Role of Neurotrophins in Orofacial Pain Modulation: A Review of the Latest Discoveries
Abstract
:1. Introduction
1.1. Orofacial Pain Classification
1.2. Orofacial Pain Differential Diagnosis
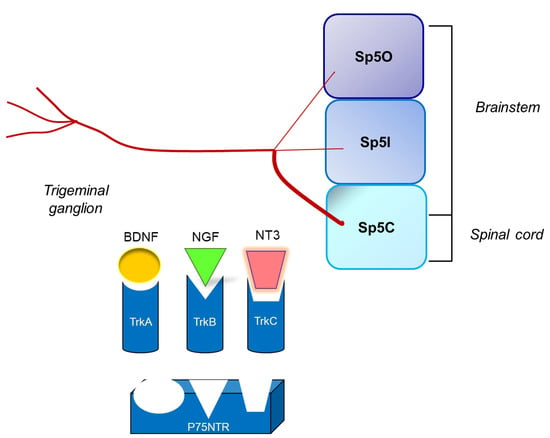
1.3. Anatomical Structures Involved in Orofacial Nociception
1.4. Neurochemistry of Orofacial Nociception: The Neurotrophins
2. Research Findings on Neurotrophins during the Last 10 Years (2013–2023)
2.1. Neurotrophins in Peripheral Orofacial Tissues
2.1.1. Nociceptive Orofacial Pain Modulation
2.1.2. Neuropathic Orofacial Pain and Migraine Modulation
2.2. Neurotrophins in Trigeminal Ganglia
2.2.1. Nociceptive Orofacial Pain Modulation
2.2.2. Neuropathic Orofacial Pain and Migraine Modulation
2.3. Neurotrophins in Brainstem Trigeminal Nucleus
2.3.1. Nociceptive Orofacial Pain Modulation
2.3.2. Neuropathic Orofacial Pain and Migraine Pain Modulation
3. Discussion
4. Conclusions
5. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Akt | protein kinase B |
| ASIC3 | acid-sensing ion channel 3 |
| Atp6v0a1 | V-type proton ATPase subunit isoform 1 |
| BAD | BCL-2-associated death promoter |
| BDNF | brain-derived neurotrophic factor |
| CCI-ION | chronic constriction injury of the infraorbital nerve |
| CCL2 | monocyte chemoattractant protein-1, MCP-1 |
| CCL19 | chemokine (C-C motif) ligand 19 |
| CGRP | calcitonin gene-related peptide |
| c-Jun | transcription factor Jun |
| COX-2 | cyclooxygenase-2 |
| CREB | cAMP response element-binding protein |
| DAG | diacylglycerol |
| DRG | dorsal root ganglion |
| ERK | extracellular signal-regulated kinase |
| FD-PRP | freeze-dried platelet-rich plasma |
| Gab1 | GRB2-associated binding protein 1 |
| GABA | gamma-aminobutyric acid |
| GDNF | glia-derived neurotrophic factors |
| GRB2 | growth factor receptor-bound protein 2 |
| GTP | guanosine 5′-triphosphate |
| IANX | inferior alveolar nerve transection |
| IASP | International Association for the Study of Pain |
| IB4 | isolectin B4 |
| ICOP | International Classification of Orofacial Pain |
| IR | idiopathic rhinitis |
| MAPK | mitogen-activated protein kinase |
| Me5 | mesencephalic nucleus |
| NF-kB | nuclear factor kappa-light-chain enhancer of activated B cells |
| NGF | nerve growth factor |
| NMDA | N-methyl-d-aspartate |
| NR2A | NMDA receptor subunit subtype 2A |
| NR2B | NMDA receptor subunit subtype 2B |
| NT-3 | neurotrophin-3 |
| NT-4 or NT-4/5 | neurotrophin-4 or neurotrophin-4/5 |
| OX1R | orexin receptor 1 |
| p38 | p38 mitogen-activated protein kinases |
| p75NTR | p75 neurotrophin receptor |
| PAC1R | PAC1 receptor |
| PACAP | pituitary adenylate cyclase-activating peptide |
| PI3K | phosphoinositide 3-kinase |
| PKA | protein kinase A |
| PKC | protein kinase C |
| PLC | phospholipase C |
| PLC-γ | phospholipase C gamma |
| Pr5 | primary nucleus |
| RAF | rapidly accelerated fibrosarcoma protein |
| RET | proto-oncogene tyrosine kinase “rearranged during transfection” |
| SHC | shc transforming protein |
| SOS | sons of sevenless |
| SP | substance-P |
| Sp5 | spinal trigeminal nucleus |
| Sp5C | spinal trigeminal subnucleus caudalis |
| Sp5I | spinal trigeminal subnucleus interpolaris |
| Sp5O | spinal trigeminal subnucleus oralis |
| TG | trigeminal ganglion |
| TMD | temporomandibular disorder |
| TMJ | temporomandibular joint |
| TN | trigeminal nucleus |
| TNF | tumor necrosis factor |
| TrkA | tropomyosin receptor kinase A |
| TrkB | tropomyosin receptor kinase B |
| TrkC | tropomyosin receptor kinase C |
| TRPA1 | transient receptor potential ankyrin 1 |
| TRPV1 | transient receptor potential vanilloid 1 |
References
- Sessle, B.J.; Baad-Hansen, L.; Exposto, F.; Svensson, P. Chapter 33: Orofacial pain. In Clinical Pain Management: A Practical Guide, 2nd ed.; Lynch, M.E., Craig, K.D., Peng, P.W., Eds.; Wiley: Hoboken, NJ, USA, 2022; pp. 343–354. [Google Scholar] [CrossRef]
- Rodella, L.F.; Merigo, C.; Borsani, E. Neurochemistry of the trigeminal system. In Clinical, Neurochemical and Experimental Aspects of Orofacial Pain in Dentistry; Rodella, L.F., Labanca, M., Eds.; Research Signpost, Transworld Research Network: Trivandrum, Kerala, India, 2010; pp. 15–34. [Google Scholar]
- Borsani, E.; Albertini, R.; Labanca, M.; Lonati, C.; Rezzani, R.; Rodella, L.F. Peripheral purinergic receptor modulation influences the trigeminal ganglia nitroxidergic system in an experimental murine model of inflammatory orofacial pain. J. Neurosci. Res. 2010, 88, 2715–2726. [Google Scholar] [CrossRef] [PubMed]
- Borsani, E.; Ballini, A.; Buffoli, B.; Muzio, L.L.; Di Domenico, M.; Boccellino, M.; Scacco, S.; Nocini, R.; Dibello, V.; Rezzani, R.; et al. Peripheral purinergic modulation in pediatric orofacial inflammatory pain affects brainstem nitroxidergic system: A translational research. BioMed Res. Int. 2022, 2022, 1326885. [Google Scholar] [CrossRef] [PubMed]
- Borsani, E.; Boninsegna, R.; Rezzani, R. Animal models to study the orofacial pain. In Clinical, Neurochemical and Experimental Aspects of Orofacial Pain in Dentistry; Rodella, L.F., Labanca, M., Eds.; Research Signpost, Transworld Research Network: Trivandrum, Kerala, India, 2010; pp. 73–97. [Google Scholar]
