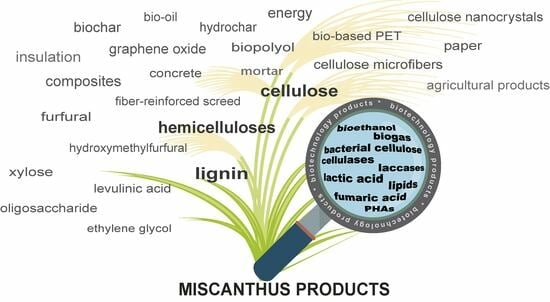
Recent Advances in Miscanthus Macromolecule Conversion: A Brief Overview
Abstract
:1. Introduction
2. Core Directions in Miscanthus Research
2.1. Miscanthus Selection
2.2. Studies on Environmental Impact of Miscanthus
2.3. Production of Various Products from Miscanthus
2.4. Miscanthus Pretreatment and Hydrolysis Processes
3. Biotechnology Products
3.1. Bioethanol
3.2. Biogas
3.3. Bacterial Cellulose
3.4. Enzymes
3.5. Lactic Acid
3.6. Lipids
3.7. Fumaric Acid
3.8. Polyhydroxyalkanoates
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, Z.; Saydaliev, H.B.; Lan, J.; Ali, S.; Anser, M.K. Assessing the Effectiveness of Biomass Energy in Mitigating CO2 Emissions: Evidence from Top-10 Biomass Energy Consumer Countries. Renew. Energy 2022, 191, 842–851. [Google Scholar] [CrossRef]
- Lu, J.; Sun, X. Carbon Regulations, Production Capacity, and Low-Carbon Technology Level for New Products with Incomplete Demand Information. J. Clean. Prod. 2021, 282, 124551. [Google Scholar] [CrossRef]
- Jiang, Q.; Ma, X. Spillovers of Environmental Regulation on Carbon Emissions Network. Technol. Forecast. Soc. Change 2021, 169, 120825. [Google Scholar] [CrossRef]
- Entezaminia, A.; Gharbi, A.; Ouhimmou, M. A Joint Production and Carbon Trading Policy for Unreliable Manufacturing Systems under Cap-and-Trade Regulation. J. Clean. Prod. 2021, 293, 125973. [Google Scholar] [CrossRef]
- Hasan, M.R.; Roy, T.C.; Daryanto, Y.; Wee, H.-M. Optimizing Inventory Level and Technology Investment under a Carbon Tax, Cap-and-Trade and Strict Carbon Limit Regulations. Sustain. Prod. Consum. 2021, 25, 604–621. [Google Scholar] [CrossRef]
- Chen, Y.; Li, B.; Zhang, G.; Bai, Q. Quantity and Collection Decisions of the Remanufacturing Enterprise under Both the Take-Back and Carbon Emission Capacity Regulations. Transp. Res. Part E Logist. Transp. Rev. 2020, 141, 102032. [Google Scholar] [CrossRef]
- Kwaśniewski, D.; Płonka, A.; Mickiewicz, P. Harvesting Technologies and Costs of Biomass Production from Energy Crops Cultivated on Farms in the Małopolska Region. Energies 2021, 15, 131. [Google Scholar] [CrossRef]
- Patrizio, P.; Fajardy, M.; Bui, M.; Dowell, N. Mac CO2 Mitigation or Removal: The Optimal Uses of Biomass in Energy System Decarbonization. iScience 2021, 24, 102765. [Google Scholar] [CrossRef]
- Shen, Z.; Tiruta-Barna, L.; Karan, S.K.; Hamelin, L. Simultaneous Carbon Storage in Arable Land and Anthropogenic Products (CSAAP): Demonstrating an Integrated Concept towards Well below 2 °C. Resour. Conserv. Recycl. 2022, 182, 106293. [Google Scholar] [CrossRef]
- Nakajima, T.; Yamada, T.; Anzoua, K.G.; Kokubo, R.; Noborio, K. Carbon Sequestration and Yield Performances of Miscanthus × Giganteus and Miscanthus Sinensis. Carbon. Manag. 2018, 9, 415–423. [Google Scholar] [CrossRef]
- Lewandowski, I.; Clifton-Brown, J.; Trindade, L.M.; van der Linden, G.C.; Schwarz, K.-U.; Müller-Sämann, K.; Anisimov, A.; Chen, C.-L.; Dolstra, O.; Donnison, I.S.; et al. Progress on Optimizing Miscanthus Biomass Production for the European Bioeconomy: Results of the EU FP7 Project OPTIMISC. Front. Plant Sci. 2016, 7, 1620. [Google Scholar] [CrossRef] [PubMed]
