Coffea arabica Extract Attenuates Atopic Dermatitis-like Skin Lesions by Regulating NLRP3 Inflammasome Expression and Skin Barrier Functions
Abstract
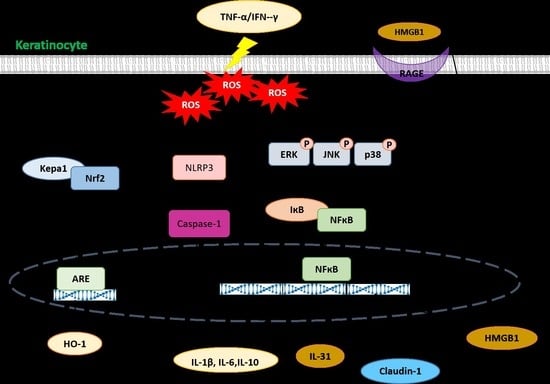
:1. Introduction
2. Results
2.1. CAE Mediates Cytotoxicity in HaCaT Cells
2.2. CAE Attenuates Oxidative Stress in HaCaT Cells
2.2.1. Effects of CAE on IFN-γ- and TNF-α-Induced Intracellular ROS Generation
2.2.2. Effects of CAE on IFN-γ- and TNF-α-Induced Phospho (p)-Extracellular Signal-Regulated Kinase (ERK) and p-p38 Expression Levels
2.3. CAE Reduces Inflammatory Signal Transduction in HaCaT Cells
2.3.1. Effects of CAE on IFN-γ- and TNF-α-Induced NF-κB Nuclear Translocation
2.3.2. Effects of CAE on IFN-γ- and TNF-α-Induced NLR Family Pyrin Domain-Containing 3 (NLRP3) Signaling Pathway
2.3.3. Effects of CAE on IFN-γ- and TNF-α-Induced IL-1β and IL-6 Cytokines Secretion
2.3.4. Effects of CAE on IFN-γ- and TNF-α-Induced High-Mobility Group Box 1 (HMGB1) and Receptor for Advanced Glycation End Products (RAGE) Expression Levels
2.4. CAE Restores the Skin Barrier Function-Related Marker Expression in HaCaT Cells
2.5. CAE Improves 2,4-Dinitrochlorobenzene (DNCB)-Induced AD-like Skin Lesions in Mice
2.5.1. Effects of CAE on DNCB-Induced Body Weights
2.5.2. Effects of CAE on DNCB-Induced a* Values
2.5.3. Effects of CAE on DNCB-Induced Ear Thickness
2.5.4. Effects of CAE on DNCB-Induced TEWL
2.5.5. Effects of CAE on DNCB-Induced Chemokine and Cytokine Levels
2.5.6. Effects of CAE on DNCB-Induced Changes in Skin Pathology
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Preparation and Quantitative Analysis of CAE
4.3. In Vitro Model
4.3.1. Cell Viability Test
4.3.2. Intracellular ROS Scavenging Assay
4.3.3. Western Blotting
4.3.4. Immunofluorescence Staining
4.3.5. Secreted Protein Measurement
4.4. In Vivo Model
4.4.1. Experimental Design
4.4.2. Body Weight Measurement
4.4.3. a* Value Measurement
4.4.4. Ear Thickness Measurement
4.4.5. TEWL Measurement
4.4.6. Determination of Chemokine and Cytokine Levels
4.4.7. Evaluation of Skin Pathology Changes
4.5. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bylund, S.; von Kobyletzki, L.B.; Svalstedt, M.; Svensson, Å. Prevalence and incidence of atopic dermatitis: A systematic review. Acta Derm.-Venereol. 2020, 100, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.; Pona, A.; Kolli, S.S.; Feldman, S.R.; Strowd, L.C. Recent insights in atopic dermatitis pathogenesis, treatment, and disease impact. J. Dermatol. Dermatol. Surg. 2019, 23, 66–72. [Google Scholar]
- Chiricozzi, A.; Maurelli, M.; Calabrese, L.; Peris, K.; Girolomoni, G. Overview of Atopic Dermatitis in Different Ethnic Groups. J. Clin. Med. 2023, 12, 2701. [Google Scholar] [CrossRef] [PubMed]
- Tollefson, M.M.; Bruckner, A.L. Atopic dermatitis: Skin-directed management. Pediatrics 2014, 134, e1735–e1744. [Google Scholar] [CrossRef] [Green Version]
- Elias, P.M.; Steinhoff, M. “Outside-to-inside”(and now back to “outside”) pathogenic mechanisms in atopic dermatitis. J. Investig. Dermatol. 2008, 128, 1067–1070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, B.E.; Leung, D.Y. Significance of skin barrier dysfunction in atopic dermatitis. Allergy Asthma Immunol. Res. 2018, 10, 207–215. [Google Scholar] [CrossRef] [Green Version]
- Goodwin, C. Anatomy and Physiology of the Skin. J. Dermatol. Nurses Assoc. 2011, 3, 203–213. [Google Scholar]
- Esche, C.; de Benedetto, A.; Beck, L.A. Keratinocytes in atopic dermatitis: Inflammatory signals. Curr. Allergy Asthma Rep. 2004, 4, 276–284. [Google Scholar] [CrossRef]