- Borsani, E.; Giovannozzi, S.; Boninsegna, R.; Rezzani, R.; Labanca, M.; Tschabitscher, M.; Rodella, L.F. Nitroxidergic system in human trigeminal ganglia neurons: A quantitative evaluation. Acta Histochem. 2010, 112, 444–4451. [Google Scholar] [CrossRef]
- Borsani, E.; Majorana, A.; Cocchi, M.A.; Conti, G.; Bonadeo, S.; Padovani, A.; Lauria, G.; Bardellini, E.; Rezzani, R.; Rodella, L.F. Epithelial expression of vanilloid and cannabinoid receptors: A potential role in burning mouth syndrome pathogenesis. Histol. Histopathol. 2014, 29, 523–533. [Google Scholar] [CrossRef]
- McFarland, A.J.; Yousuf, M.S.; Shiers, S.; Price, T.J. Neurobiology of SARS-CoV-2 interactions with the peripheral nervous system: Implications for COVID-19 and pain. Pain Rep. 2021, 6, e885. [Google Scholar] [CrossRef]
- Biamonte, F.; Re, A.; Balzamino, B.O.; Ciasca, G.; Santucci, D.; Napodano, C.; Nocca, G.; Fiorita, A.; Marino, M.; Basile, U.; et al. Circulating and salivary NGF and BDNF levels in SARS-CoV-2 infection: Potential predictor biomarkers of COVID-19 disease-preliminary data. J. Pers. Med. 2022, 12, 1877. [Google Scholar] [CrossRef] [PubMed]
- Petrella, C.; Zingaropoli, M.A.; Ceci, F.M.; Pasculli, P.; Latronico, T.; Liuzzi, G.M.; Ciardi, M.R.; Angeloni, A.; Ettorre, E.; Menghi, M.; et al. COVID-19 affects serum brain-derived neurotrophic factor and neurofilament light chain in aged men: Implications for morbidity and mortality. Cells 2023, 12, 655. [Google Scholar] [CrossRef] [PubMed]
- Emodi-Perlman, A.; Eli, I.; Smardz, J.; Uziel, N.; Wieckiewicz, G.; Gilon, E.; Grychowska, N.; Wieckiewicz, M. Temporomandibular disorders and bruxism outbreak as a possible factor of orofacial pain worsening during the COVID-19 pandemic-concomitant research in two countries. J. Clin. Med. 2020, 9, 3250. [Google Scholar] [CrossRef] [PubMed]
- Saki, M.; Shadmanpour, M.; Zarif Najafi, H. Are individuals with orofacial pain more prone to psychological distress during the COVID-19 pandemic? Dent. Med. Probl. 2021, 58, 17–25. [Google Scholar] [CrossRef]
- Treede, R.D.; Jensen, T.S.; Campbell, J.N.; Cruccu, G.; Dostrovsky, J.O.; Griffin, J.W.; Hansson, P.; Hughes, R.; Nurmikko, T.; Serra, J. Neuropathic pain: Redefinition and a grading system for clinical and research purposes. Neurology 2008, 70, 1630–1635. [Google Scholar] [CrossRef]
- Khan, N.; Smith, M.T. Neurotrophins and neuropathic pain: Role in pathobiology. Molecules 2015, 20, 10657–10688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merskey, H.; Bogduk, N. Classification of Chronic Pain, 2nd ed.; IASP Press: Seattle, WA, USA, 1994; 394p. [Google Scholar]
- International Classification of Orofacial Pain, 1st edition (ICOP). Cephalalgia 2020, 40, 129–221. [CrossRef] [PubMed] [Green Version]
- Benedet, T.; Gonzalez, P.; Oliveros, J.C.; Dopazo, J.M.; Ghimire, K.; Palczewska, M.; Mellstrom, B.; Naranjo, J.R. Transcriptional repressor DREAM regulates trigeminal noxious perception. J. Neurochem. 2017, 141, 544–552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dubner, R.; Sessle, B.J.; Storey, A.T. The Neural Basis of Oral and Facial Function; Plenum Press: New York, NY, USA, 1978. [Google Scholar]
- Svensson, P.; Sessle, B.J. Chapter 4: Orofacial Pain. In Clinical Oral Physiology; Miles, T.S., Nauntofte, B., Svensson, P., Eds.; Quintessence: Copenhagen, Denmark, 2004; pp. 93–139. ISBN 978-1-85097-091-0. [Google Scholar]
- Sessle, B.J. Acute and chronic craniofacial pain: Brainstem mechanisms of nociceptive transmission and neuroplasticity, and their clinical correlates. Crit. Rev. Oral Biol. Med. 2000, 11, 57–91. [Google Scholar] [CrossRef] [PubMed]
- DaSilva, A.F.; Becerra, L.; Makris, N.; Strassman, A.M.; Gonzalez, R.G.; Geatrakis, N.; Borsook, D. Somatotopic activation in the human trigeminal pain pathway. J. Neurosci. 2002, 22, 8183–8192. [Google Scholar] [CrossRef] [Green Version]
- Dubner, R.; Bennett, G.J. Spinal and trigeminal mechanisms of nociception. Annu. Rev. Neurosci. 1983, 6, 381–418. [Google Scholar] [CrossRef]
- Ren, K.; Dubner, R. Central nervous system plasticity and persistent pain. J. Orofac. Pain. 1999, 13, 155–171. [Google Scholar]
- Sessle, B.J. Brainstem Mechanisms of Orofacial Pain; Fricton, J.R., Dubner, R., Eds.; Raven Press: New York, NY, USA, 1995; p. 45. [Google Scholar]
- Capra, N.F.; Dessem, D. Central connections of trigeminal primary afferent neurons: Topographical and functional considerations. Crit. Rev. Oral Biol. Med. 1992, 4, 1–52. [Google Scholar] [CrossRef] [Green Version]
- Capra, N.F. Localization and central projections of primary afferent neurons that innervate the temporomandibular joint in cats. Somatosens. Res. 1987, 4, 201–213. [Google Scholar] [CrossRef]
- Li, J.L.; Wang, D.; Kaneko, T.; Shigemoto, R.; Nomura, S.; Mizuno, N. The relationship between neurokinin-1 receptor and substance P in the medullary dorsal horn: A light and electron microscopic immunohistochemical study in the rat. Neurosci. Res. 2000, 36, 327–334. [Google Scholar] [CrossRef]
- Sugimoto, T.; Funahashi, M.; Xiao, C.; Adachi, A.; Ichikawa, H. Exaggerated C-fiber activation prevents peripheral nerve injury-induced hyperinducibility of c-Fos in partially deafferented spinal dorsal horn. Neurosci. Res. 1997, 27, 161–167. [Google Scholar] [CrossRef]