- Agostini, A.; Serra, P.; Giuntoli, J.; Martani, E.; Ferrarini, A.; Amaducci, S. Biofuels from Perennial Energy Crops on Buffer Strips: A Win-Win Strategy. J. Clean. Prod. 2021, 297, 126703. [Google Scholar] [CrossRef]
- Lask, J.; Wagner, M.; Trindade, L.M.; Lewandowski, I. Life Cycle Assessment of Ethanol Production from Miscanthus: A Comparison of Production Pathways at Two European Sites. GCB Bioenergy 2019, 11, 269–288. [Google Scholar] [CrossRef]
- Danielewicz, D.; Surma-Ślusarska, B. Miscanthus × Giganteus Stalks as a Potential Non-Wood Raw Material for the Pulp and Paper Industry. Influence of Pulping and Beating Conditions on the Fibre and Paper Properties. Ind. Crops Prod. 2019, 141, 111744. [Google Scholar] [CrossRef]
- Feng, H.; Lin, C.; Liu, W.; Xiao, L.; Zhao, X.; Kang, L.; Liu, X.; Sang, T.; Yi, Z.; Yan, J.; et al. Transcriptomic Characterization of Miscanthus Sacchariflorus × M. Lutarioriparius and Its Implications for Energy Crop Development in the Semiarid Mine Area. Plants 2022, 11, 1568. [Google Scholar] [CrossRef]
- von Hellfeld, R.; Hastings, A.; Kam, J.; Rowe, R.; Clifton-Brown, J.; Donnison, I.; Shepherd, A. Expanding the Miscanthus Market in the UK: Growers in Profile and Experience, Benefits and Drawbacks of the Bioenergy Crop. GCB Bioenergy 2022, 14, 1205–1218. [Google Scholar] [CrossRef]
- Lewandowski, I.; Clifton-Brown, J.; Kiesel, A.; Hastings, A.; Iqbal, Y. Miscanthus. In Perennial Grasses for Bioenergy and Bioproducts; Elsevier: Amsterdam, The Netherlands, 2018; pp. 35–59. [Google Scholar]
- Hao, M.; Chen, S.; Qian, Y.; Jiang, D.; Ding, F. Using Machine Learning to Identify the Potential Marginal Land Suitable for Giant Silvergrass (Miscanthus × Giganteus). Energies 2022, 15, 591. [Google Scholar] [CrossRef]
- Hong, C.; Fang, J.; Jin, A.; Cai, J.; Guo, H.; Ren, J.; Shao, Q.; Zheng, B. Comparative Growth, Biomass Production and Fuel Properties Among Different Perennial Plants, Bamboo and Miscanthus. Bot. Rev. 2011, 77, 197–207. [Google Scholar] [CrossRef]
- Klímek, P.; Wimmer, R.; Meinlschmidt, P.; Kúdela, J. Utilizing Miscanthus Stalks as Raw Material for Particleboards. Ind. Crops Prod. 2018, 111, 270–276. [Google Scholar] [CrossRef]
- Wójciak, A.; Joachimiak, K.; Wojech, R. Comparison of Miscanthus Giganteus and Birch Wood NSSC Pulping. Part 1: The Effects of Technological Conditions on Certain Pulp Properties. Wood Res. 2019, 64, 49–58. [Google Scholar]
- Doczekalska, B.; Bartkowiak, M.; Waliszewska, B.; Orszulak, G.; Cerazy-Waliszewska, J.; Pniewski, T. Characterization of Chemically Activated Carbons Prepared from Miscanthus and Switchgrass Biomass. Materials 2020, 13, 1654. [Google Scholar] [CrossRef]
- Waliszewska, B.; Grzelak, M.; Gaweł, E.; Spek-Dźwigała, A.; Sieradzka, A.; Czekała, W. Chemical Characteristics of Selected Grass Species from Polish Meadows and Their Potential Utilization for Energy Generation Purposes. Energies 2021, 14, 1669. [Google Scholar] [CrossRef]
