Dhawan, P.; Verstuyf, A.; Verlinden, L.; Carmeliet, G. Vitamin D: Metabolism, Molecular Mechanism of Action, and Pleiotropic Effects. Physiol. Rev. 2016, 96, 365–408. [Google Scholar] [CrossRef] [Green Version]
- Schottker, B.; Jorde, R.; Peasey, A.; Thorand, B.; Jansen, E.H.; Groot, L.; Streppel, M.; Gardiner, J.; Ordonez-Mena, J.M.; Perna, L.; et al. Vitamin D and mortality: Meta-analysis of individual participant data from a large consortium of cohort studies from Europe and the United States. BMJ 2014, 348, g3656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Callejo, M.; Mondejar-Parreño, G.; Esquivel-Ruiz, S.; Olivencia, M.A.; Moreno, L.; Blanco, I.; Escribano-Subias, P.; Cogolludo, A.; Barbera, J.A.; Perez-Vizcaino, F. Total, Bioavailable, and Free Vitamin D Levels and Their Prognostic Value in Pulmonary Arterial Hypertension. J. Clin. Med. 2020, 9, 448. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, H.; Kataoka, M.; Isobe, S.; Yamamoto, T.; Shirakawa, K.; Endo, J.; Satoh, T.; Hakamata, Y.; Kobayashi, E.; Sano, M.; et al. Therapeutic impact of dietary vitamin D supplementation for preventing right ventricular remodeling and improving survival in pulmonary hypertension. PLoS ONE 2017, 12, e0180615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Callejo, M.; Mondejar-Parreno, G.; Morales-Cano, D.; Barreira, B.; Esquivel-Ruiz, S.; Olivencia, M.A.; Manaud, G.; Perros, F.; Duarte, J.; Moreno, L.; et al. Vitamin D deficiency downregulates TASK-1 channels and induces pulmonary vascular dysfunction. Am. J. Physiol. Lung Cell. Mol. Physiol. 2020, 319, L627–L640. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Lu, R.; Zhang, Y.G.; Sun, J. Vitamin D Receptor Deletion Leads to the Destruction of Tight and Adherens Junctions in Lungs. Tissue Barriers 2018, 6, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Meca, A.D.; Stefanescu, S.; Bogdan, M.; Turcu-Stiolica, A.; Nitu, F.M.; Matei, M.; Cioboata, R.; Buga, A.M.; Pisoschi, C.G. Crosstalk between vitamin D axis, inflammation and host immunity mechanisms: A prospective study. Exp. Ther. Med. 2021, 21, 608. [Google Scholar] [CrossRef] [PubMed]
- Roth, M.; Sun, Q.; Tamm, M. Up-Regulated Vitamin D Receptor by Pelargonium sidoides Extract EPs((R)) 7630 Contributes to Rhinovirus Defense in Bronchial Epithelial Cells. Pharmaceuticals 2021, 14, 172. [Google Scholar] [CrossRef]
- Fei, J.; Fu, L.; Cao, W.; Hu, B.; Zhao, H.; Li, J.B. Low Vitamin D Status Is Associated with Epithelial-Mesenchymal Transition in Patients with Chronic Obstructive Pulmonary Disease. J. Immunol. 2019, 203, 1428–1435. [Google Scholar] [CrossRef]
- Tzilas, V.; Bouros, E.; Barbayianni, I.; Karampitsakos, T.; Kourtidou, S.; Ntassiou, M.; Ninou, I.; Aidinis, V.; Bouros, D.; Tzouvelekis, A. Vitamin D prevents experimental lung fibrosis and predicts survival in patients with idiopathic pulmonary fibrosis. Pulm. Pharmacol. Ther. 2019, 55, 17–24. [Google Scholar] [CrossRef]
- Callejo, M. Vitamin D in Pulmonary Hypertension. Doctoral Thesis, Universidad Complutense de Madrid, Madrid, Spain, 2021. [Google Scholar]
- Li, Y.C.; Amling, M.; Pirro, A.E.; Priemel, M.; Meuse, J.; Baron, R.; Delling, G.; Demay, M.B. Normalization of mineral ion homeostasis by dietary means prevents hyperparathyroidism, rickets, and osteomalacia, but not alopecia in vitamin D receptor-ablated mice. Endocrinology 1998, 139, 4391–4396. [Google Scholar] [CrossRef]
- Ferrer-Mayorga, G.; Gomez-Lopez, G.; Barbachano, A.; Fernandez-Barral, A.; Pena, C.; Pisano, D.G.; Cantero, R.; Rojo, F.; Munoz, A.; Larriba, M.J. Vitamin D receptor expression and associated gene signature in tumour stromal fibroblasts predict clinical outcome in colorectal cancer. Gut 2017, 66, 1449–1462. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.C.; Pirro, A.E.; Amling, M.; Delling, G.; Baron, R.; Bronson, R.; Demay, M.B. Targeted ablation of the vitamin D receptor: An animal model of vitamin D-dependent rickets type II with alopecia. Proc. Natl. Acad. Sci. USA 1997, 94, 9831–9835. [Google Scholar] [CrossRef]