- Bereiter, D.A.; Shen, S.; Benetti, A.P. Sex differences in amino acid release from rostral trigeminal subnucleus caudalis after acute injury to the TMJ region. Pain 2002, 98, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Imbe, H.; Dubner, R.; Ren, K. Masseteric inflammation-induced Fos protein expression in the trigeminal interpolaris/caudalis transition zone: Contribution of somatosensory-vagal-adrenal integration. Brain Res. 1999, 845, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Sugiyo, S.; Takemura, M.; Dubner, R.; Ren, K. Trigeminal transition zone/rostral ventral medial medulla connections and facilitation of orofacial hyperalgesia after masseter muscle inflammation in rats. J. Comp. Neurol. 2005, 493, 510–523. [Google Scholar] [CrossRef] [PubMed]
- Ren, K.; Dubner, R. The role of trigeminal interpolaris-caudalis transition zone in persistent orofacial pain. Int. Rev. Neurobiol. 2011, 97, 207–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tyler, W.J.; Perrett, S.P.; Pozzo-Miller, L.D. The role of neurotrophins in neurotransmitter release. Neuroscientist 2002, 8, 524–531. [Google Scholar] [CrossRef] [Green Version]
- Huang, E.J.; Reichardt, L.F. Neurotrophins: Roles in neuronal development and function. Annu. Rev. Neurosci. 2001, 24, 677–736. [Google Scholar] [CrossRef] [Green Version]
- Skaper, S.D. The neurotrophin family of neurotrophic factors: An overview. Methods Mol. Biol. 2012, 846, 1–12. [Google Scholar] [CrossRef]
- Ceni, C.; Unsain, N.; Zeinieh, M.P.; Barker, P.A. Neurotrophins in the regulation of cellular survival and death. Handb. Exp. Pharmacol. 2014, 220, 193–221. [Google Scholar] [CrossRef]
- Patapoutian, A.; Reichardt, L.F. Trk receptors: Mediators of neurotrophin action. Curr. Opin. Neurobiol. 2001, 11, 272–280. [Google Scholar] [CrossRef]
- Davies, A.M.; Minichiello, L.; Klein, R. Developmental changes in NT3 signalling via TrkA and TrkB in embryonic neurons. EMBO J. 1995, 14, 4482–4489. [Google Scholar] [CrossRef] [PubMed]
- Fariñas, I.; Wilkinson, G.A.; Backus, C.; Reichardt, L.F.; Patapoutian, A. Characterization of neurotrophin and Trk receptor functions in developing sensory ganglia: Direct NT-3 activation of TrkB neurons in vivo. Neuron 1998, 21, 325–334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, E.J.; Wilkinson, G.A.; Fariñas, I.; Backus, C.; Zang, K.; Wong, S.L.; Reichardt, L.F. Expression of Trk receptors in the developing mouse trigeminal ganglion: In vivo evidence for NT-3 activation of TrkA and TrkB in addition to TrkC. Development 1999, 126, 2191–2203. [Google Scholar] [CrossRef]
- Enokido, Y.; Wyatt, S.; Davies, A.M. Developmental changes in the response of trigeminal neurons to neurotrophins: Influence of birthdate and the ganglion environment. Development 1999, 126, 4365–4373. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Pareek, V.; Faiq, M.A.; Kumar, P.; Raza, K.; Prasoon, P.; Dantham, S.; Mochan, S. Regulatory role of NGFs in neurocognitive functions. Rev. Neurosci. 2017, 28, 649–673. [Google Scholar] [CrossRef]
- Indo, Y. Nerve growth factor, pain, itch and inflammation: Lessons from congenital insensitivity to pain with anhidrosis. Expert. Rev. Neurother. 2010, 10, 1707–1724. [Google Scholar] [CrossRef]
- Pezet, S.; McMahon, S.B. Neurotrophins: Mediators and modulators of pain. Ann. Rev. Neurosci. 2006, 29, 507–538. [Google Scholar] [CrossRef]
- Ikoma, A.; Steinhoff, M.; Ständer, S.; Yosipovitch, G.; Schmelz, M. The neurobiology of itch. Nat. Rev. Neurosci. 2006, 7, 535–547. [Google Scholar] [CrossRef]
- Diogenes, A.; Akopian, A.N.; Hargreaves, K.M. NGF up-regulates TRPA1: Implications for orofacial pain. J. Dent. Res. 2007, 86, 550–555. [Google Scholar] [CrossRef]
- Boucher, T.J.; McMahon, S.B. Neurotrophic factors and neuropathic pain. Curr. Opin. Pharmacol. 2001, 1, 66–72. [Google Scholar] [CrossRef]
- Takeda, M.; Takahashi, M.; Kitagawa, J.; Kanazawa, T.; Nasu, M.; Matsumoto, S. Brain-derived neurotrophic factor enhances the excitability of small-diameter trigeminal ganglion neurons projecting to the trigeminal nucleus interpolaris/caudalis transition zone following masseter muscle inflammation. Mol. Pain. 2013, 9, 49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarhan, M.; Pawlowski, S.A.; Barthas, F.; Yalcin, I.; Kaufling, J.; Dardente, H.; Zachariou, V.; Dileone, R.J.; Barrot, M.; Veinante, P. BDNF parabrachio-amygdaloid pathway in morphine-induced analgesia. Int. J. Neuropsychopharmacol. 2013, 16, 1649–1660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eaton, M.J.; Blits, B.; Ruitenberg, M.J.; Verhaagen, J.; Oudega, M. Amelioration of chronic neuropathic pain after partial nerve injury by adeno-associated viral (AAV) vector-mediated over-expression of BDNF in the rat spinal cord. Gene Ther. 2002, 9, 1387–1395. [Google Scholar] [CrossRef] [Green Version]
- Cejas, P.J.; Martinez, M.; Karmally, S.; McKillop, M.; McKillop, J.; Plunkett, J.A.; Oudega, M.; Eaton, M.J. Lumbar transplant of neurons genetically modified to secrete brain-derived neurotrophic factor attenuates allodynia and hyperalgesia after sciatic nerve constriction. Pain 2000, 86, 195–210. [Google Scholar] [CrossRef] [PubMed]