- Meagher, L.J.; Wines, N.Y.; Cooper, A.J. Atopic dermatitis: Review of immunopathogenesis and advances in immunosuppressive therapy. Aust. J. Dermatol. 2002, 43, 247–254. [Google Scholar] [CrossRef]
- Leung, D.Y.; Boguniewicz, M.; Howell, M.D.; Nomura, I.; Hamid, Q.A. New insights into atopic dermatitis. J. Clin. Investig. 2004, 113, 651–657. [Google Scholar] [CrossRef]
- Leung, D.Y. Atopic dermatitis: New insights and opportunities for therapeutic intervention. J. Allergy Clin. Immunol. 2000, 105, 860–876. [Google Scholar] [CrossRef] [PubMed]
- Cabanillas, B.; Novak, N. Atopic dermatitis and filaggrin. Curr. Opin. Immunol. 2016, 42, 1–8. [Google Scholar] [CrossRef]
- Palmer, C.N.; Irvine, A.D.; Terron-Kwiatkowski, A.; Zhao, Y.; Liao, H.; Lee, S.P.; Goudie, D.R.; Sandilands, A.; Campbell, L.E.; Smith, F.J.; et al. Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nat. Genet. 2006, 38, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Jakasa, I.; Koster, E.S.; Calkoen, F.; McLean, W.H.; Campbell, L.E.; Bos, J.D.; Verberk, M.M.; Kezic, S. Skin barrier function in healthy subjects and patients with atopic dermatitis in relation to filaggrin loss-of-function mutations. J. Investig. Dermatol. 2011, 131, 540–542. [Google Scholar] [CrossRef] [Green Version]
- Tokumasu, R.; Yamaga, K.; Yamazaki, Y.; Murota, H.; Suzuki, K.; Tamura, A.; Bando, K.; Furuta, Y.; Katayama, I.; Tsukita, S. Dose-dependent role of claudin-1 In Vivo in orchestrating features of atopic dermatitis. Proc. Natl. Acad. Sci. USA 2016, 113, E4061–E4068. [Google Scholar] [CrossRef]
- Coondoo, A.; Phiske, M.; Verma, S.; Lahiri, K. Side-effects of topical steroids: A long overdue revisit. Indian Dermatol. Online J. 2014, 5, 416–425. [Google Scholar] [CrossRef]
- Ngamsuk, S.; Huang, T.-C.; Hsu, J.-L. Determination of phenolic compounds, procyanidins, and antioxidant activity in processed Coffea arabica L. leaves. Foods 2019, 8, 389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, C.-S.; Chiang, H.-M.; Chen, Y.; Chen, C.-Y.; Chen, H.-F.; Su, W.-C.; Wang, W.-J.; Chou, Y.-C.; Chang, W.-C.; Wang, S.-C. Prospects of Coffee Leaf against SARS-CoV-2 Infection. Int. J. Biol. Sci. 2022, 18, 4677. [Google Scholar] [CrossRef]
- Chiang, H.-M.; Lin, T.-J.; Chiu, C.-Y.; Chang, C.-W.; Hsu, K.-C.; Fan, P.-C.; Wen, K.-C. Coffea arabica extract and its constituents prevent photoaging by suppressing MMPs expression and MAP kinase pathway. Food Chem. Toxicol. 2011, 49, 309–318. [Google Scholar] [CrossRef]
- Wu, P.-Y.; Huang, C.-C.; Chu, Y.; Huang, Y.-H.; Lin, P.; Liu, Y.-H.; Wen, K.-C.; Lin, C.-Y.; Hsu, M.-C.; Chiang, H.-M. Alleviation of ultraviolet B-induced photodamage by Coffea arabica extract in human skin fibroblasts and hairless mouse skin. Int. J. Mol. Sci. 2017, 18, 782. [Google Scholar] [CrossRef] [Green Version]
- Thomsen, S.F. Atopic dermatitis: Natural history, diagnosis, and treatment. Int. Sch. Res. Not. 2014, 2014, 354250. [Google Scholar] [CrossRef] [Green Version]
- Wollenberg, A.; Bieber, T. Atopic dermatitis: From the genes to skin lesions. Allergy 2000, 55, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Mesjasz, A.; Zawadzka, M.; Chałubiński, M.; Trzeciak, M. Is Atopic Dermatitis Only a Skin Disease? Int. J. Mol. Sci. 2023, 24, 837. [Google Scholar] [CrossRef] [PubMed]