- Xu, P.; Cheng, S.; Han, Y.; Zhao, D.; Li, H.; Wang, Y.; Zhang, G.; Chen, C. Natural Variation of Lignocellulosic Components in Miscanthus Biomass in China. Front. Chem. 2020, 8, 595143. [Google Scholar] [CrossRef]
- Briones, M.J.I.; Massey, A.; Elias, D.M.O.; McCalmont, J.P.; Farrar, K.; Donnison, I.; McNamara, N.P. Species Selection Determines Carbon Allocation and Turnover in Miscanthus Crops: Implications for Biomass Production and C Sequestration. Sci. Total Environ. 2023, 887, 164003. [Google Scholar] [CrossRef]
- Hassan, E.-S.R.E.; Mutelet, F. Evaluation of Miscanthus Pretreatment Effect by Choline Chloride Based Deep Eutectic Solvents on Bioethanol Production. Bioresour. Technol. 2022, 345, 126460. [Google Scholar] [CrossRef]
- Li, H.; Wang, B.; Shui, H.; Wei, Q.; Xu, C.C. Preparation of Bio-Based Polyurethane Hydroponic Foams Using 100% Bio-Polyol Derived from Miscanthus through Organosolv Fractionation. Ind. Crops Prod. 2022, 181, 114774. [Google Scholar] [CrossRef]
- Turner, W.; Greetham, D.; Mos, M.; Squance, M.; Kam, J.; Du, C. Exploring the Bioethanol Production Potential of Miscanthus Cultivars. Appl. Sci. 2021, 11, 9949. [Google Scholar] [CrossRef]
- Gismatulina, Y.A.; Budaeva, V.V.; Kortusov, A.N.; Kashcheyeva, E.I.; Gladysheva, E.K.; Mironova, G.F.; Skiba, E.A.; Shavyrkina, N.A.; Korchagina, A.A.; Zolotukhin, V.N.; et al. Evaluation of Chemical Composition of Miscanthus × Giganteus Raised in Different Climate Regions in Russia. Plants 2022, 11, 2791. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, J.; He, W.-Q.; Li, Q.-M.; Chen, L.; Wu, X.-F.; Su, X.-J. Separation of Lignocellulose and Preparation of Xylose from Miscanthus Lutarioriparius with a Formic Acid Method. Appl. Sci. 2022, 12, 1432. [Google Scholar] [CrossRef]
- Dorogina, O.V.; Vasilyeva, O.Y.; Nuzhdina, N.S.; Buglova, L.V.; Gismatulina, Y.A.; Zhmud, E.V.; Zueva, G.A.; Komina, O.V.; Tsybchenko, E.A. Resource Potential of Some Species of the Genus Miscanthus Anderss. under Conditions of Continental Climate of West Siberian Forest-Steppe. Vavilov J. Genet. Breed. 2018, 22, 553–559. [Google Scholar] [CrossRef]
- van der Cruijsen, K.; Al Hassan, M.; van Erven, G.; Dolstra, O.; Trindade, L.M. Breeding Targets to Improve Biomass Quality in Miscanthus. Molecules 2021, 26, 254. [Google Scholar] [CrossRef]
- Bhatia, R.; Timms-Taravella, E.; Roberts, L.A.; Moron-Garcia, O.M.; Hauck, B.; Dalton, S.; Gallagher, J.A.; Wagner, M.; Clifton-Brown, J.; Bosch, M. Transgenic ZmMYB167 Miscanthus Sinensis with Increased Lignin to Boost Bioenergy Generation for the Bioeconomy. Biotechnol. Biofuels Bioprod. 2023, 16, 29. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Kong, Y.; Hu, R.; Zhou, G. Miscanthus: A Fast-growing Crop for Environmental Remediation and Biofuel Production. GCB Bioenergy 2021, 13, 58–69. [Google Scholar] [CrossRef]