- Tu, W.; Wang, W.; Xi, H.; He, R.; Gao, L.; Jiang, S. Regulation of neurotrophin-3 and interleukin-1 and inhibition of spinal glial activation contribute to the analgesic effect of electroacupuncture in chronic neuropathic pain states of rats. Evid. Based Complement. Altern. Med. 2015, 2015, 642081. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.F.; Deng, Y.S.; Xian, C.J.; Zhong, J.H. Neurotrophins from dorsal root ganglia trigger allodynia after spinal nerve injury in rats. Eur. J. Neurosci. 2000, 12, 100–105. [Google Scholar] [CrossRef]
- White, D.M. Contribution of neurotrophin-3 to the neuropeptide Y-induced increase in neurite outgrowth of rat dorsal root ganglion cells. Neuroscience 1998, 86, 257–263. [Google Scholar] [CrossRef]
- Gotz, R.; Koster, R.; Winkler, C.; Raulf, F.; Lottspeich, F.; Schartl, M.; Thoenen, H. Neurotrophin-6 is a new member of the nerve growth factor family. Nature 1994, 372, 266–269. [Google Scholar] [CrossRef] [Green Version]
- Nilsson, A.S.; Fainzilber, M.; Falck, P.; Ibanez, C.F. Neurotrophin-7: A novel member of the neurotrophin family from the zebrafish. FEBS Lett. 1998, 424, 285–290. [Google Scholar] [CrossRef] [Green Version]
- Lohof, A.M.; Ip, N.Y.; Poo, M.M. Potentiation of developing neuromuscular synapses by the neurotrophins NT-3 and BDNF. Nature 1993, 363, 350–353. [Google Scholar] [CrossRef]
- Carmignoto, G.; Pizzorusso, T.; Tia, S.; Vicini, S. Brain-derived neurotrophic factor and nerve growth factor potentiate excitatory synaptic transmission in the rat visual cortex. J. Physiol. 1997, 498, 153–164. [Google Scholar] [CrossRef]
- Zhang, J.; Yi, Q.T.; Gong, M.; Zhang, Y.Q.; Liu, D.; Zhu, R.J. Upregulation of TRPV1 in spinal dorsal root ganglion by activating NGF-TrkA pathway contributes to pelvic organ cross-sensitisation in rats with experimental autoimmune prostatitis. Andrologia 2019, 51, e13302. [Google Scholar] [CrossRef] [PubMed]
- Kitayama, T. The role of K+-Cl−-cotransporter-2 in neuropathic pain. Neurochem. Res. 2018, 43, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.K.; You, Y.; Gupta, V.B.; Klistorner, A.; Graham, S.L. TrkB receptor signalling: Implications in neurodegenerative, psychiatric and proliferative disorders. Int. J. Mol. Sci. 2013, 14, 10122–10142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korsching, S. The neurotrophic factor concept: A reexamination. J. Neurosci. 1993, 13, 2739–2748. [Google Scholar] [CrossRef] [Green Version]
- Mannion, R.J.; Costigan, M.; Decosterd, I.; Amaya, F.; Ma, Q.P.; Holstege, J.C.; Ji, R.R.; Acheson, A.; Lindsay, R.M.; Wilkinson, G.A.; et al. Neurotrophins: Peripherally and centrally acting modulators of tactile stimulus-induced inflammatory pain hypersensitivity. Proc. Natl. Acad. Sci. USA 1999, 96, 9385–9390. [Google Scholar] [CrossRef]
- Brady, R.; Zaidi, S.I.; Mayer, C.; Katz, D.M. BDNF is a target-derived survival factor for arterial baroreceptor and chemoafferent primary sensory neurons. J. Neurosci. 1999, 19, 2131–2142. [Google Scholar] [CrossRef] [Green Version]
- Sasi, M.; Vignoli, B.; Canossa, M.; Blum, R. Neurobiology of local and intercellular BDNF signaling. Pflug. Arch. 2017, 469, 593–610. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.F.; Rush, R.A. Localization of neurotrophin-3-like immunoreactivity in the rat central nervous system. Brain Res. 1994, 643, 162–172. [Google Scholar] [CrossRef]
- Nishio, T.; Furukawa, S.; Akiguchi, I.; Oka, N.; Ohnishi, K.; Tomimoto, H.; Nakamura, S.; Kimura, J. Cellular localization of nerve growth factor-like immunoreactivity in adult rat brain: Quantitative and immunohistochemical study. Neuroscience 1994, 60, 67–84. [Google Scholar] [CrossRef]
- Lewin, G.R.; Barde, Y.A. Physiology of the neurotrophins. Annu. Rev. Neurosci. 1996, 19, 289–317. [Google Scholar] [CrossRef]
- Hofer, M.; Pagliusi, S.R.; Hohn, A.; Leibrock, J.; Barde, Y.A. Regional distribution of brain-derived neurotrophic factor mRNA in the adult mouse brain. EMBO J. 1990, 9, 2459–2464. [Google Scholar] [CrossRef] [PubMed]
- Biane, J.; Conner, J.M.; Tuszynski, M.H. Nerve growth factor is primarily produced by GABAergic neurons of the adult rat cortex. Front. Cell Neurosci. 2014, 8, 220. [Google Scholar] [CrossRef] [Green Version]
- Fernández-García, S.; Sancho-Balsells, A.; Longueville, S.; Hervé, D.; Gruart, A.; Delgado-García, J.M.; Alberch, J.; Giralt, A. Astrocytic BDNF and TrkB regulate severity and neuronal activity in mouse models of temporal lobe epilepsy. Cell Death Dis. 2020, 11, 411. [Google Scholar] [CrossRef]
- Parkhurst, C.N.; Yang, G.; Ninan, I.; Savas, J.N.; Yates, J.R., 3rd; Lafaille, J.J.; Hempstead, B.L.; Littman, D.R.; Gan, W.B. Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. Cell 2013, 155, 1596–1609. [Google Scholar] [CrossRef] [Green Version]
- Trang, T.; Beggs, S.; Wan, X.; Salter, M.W. P2X4-receptor-mediated synthesis and release of brain-derived neurotrophic factor in microglia is dependent on calcium and p38-mitogen-activated protein kinase activation. J. Neurosci. 2009, 29, 3518–3528. [Google Scholar] [CrossRef] [Green Version]
- Acheson, A.; Conover, J.C.; Fandl, J.P.; DeChiara, T.M.; Russell, M.; Thadani, A.; Squinto, S.P.; Yancopoulos, G.D.; Lindsay, R.M. A BDNF autocrine loop in adult sensory neurons prevents cell death. Nature 1995, 374, 450–453. [Google Scholar] [CrossRef]
- Robinson, M.; Buj-Bello, A.; Davies, A.M. Paracrine interactions of BDNF involving NGF-dependent embryonic sensory neurons. Mol. Cell Neurosci. 1996, 7, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Walsh, G.S.; Krol, K.M.; Kawaja, M.D. Absence of the p75 neurotrophin receptor alters the pattern of sympathosensory sprouting in the trigeminal ganglia of mice overexpressing nerve growth factor. J. Neurosci. 1999, 19, 258–273. [Google Scholar] [CrossRef]