- Makowska, K.; Nowaczyk, J.; Blicharz, L.; Waśkiel-Burnat, A.; Czuwara, J.; Olszewska, M.; Rudnicka, L. Immunopathogenesis of Atopic Dermatitis: Focus on Interleukins as Disease Drivers and Therapeutic Targets for Novel Treatments. Int. J. Mol. Sci. 2023, 24, 781. [Google Scholar] [CrossRef]
- Ji, H.; Li, X.-K. Oxidative stress in atopic dermatitis. Oxid. Med. Cell. Longev. 2016, 2016, 2721469. [Google Scholar] [CrossRef]
- Pan, Z.; Dai, Y.; Akar-Ghibril, N.; Simpson, J.; Ren, H.; Zhang, L.; Hou, Y.; Wen, X.; Chang, C.; Tang, R. Impact of Air Pollution on Atopic Dermatitis: A Comprehensive Review. Clin. Rev. Allergy Immunol. 2023, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.-H.; Chiang, H.-L.; Wu, P.-Y.; Chu, Y.; Chang, Q.-X.; Wen, K.-C.; Lin, C.-Y.; Chiang, H.-M. Protection against Ultraviolet A-Induced Skin Apoptosis and Carcinogenesis through the Oxidative Stress Reduction Effects of N-(4-bromophenethyl) Caffeamide, A Propolis Derivative. Antioxidants 2020, 9, 335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bentrad, N.; Gaceb-Terrak, R. Evaluation of the level of biomolecules isolated from date palm seeds (Phoenix dactylifera L) and in vitro Antioxidant property. BioMedicine 2020, 10, 23–29. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, H.; Qi, C.; Guo, H.; Jiao, X.; Yan, J.; Wang, Y.; Li, Q.; Zhao, M.; Guo, X. Ursolic acid ameliorates DNCB-induced atopic dermatitis-like symptoms in mice by regulating TLR4/NF-κB and Nrf2/HO-1 signaling pathways. Int. Immunopharmacol. 2023, 118, 110079. [Google Scholar] [CrossRef]
- Tang, L.; Zhou, F. Inflammasomes in common immune-related skin diseases. Front. Immunol. 2020, 11, 882. [Google Scholar] [CrossRef]
- Shao, B.-Z.; Xu, Z.-Q.; Han, B.-Z.; Su, D.-F.; Liu, C. NLRP3 inflammasome and its inhibitors: A review. Front. Pharmacol. 2015, 6, 262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dinarello, C.A. A clinical perspective of IL-1β as the gatekeeper of inflammation. Eur. J. Immunol. 2011, 41, 1203–1217. [Google Scholar] [CrossRef] [PubMed]
- Erlandsson Harris, H.; Andersson, U. Mini-review: The nuclear protein HMGB1 as a proinflammatory mediator. Eur. J. Immunol. 2004, 34, 1503–1512. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; You, H.; Li, X.-M.; Liu, T.-H.; Wang, P.; Wang, B.-E. HMGB1 promotes the synthesis of pro-IL-1β and pro-IL-18 by activation of p38 MAPK and NF-κB through receptors for advanced glycation end-products in macrophages. Asian Pac. J. Cancer Prev. 2012, 13, 1365–1370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qu, D.; Ling, Z.; Tan, X.; Chen, Y.; Huang, Q.; Li, M.; Liu, T.; Hou, C.; Chen, Y. High mobility group protein B1 (HMGB1) interacts with receptor for advanced glycation end products (RAGE) to promote airway smooth muscle cell proliferation through ERK and NF-κB pathways. Int. J. Clin. Exp. Pathol. 2019, 12, 3268. [Google Scholar] [PubMed]
- Ko, K.I.; Merlet, J.J.; DerGarabedian, B.P.; Zhen, H.; Suzuki-Horiuchi, Y.; Hedberg, M.L.; Hu, E.; Nguyen, A.T.; Prouty, S.; Alawi, F. NF-κB perturbation reveals unique immunomodulatory functions in Prx1+ fibroblasts that promote development of atopic dermatitis. Sci. Transl. Med. 2022, 14, eabj0324. [Google Scholar] [CrossRef]
- Zainal, H.; Jamil, A.; Md Nor, N.; Tang, M.M. Skin pH mapping and its relationship with transepidermal water loss, hydration and disease severity in adult patients with atopic dermatitis. Ski. Res. Technol. 2020, 26, 91–98. [Google Scholar] [CrossRef]