- Gomez-Arroyo, J.; Saleem, S.J.; Mizuno, S.; Syed, A.A.; Bogaard, H.J.; Abbate, A.; Taraseviciene-Stewart, L.; Sung, Y.; Kraskauskas, D.; Farkas, D.; et al. A brief overview of mouse models of pulmonary arterial hypertension: Problems and prospects. Am. J. Physiol. Lung Cell. Mol. Physiol. 2012, 302, L977–L991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mondejar-Parreno, G.; Moral-Sanz, J.; Barreira, B.; De la Cruz, A.; Gonzalez, T.; Callejo, M.; Esquivel-Ruiz, S.; Morales-Cano, D.; Moreno, L.; Valenzuela, C.; et al. Activation of K(v) 7 channels as a novel mechanism for NO/cGMP-induced pulmonary vasodilation. Br. J. Pharmacol. 2019, 176, 2131–2145. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Vishwakarma, V.K.; Arava, S.K.; Mridha, A.R.; Yadav, R.K.; Seth, S.; Bhatia, J.; Hote, M.P.; Arya, D.S.; Yadav, H.N. Differential effect of basal vitamin D status in monocrotaline induced pulmonary arterial hypertension in normal and vitamin D deficient rats: Possible involvement of eNOS/TGF-beta/alpha-SMA signaling pathways. J. Nutr. Biochem. 2023, 113, 109246. [Google Scholar] [CrossRef]
- Mondejar-Parreno, G.; Cogolludo, A.; Perez-Vizcaino, F. Potassium (K(+)) channels in the pulmonary vasculature: Implications in pulmonary hypertension Physiological, pathophysiological and pharmacological regulation. Pharmacol. Ther. 2021, 225, 107835. [Google Scholar] [CrossRef]
- Perez-Vizcaino, F.; Cogolludo, A.; Mondejar-Parreno, G. Transcriptomic profile of cationic channels in human pulmonary arterial hypertension. Sci. Rep. 2021, 11, 15829. [Google Scholar] [CrossRef]
- Manoury, B.; Lamalle, C.; Oliveira, R.; Reid, J.; Gurney, A.M. Contractile and electrophysiological properties of pulmonary artery smooth muscle are not altered in TASK-1 knockout mice. J. Physiol. 2011, 589, 3231–3246. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.T.; Tavera-Mendoza, L.E.; Laperriere, D.; Libby, E.; MacLeod, N.B.; Nagai, Y.; Bourdeau, V.; Konstorum, A.; Lallemant, B.; Zhang, R.; et al. Large-scale in silico and microarray-based identification of direct 1,25-dihydroxyvitamin D3 target genes. Mol. Endocrinol. 2005, 19, 2685–2695. [Google Scholar] [CrossRef] [Green Version]
- Morales-Cano, D.; Menendez, C.; Moreno, E.; Moral-Sanz, J.; Barreira, B.; Galindo, P.; Pandolfi, R.; Jimenez, R.; Moreno, L.; Cogolludo, A.; et al. The flavonoid quercetin reverses pulmonary hypertension in rats. PLoS ONE 2014, 9, e114492. [Google Scholar] [CrossRef] [Green Version]
- Meyrick, B.; Hislop, A.; Reid, L. Pulmonary arteries of the normal rat: The thick walled oblique muscle segment. J. Anat. 1978, 125, 209–221. [Google Scholar]
- Meyrick, B.; Reid, L. Pulmonary arterial and alveolar development in normal postnatal rat lung. Am. Rev. Respir. Dis. 1982, 125, 468–473. [Google Scholar] [CrossRef] [PubMed]
- Cogolludo, A.; Moreno, L.; Lodi, F.; Frazziano, G.; Cobeno, L.; Tamargo, J.; Perez-Vizcaino, F. Serotonin inhibits voltage-gated K+ currents in pulmonary artery smooth muscle cells: Role of 5-HT2A receptors, caveolin-1, and KV1.5 channel internalization. Circ. Res. 2006, 98, 931–938. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Moreira, B. 3D-footprint: A database for the structural analysis of protein-DNA complexes. Nucleic Acids Res. 2010, 38, D91–D97. [Google Scholar] [CrossRef] [PubMed] [Green Version]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olivencia, M.A.; Villegas-Esguevillas, M.; Sancho, M.; Barreira, B.; Paternoster, E.; Adão, R.; Larriba, M.J.; Cogolludo, A.; Perez-Vizcaino, F. Vitamin D Receptor Deficiency Upregulates Pulmonary Artery Kv7 Channel Activity. Int. J. Mol. Sci. 2023, 24, 12350. https://doi.org/10.3390/ijms241512350
Olivencia MA, Villegas-Esguevillas M, Sancho M, Barreira B, Paternoster E, Adão R, Larriba MJ, Cogolludo A, Perez-Vizcaino F. Vitamin D Receptor Deficiency Upregulates Pulmonary Artery Kv7 Channel Activity. International Journal of Molecular Sciences. 2023; 24(15):12350. https://doi.org/10.3390/ijms241512350
Chicago/Turabian StyleOlivencia, Miguel A., Marta Villegas-Esguevillas, Maria Sancho, Bianca Barreira, Elena Paternoster, Rui Adão, María Jesús Larriba, Angel Cogolludo, and Francisco Perez-Vizcaino. 2023. "Vitamin D Receptor Deficiency Upregulates Pulmonary Artery Kv7 Channel Activity" International Journal of Molecular Sciences 24, no. 15: 12350. https://doi.org/10.3390/ijms241512350
APA StyleOlivencia, M. A., Villegas-Esguevillas, M., Sancho, M., Barreira, B., Paternoster, E., Adão, R., Larriba, M. J., Cogolludo, A., & Perez-Vizcaino, F. (2023). Vitamin D Receptor Deficiency Upregulates Pulmonary Artery Kv7 Channel Activity. International Journal of Molecular Sciences, 24(15), 12350. https://doi.org/10.3390/ijms241512350