- Kovačič, U.; Tesovnik, B.; Molnar, N.; Cör, A.; Skalerič, U.; Gašperšič, R. Dental pulp and gingivomucosa in rats are innervated by two morphologically and neurochemically different populations of nociceptors. Arch. Oral Biol. 2013, 58, 788–795. [Google Scholar] [CrossRef] [PubMed]
- Wong, H.; Kang, I.; Dong, X.D.; Christidis, N.; Ernberg, M.; Svensson, P.; Cairns, B.E. NGF-induced mechanical sensitization of the masseter muscle is mediated through peripheral NMDA receptors. Neuroscience 2014, 269, 232–244. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.W.; Hao, T.; Kou, X.X.; Gan, Y.H.; Ma, X.C. Synovial TRPV1 is upregulated by 17-β-estradiol and involved in allodynia of inflamed temporomandibular joints in female rats. Arch. Oral Biol. 2015, 60, 1310–1318. [Google Scholar] [CrossRef] [PubMed]
- Van Gerven, L.; Alpizar, Y.A.; Steelant, B.; Callebaut, I.; Kortekaas Krohn, I.; Wouters, M.; Vermeulen, F.; Boeckxstaens, G.; Talavera, K.; Hellings, P.W. Enhanced chemosensory sensitivity in patients with idiopathic rhinitis and its reversal by nasal capsaicin treatment. J. Allergy Clin. Immunol. 2017, 140, 437–446.e2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Exposto, F.G.; Masuda, M.; Castrillon, E.E.; Svensson, P. Effects of nerve growth factor experimentally-induced craniofacial muscle sensitization on referred pain frequency and number of headache days: A double-blind, randomized placebo-controlled study. Cephalalgia 2018, 38, 2006–2016. [Google Scholar] [CrossRef]
- Jasim, H.; Ghafouri, B.; Gerdle, B.; Hedenberg-Magnusson, B.; Ernberg, M. Altered levels of salivary and plasma pain related markers in temporomandibular disorders. J. Headache Pain. 2020, 21, 105. [Google Scholar] [CrossRef]
- Alhilou, A.M.; Shimada, A.; Svensson, C.I.; Svensson, P.; Ernberg, M.; Cairns, B.E.; Christidis, N. Sex-related differences in response to masseteric injections of glutamate and nerve growth factor in healthy human participants. Sci. Rep. 2021, 11, 13873. [Google Scholar] [CrossRef]
- Gao, M.; Yan, X.; Lu, Y.; Ren, L.; Zhang, S.; Zhang, X.; Kuang, Q.; Liu, L.; Zhou, J.; Wang, Y.; et al. Retrograde nerve growth factor signaling modulates tooth mechanical hyperalgesia induced by orthodontic tooth movement via acid-sensing ion channel 3. Int. J. Oral Sci. 2021, 13, 18. [Google Scholar] [CrossRef]
- Boscato, N.; Exposto, F.G.; Costa, Y.M.; Svensson, P. Effect of standardized training in combination with masseter sensitization on corticomotor excitability in bruxer and control individuals: A proof of concept study. Sci. Rep. 2022, 12, 17469. [Google Scholar] [CrossRef]
- Grayson, M.; Arris, D.; Wu, P.; Merlo, J.; Ibrahim, T.; Fang-Mei, C.; Valenzuela, V.; Ganatra, S.; Ruparel, S. Oral squamous cell carcinoma-released brain-derived neurotrophic factor contributes to oral cancer pain by peripheral tropomyosin receptor kinase B activation. Pain 2022, 163, 496–507. [Google Scholar] [CrossRef]
- Evans, L.J.; Loescher, A.R.; Boissonade, F.M.; Whawell, S.A.; Robinson, P.P.; Andrew, D. Temporal mismatch between pain behaviour, skin nerve growth factor and intra-epidermal nerve fibre density in trigeminal neuropathic pain. BMC Neurosci. 2014, 15, 1. [Google Scholar] [CrossRef] [Green Version]
- Rahmi, R.D.; Soebadi, B.; Parmadiati, A.E.; Winias, S. Nerve growth factor and S100B: Molecular marker of neuroregeneration after injection of freeze-Dried platelet rich plasma. J. Oral Biol. Craniofac. Res. 2022, 12, 570–574. [Google Scholar] [CrossRef] [PubMed]
- Canner, J.P.; Linsenmayer, T.F.; Kubilus, J.K. Developmental regulation of trigeminal TRPA1 by the cornea. Investig. Ophthalmol. Vis. Sci. 2014, 56, 29–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, M.K.; Park, J.; Asgar, J.; Ro, J.Y. Transcriptome analysis of trigeminal ganglia following masseter muscle inflammation in rats. Mol. Pain. 2016, 12, 744806916668526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rivas, M.; Aurrekoetxea, K.; Mellström, B.; Naranjo, J.R. Redox signaling regulates transcriptional activity of the Ca2+-dependent repressor DREAM. Antioxid. Redox Signal. 2011, 14, 1237–1243. [Google Scholar] [CrossRef]
- O’Leary, V.B.; O’Connell, M.; Antyborzec, I.; Ntziachristos, V.; Oliver Dolly, J.; Ovsepian, S.V. Alleviation of trigeminal nociception using p75 neurotrophin receptor targeted lentiviral interference therapy. Neurotherapeutics 2018, 15, 489–499. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Chung, M.K. Orthodontic force induces nerve injury-like transcriptomic changes driven by TRPV1-expressing afferents in mouse trigeminal ganglia. Mol. Pain. 2020, 16, 1744806920973141. [Google Scholar] [CrossRef]
- Tao, T.; Liu, Y.; Zhang, J.; Lai, W.; Long, H. NGF-induced upregulation of CGRP in orofacial pain induced by tooth movement is dependent on Atp6v0a1 and vesicle release. Int. J. Mol. Sci. 2022, 23, 11440. [Google Scholar] [CrossRef]
- Wang, H.; Wei, Y.; Pu, Y.; Jiang, D.; Jiang, X.; Zhang, Y.; Tao, J. Brain-derived neurotrophic factor stimulation of T-type Ca2+ channels in sensory neurons contributes to increased peripheral pain sensitivity. Sci. Signal. 2019, 12, eaaw2300. [Google Scholar] [CrossRef]