- Montero-Vilchez, T.; Segura-Fernández-Nogueras, M.-V.; Pérez-Rodríguez, I.; Soler-Gongora, M.; Martinez-Lopez, A.; Fernández-González, A.; Molina-Leyva, A.; Arias-Santiago, S. Skin barrier function in psoriasis and atopic dermatitis: Transepidermal water loss and temperature as useful tools to assess disease severity. J. Clin. Med. 2021, 10, 359. [Google Scholar] [CrossRef]
- Yamada, K.; Matsushita, K.; Jingshu, W.; Kanekura, T. Topical Glucose Induces Claudin-1 and Filaggrin Expression in a Mouse Model of Atopic Dermatitis and in Keratinocyte Culture, Exerting Anti-inflammatory Effects by Repairing Skin Barrier Function. Acta Derm. Venereol. 2018, 98, 19–25. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.-M.; Kang, Y.-M.; Jin, B.-R.; Lee, H.; Lee, D.-S.; Lee, M.; An, H.-J. Morus alba fruits attenuates atopic dermatitis symptoms and pathology in vivo and in vitro via the regulation of barrier function, immune response and pruritus. Phytomedicine 2023, 109, 154579. [Google Scholar] [CrossRef]
- Furuse, M.; Hata, M.; Furuse, K.; Yoshida, Y.; Haratake, A.; Sugitani, Y.; Noda, T.; Kubo, A.; Tsukita, S. Claudin-based tight junctions are crucial for the mammalian epidermal barrier a lesson from claudin-1–deficient mice. J. Cell Biol. 2002, 156, 1099–1111. [Google Scholar] [CrossRef]
- De Benedetto, A.; Slifka, M.K.; Rafaels, N.M.; Kuo, I.-H.; Georas, S.N.; Boguniewicz, M.; Hata, T.; Schneider, L.C.; Hanifin, J.M.; Gallo, R.L. Reductions in claudin-1 may enhance susceptibility to herpes simplex virus 1 infections in atopic dermatitis. J. Allergy Clin. Immunol. 2011, 128, 242–246.e245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chu, Y.; Wu, P.-Y.; Chen, C.-W.; Lyu, J.-L.; Liu, Y.-J.; Wen, K.-C.; Lin, C.-Y.; Kuo, Y.-H.; Chiang, H.-M. Protective effects and mechanisms of N-Phenethyl Caffeamide from UVA-induced skin damage in human epidermal keratinocytes through Nrf2/HO-1 regulation. Int. J. Mol. Sci. 2019, 20, 164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, P.-Y.; Lin, T.-Y.; Hou, C.-W.; Chang, Q.-X.; Wen, K.-C.; Lin, C.-Y.; Chiang, H.-M. 1,2-Bis[(3-methoxyphenyl)methyl]ethane-1,2-dicarboxylic acid reduces UVB-induced photodamage in vitro and in vivo. Antioxidants 2019, 8, 452. [Google Scholar] [CrossRef] [PubMed] [Green Version]


















Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, Q.-X.; Lyu, J.-L.; Wu, P.-Y.; Wen, K.-C.; Chang, C.-C.; Chiang, H.-M. Coffea arabica Extract Attenuates Atopic Dermatitis-like Skin Lesions by Regulating NLRP3 Inflammasome Expression and Skin Barrier Functions. Int. J. Mol. Sci. 2023, 24, 12367. https://doi.org/10.3390/ijms241512367
Chang Q-X, Lyu J-L, Wu P-Y, Wen K-C, Chang C-C, Chiang H-M. Coffea arabica Extract Attenuates Atopic Dermatitis-like Skin Lesions by Regulating NLRP3 Inflammasome Expression and Skin Barrier Functions. International Journal of Molecular Sciences. 2023; 24(15):12367. https://doi.org/10.3390/ijms241512367
Chicago/Turabian StyleChang, Qiao-Xin, Jia-Ling Lyu, Po-Yuan Wu, Kuo-Ching Wen, Chang-Cheng Chang, and Hsiu-Mei Chiang. 2023. "Coffea arabica Extract Attenuates Atopic Dermatitis-like Skin Lesions by Regulating NLRP3 Inflammasome Expression and Skin Barrier Functions" International Journal of Molecular Sciences 24, no. 15: 12367. https://doi.org/10.3390/ijms241512367
APA StyleChang, Q. -X., Lyu, J. -L., Wu, P. -Y., Wen, K. -C., Chang, C. -C., & Chiang, H. -M. (2023). Coffea arabica Extract Attenuates Atopic Dermatitis-like Skin Lesions by Regulating NLRP3 Inflammasome Expression and Skin Barrier Functions. International Journal of Molecular Sciences, 24(15), 12367. https://doi.org/10.3390/ijms241512367