- Guo, R.; Chen, Y.; Liu, L.; Wen, J.; Yang, H.; Zhu, Y.; Gao, M.; Liang, H.; Lai, W.; Long, H. Nerve growth factor enhances tooth mechanical hyperalgesia through C-C chemokine ligand 19 in rats. Front. Neurol. 2021, 12, 540660. [Google Scholar] [CrossRef]
- Costa, G.M.F.; Rocha, L.P.C.; Siqueira, S.R.D.T.; Moreira, P.R.; Almeida-Leite, C.M. No Association of Polymorphisms in Nav1.7 or Nerve Growth Factor Receptor Genes with Trigeminal Neuralgia. Pain. Med. 2019, 20, 1362–1369. [Google Scholar] [CrossRef]
- Virtuoso, A.; Herrera-Rincon, C.; Papa, M.; Panetsos, F. Dependence of Neuroprosthetic Stimulation on the Sensory Modality of the Trigeminal Neurons Following Nerve Injury. Implications in the Design of Future Sensory Neuroprostheses for Correct Perception and Modulation of Neuropathic Pain. Front. Neurosci. 2019, 13, 389. [Google Scholar] [CrossRef] [PubMed]
- Flowerdew, S.E.; Wick, D.; Himmelein, S.; Horn, A.K.; Sinicina, I.; Strupp, M.; Brandt, T.; Theil, D.; Hüfner, K. Characterization of neuronal populations in the human trigeminal ganglion and their association with latent herpes simplex virus-1 infection. PLoS ONE 2013, 8, e83603. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Luo, L.; Lin, R.; Lin, L.; Lin, Q. Brain-derived neurotrophic factor and Glial cell line-derived neurotrophic factor expressions in the trigeminal root entry zone and trigeminal ganglion neurons of a trigeminal neuralgia rat model. Anat. Rec. 2020, 303, 3014–3023. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; He, L.; Yin, C.; Huang, R.; Shen, W.; Ge, H.; Sun, M.; Li, S.; Gao, Y.; Xiong, W. Effects of palmatine on BDNF/TrkB-mediated trigeminal neuralgia. Sci. Rep. 2020, 10, 4998. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.Y.; Liu, F.; Fang, Z.H.; Li, Y.L.; Liao, H.L.; Song, Q.X.; Zhou, C.; Shen, J.F. Differential roles of NMDAR subunits 2A and 2B in mediating peripheral and central sensitization contributing to orofacial neuropathic pain. Brain Behav. Immun. 2022, 106, 129–146. [Google Scholar] [CrossRef]
- Finamor, F.; Scarabelot, V.L.; Medeiros, L.F.; Stein, D.J.; da Silva, M.D.; Callai, E.; Caumo, W.; de Souza, A.; Torres, I.L.S. Involvement of GABAergic, glutamatergic, opioidergic, and brain-derived neurotrophic factor systems in the trigeminal neuropathic pain process. Neurosci. Lett. 2023, 793, 136970. [Google Scholar] [CrossRef]
- Kooshki, R.; Abbasnejad, M.; Esmaeili Mahani, S.; Raoof, M.; Moeini Aghtaei, M.M.; Dabiri, S. Orexin-A inhibits capsaicin-induced changes in cyclooxygenase-2 and brain-derived neurotrophic factor expression in trigeminal nucleus caudalis of rats. Korean J. Pain. 2018, 31, 174–182. [Google Scholar] [CrossRef]
- Scarabelot, V.L.; de Oliveira, C.; Medeiros, L.F.; de Macedo, I.C.; Cioato, S.G.; Adachi, L.N.S.; Paz, A.H.; de Souza, A.; Caumo, W.; Torres, I.L.S. Transcranial direct-current stimulation reduces nociceptive behaviour in an orofacial pain model. J. Oral Rehabil. 2019, 46, 40–50. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, Y.; Yang, L.; Wang, Y.; Xiao, Z. PACAP6-38 improves nitroglycerin-induced central sensitization by modulating synaptic plasticity at the trigeminal nucleus caudalis in a male rat model of chronic migraine. J. Headache Pain. 2023, 24, 66. [Google Scholar] [CrossRef]
- Finkbeiner, S.; Tavazoie, S.F.; Maloratsky, A.; Jacobs, K.M.; Harris, K.M.; Greenberg, M.E. CREB: A major mediator of neuronal neurotrophin responses. Neuron 1997, 19, 1031–1047. [Google Scholar] [CrossRef] [Green Version]
- Madhu, L.N.; Kodali, M.; Attaluri, S.; Shuai, B.; Melissari, L.; Rao, X.; Shetty, A.K. Melatonin improves brain function in a model of chronic Gulf War Illness with modulation of oxidative stress, NLRP3 inflammasomes, and BDNF-ERK-CREB pathway in the hippocampus. Redox Biol. 2021, 43, 101973. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhang, Y.; Liu, Q.; Jiang, L.; Li, M.; Wang, S.; Long, T.; He, W.; Kong, X.; Qin, G.; et al. P2X4-receptor participates in EAAT3 regulation via BDNF-TrkB signaling in a model of trigeminal allodynia. Mol. Pain. 2018, 14, 1744806918795930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Oliveira, C.L.; Medeiros, L.F.; de Souza, V.S.; Lopes, B.C.; de Oliveira, F.F.; Marques, L.X.; da Silva Torres, I.L.; de Souza, A. Low-dose naltrexone reverses facial mechanical allodynia in a rat model of trigeminal neuralgia. Neurosci. Lett. 2020, 736, 135248. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhang, L.; Hao, Y.; Yang, L.; Fan, S.; Xiao, Z. FKN/CX3CR1 axis facilitates migraine-Like behaviour by activating thalamic-cortical network microglia in status epilepticus model rats. J. Headache Pain. 2022, 23, 42. [Google Scholar] [CrossRef]
- Curia, G.; Longo, D.; Biagini, G.; Jones, R.S.; Avoli, M. The pilocarpine model of temporal lobe epilepsy. J. Neurosci. Methods 2008, 172, 143–157. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, H.; Yabuuchi, T.; Jin, H.W.; Terayama, R.; Yamaai, T.; Deguchi, T.; Kamioka, H.; Takano-Yamamoto, T.; Sugimoto, T. Brain-derived neurotrophic factor-immunoreactive primary sensory neurons in the rat trigeminal ganglion and trigeminal sensory nuclei. Brain Res. 2006, 1081, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Long, H.; Liao, L.; Zhou, Y.; Shan, D.; Gao, M.; Huang, R.; Yang, X.; Lai, W. A novel technique of delivering viral vectors to trigeminal ganglia in rats. Eur. J. Oral Sci. 2017, 125, 1–7. [Google Scholar] [CrossRef]
- Sorge, R.E.; Mapplebeck, J.C.; Rosen, S.; Beggs, S.; Taves, S.; Alexander, J.K.; Martin, L.J.; Austin, J.S.; Sotocinal, S.G.; Chen, D.; et al. Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nat. Neurosci. 2015, 18, 1081–1083. [Google Scholar] [CrossRef] [Green Version]
- Mapplebeck, J.C.S.; Beggs, S.; Salter, M.W. Sex differences in pain: A tale of two immune cells. Pain 2016, 157, S2–S6. [Google Scholar] [CrossRef]





| Reference | Key Findings | Possible Clinical Implications |
|---|---|---|
| Alhilou et al., 2021 [83] | NGF injection into the human masseter muscle displays a greater magnitude of NGF-induced mechanical sensitization in women compared to men. This sensitization is associated with nerve fibers expression of NMDA-receptors. | Women could be at greater potential risk of developing NGF muscle pain than men. |
| Benedet et al., 2017 [17] | DREAM regulates trigeminal nociception in TG, at least in part, through the control of BDNF expression levels. | Evidence of BDNF and DREAM role in trigeminal nociception and individuation of new potential molecular targets. |
| Boscato et al., 2022 [85] | Bruxers do not significantly change the central modulation of motor pathways after NGF injection in masseter muscle while it is altered in control subjects. | NGF-induce sensitization could have therapeutic implications for the potential to “detrain” and manage bruxism |
| Canner et al., 2014 [89] | First-time description of a relationship between NT-3 and TRPA1 regulation. | Individuation of NT-3/TRPA1 signaling as a potential target in nociception. |
| Chung et al., 2016 [90] | Masseter inflammation alters the expression of multiple nociceptor genes, among which Bdnf, is involved in craniofacial hyperalgesia. | Identification of multiple novel potential pharmacological targets in persistent craniofacial muscle pain. |
| Costa et al., 2019 [97] | No association is found between TrkA gene polymorphisms and trigeminal neuralgia in humans. | The data do not exclude the possibility that other genotypes affecting the expression of TrkA are associated with the disease. |
| de Oliveira et al., 2020 [110] | Low-dose naltrexone exerts an analgesic effect in a trigeminal neuralgia rat model involving BDNF modulation in the spinal cord but not in the brainstem. | Administration of low-dose naltrexone may be an option for treating trigeminal neuralgia. |
| Evans et al., 2014 [87] | Temporal mismatch among the behavioral signs of rat neuropathic pain, skin NGF, and phenotypic changes in cutaneous nerve fibers expressing TrkA. | Skin NGF and nerve fiber TrkA increase are not sufficient to cause hyperalgesia. These results should be considered in future therapeutic approaches. |
| Exposto et al., 2018 [81] |
| Healthy individuals’ referred pain following palpation may be an epiphenomenon of the muscle in response to NGF noxious input. NGF seems not to be involved in the nociceptive pathway. |
| Finamor et al., 2023 [103] | BDNF is increased in rat TG in a trigeminal neuralgia model. In the brainstem, BDNF increased also in sham-operated rats. | Inhibition of local BDNF could be a target of future therapies for trigeminal neuralgia. |
| Gao et al., 2021 [84] | Tooth mechanical hyperalgesia is alleviated by NGF-neutralizing antibody injection in TG. | NGF-based gene therapy is a viable method for alleviating tooth mechanical hyperalgesia. |
| Grayson et al., 2022 [86] |
| BDNF contributes to pain-like behaviors at the site of tumor growth. Inhibitors for BDNF/TrkB could have significant potential in pain treatments allowing for considerable improvement in head and neck cancer patient quality of life. |
| Guo et al., 2021 [96] |
| Tooth-movement orofacial pain may be modulated by NGF through CCL19. |
| Jasim et al., 2020 [82] |
| Potential use of salivary BDNF and NGF as indicative biomarkers for TMD-myalgia. |
| Kooshki et al., 2018 [104] |
| Orexin-A may be a potential treatment for trigeminal pain. |
| Kovačič et al., 2013 [77] |
| Different sensitivity of gingivomucosa and dental pulp during normal and pathological conditions suggests its potential clinical applications in tissue-specific modulation of nociception. |
| Liu et al., 2018 [109] |
| Microglia activation, modulation of purinergic P2X4 receptor activation, and BDNF-TrkB signaling are potential therapeutic targets for treating trigeminal allodynia. |
| Liu et al., 2020 [101] | Palmatine administration increases the mechanical pain threshold in a rat model of trigeminal neuralgia by reducing the expressions of BDNF and TrkB and by inhibiting the ERK1/2 pathway in TG. | Palmatine and its analgesic mechanism could act as a potential pharmacotherapy in the treatment of trigeminal neuralgia and other chronic pain conditions. |
| Luo et al., 2020 [100] |
| BDNF and GDNF signaling could be pharmacological targets in trigeminal neuralgia. |
| O’Leary et al., 2018 [92] | p75NTR targeted lentiviral interference therapy is proposed to alleviate trigeminal nociception to achieve targeted depletion of TRPV1 in rat TG sensory neurons with novel and positive results monitoring TG and Sp5C. | This study introduced a therapeutic targeting strategy that provided a means to post-transcriptionally downregulate TRPV1 in TG nociceptors of adult rats in vivo. |
| Rahmi et al., 2022 [88] |
| Neuroregeneration is a process needed for the treatment of neuropathic pain. FD-PRP injection is effective in inducing neuroregeneration by increasing NGF and S100B expression. |
| Scarabelot et al., 2019 [105] | Transcranial direct-current stimulation reduces mechanical and thermal hyperalgesia and also the BDNF and NGF increase in the brainstem of a rat model of TMJ pain. | Transcranial direct-current stimulation may be a non-pharmacological and non-invasive therapeutic tool against orofacial pain. |
| Takeda et al., 2013 [48] | BDNF enhances the excitability of the small-diameter TG neurons projecting onto the Sp5I/Sp5C following a rat model of masseter muscle inflammation. | TG BDNF-TrkB signaling could be a therapeutic target for the treatment of trigeminal inflammatory hyperalgesia. |
| Tao et al., 2022 [94] | In an experimental model of orofacial pain induced by tooth movement in rats, NGF regulates CGRP expression both in TG and Sp5C. | NGF and CGRP are involved in the transmission of nociceptive information in orofacial pain, so they could be the targets for future therapies. |
| Van Gerven et al., 2017 [80] |
| NGF seems not involved in idiopathic rhinitis and so it couldn’t be considered a potential therapeutic target. |
| Virtuoso et al., 2019 [98] | Following nerve axotomy in a rat model and electrical stimulation of the infraorbital nerve, RET- and Trk-expression patterns indicate that sensory TG neurons express NGF, BDNF/NT-4, GDNF, and NT-3 receptors at levels similar to those found in physiological conditions, although they have presumably switched to regeneration-repair state due to the injury. | Neurostimulation protocols, either for therapeutic applications in neuropathic pain or for the development of nerve-machine sensory neuroprostheses, should be designed considering the sensory modality of target-ganglion neurons and the specific alterations they will elicit on each fiber/neuron type. |
| Wang et al., 2019 [95] | BDNF/TrkB enhances the T-type channel through the PI3K-p38-PKA signaling cascade resulting in TG pain hypersensitivity in rats. | BDNF-TrkB pathway in TG neurons may be a tool for developing pain therapeutics in clinical applications. |
| Wang and Chung, 2020 [93] |
| The results lead to the identification of targets for better management of pain and sensory disturbances during orthodontic treatment. |
| Wong et al., 2014 [78] |
|
|
| Wu et al., 2015 [79] |
| The study results could potentially help clinicians understand the sexual dimorphism of TMD pain. |
| Zhang et al., 2023 [106] |
| The findings may suggest PACAP/PAC1R inhibition as a potential therapeutic target for migraine. |
| Zhang et al., 2022 [102] | Differential roles of NR2A and NR2B in mediating peripheral sensitization in the TG and central sensitization in the Sp5C and contributing to orofacial neuropathic pain in a mice model also influencing BDNF release. | The results may be a fundamental basis for advancing knowledge of the neural mechanisms’ reaction to nerve injury with future translational research in clinical studies. |
| Zhou et al., 2022 [111] | The data indicate that epilepsy favors migraine axis-mediated microglial activation in the cortex/thalamus/Sp5C and is accompanied by the BDNF release. | This information could be used to develop potential therapeutic strategies for preventing and treating migraine in patients with epilepsy. |
| Anatomical Structures | Type of Pain | Neurotrophins | References |
|---|---|---|---|
| Peripheral tissues | Nociceptive pain | BDNF | [77,78,79,80,81,82,83,84,85,86] |
| NGF | |||
| Neuropathic pain and migraine | BDNF | [87,88] | |
| NGF | |||
| Trigeminal ganglion | Nociceptive pain | BDNF | [17,48,77,78,84,89,90,92,93,94,95] |
| NGF | |||
| Neuropathic pain and migraine | BDNF | [81,96,97,98,100,101,103] | |
| NGF | |||
| Trigeminal nucleus (Sp5C) | Nociceptive pain | BDNF | [92,94,104,105] |
| NGF | |||
| Neuropathic pain and migraine | BDNF | [102,103,106,109,110,111] | |
| NGF |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonomini, F.; Favero, G.; Castrezzati, S.; Borsani, E. Role of Neurotrophins in Orofacial Pain Modulation: A Review of the Latest Discoveries. Int. J. Mol. Sci. 2023, 24, 12438. https://doi.org/10.3390/ijms241512438
Bonomini F, Favero G, Castrezzati S, Borsani E. Role of Neurotrophins in Orofacial Pain Modulation: A Review of the Latest Discoveries. International Journal of Molecular Sciences. 2023; 24(15):12438. https://doi.org/10.3390/ijms241512438
Chicago/Turabian StyleBonomini, Francesca, Gaia Favero, Stefania Castrezzati, and Elisa Borsani. 2023. "Role of Neurotrophins in Orofacial Pain Modulation: A Review of the Latest Discoveries" International Journal of Molecular Sciences 24, no. 15: 12438. https://doi.org/10.3390/ijms241512438
APA StyleBonomini, F., Favero, G., Castrezzati, S., & Borsani, E. (2023). Role of Neurotrophins in Orofacial Pain Modulation: A Review of the Latest Discoveries. International Journal of Molecular Sciences, 24(15), 12438. https://doi.org/10.3390/ijms241512438