- Lask, J.; Kam, J.; Weik, J.; Kiesel, A.; Wagner, M.; Lewandowski, I. A Parsimonious Model for Calculating the Greenhouse Gas Emissions of Miscanthus Cultivation Using Current Commercial Practice in the United Kingdom. GCB Bioenergy 2021, 13, 1087–1098. [Google Scholar] [CrossRef]
- Collura, S.; Azambre, B.; Finqueneisel, G.; Zimny, T.; Victor Weber, J. Miscanthus × Giganteus Straw and Pellets as Sustainable Fuels. Environ. Chem. Lett. 2006, 4, 75–78. [Google Scholar] [CrossRef]
- Bilandžija, N.; Zgorelec, Ž.; Pezo, L.; Grubor, M.; Velaga, A.G.; Krička, T. Solid Biofuels Properties of Miscanthus × Giganteus Cultivated on Contaminated Soil after Phytoremediation Process. J. Energy Inst. 2022, 101, 131–139. [Google Scholar] [CrossRef]
- Lee, J.-K.; Hong, D.; Chae, H.; Lee, D.-H. Prediction of Storage Conditions to Increase the Bioenergy Efficiency of Giant Miscanthus Pellets Produced through On-Site Integrated Pretreatment Machines. Energies 2023, 16, 2422. [Google Scholar] [CrossRef]
- Lakshman, V.; Brassard, P.; Hamelin, L.; Raghavan, V.; Godbout, S. Pyrolysis of Miscanthus: Developing the Mass Balance of a Biorefinery through Experimental Tests in an Auger Reactor. Bioresour. Technol. Rep. 2021, 14, 100687. [Google Scholar] [CrossRef]
- Singh, A.; Nanda, S.; Guayaquil-Sosa, J.F.; Berruti, F. Pyrolysis of Miscanthus and Characterization of Value-added Bio-oil and Biochar Products. Can. J. Chem. Eng. 2021, 99, S55–S68. [Google Scholar] [CrossRef]
- Pidlisnyuk, V.; Newton, R.A.; Mamirova, A. Miscanthus Biochar Value Chain—A Review. J. Environ. Manag. 2021, 290, 112611. [Google Scholar] [CrossRef]
- Xu, Y.; Wu, S.; Huang, F.; Huang, H.; Yi, Z.; Xue, S. Biomodification of Feedstock for Quality-Improved Biochar: A Green Method to Enhance the Cd Sorption Capacity of Miscanthus Lutarioriparius-Derived Biochar. J. Clean. Prod. 2022, 350, 131241. [Google Scholar] [CrossRef]
- Ivanovski, M.; Goričanec, D.; Urbancl, D. The Evaluation of Torrefaction Efficiency for Lignocellulosic Materials Combined with Mixed Solid Wastes. Energies 2023, 16, 3694. [Google Scholar] [CrossRef]
- Georgiou, E.; Mihajlović, M.; Petrović, J.; Anastopoulos, I.; Dosche, C.; Pashalidis, I.; Kalderis, D. Single-Stage Production of Miscanthus Hydrochar at Low Severity Conditions and Application as Adsorbent of Copper and Ammonium Ions. Bioresour. Technol. 2021, 337, 125458. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Manickam, S.; Lester, E.; Wu, T.; Pang, C.H. Synthesis of Graphene Oxide and Graphene Quantum Dots from Miscanthus via Ultrasound-Assisted Mechano-Chemical Cracking Method. Ultrason. Sonochem 2021, 73, 105519. [Google Scholar] [CrossRef]
- Lemaire, T.; Rodi, E.G.; Langlois, V.; Renard, E.; Sansalone, V. Study of Mechanical Properties of PHBHV/Miscanthus Green Composites Using Combined Experimental and Micromechanical Approaches. Polymers 2021, 13, 2650. [Google Scholar] [CrossRef] [PubMed]
- Delpouve, N.; Faraj, H.; Demarest, C.; Dontzoff, E.; Garda, M.-R.; Delbreilh, L.; Berton, B.; Dargent, E. Water-Induced Breaking of Interfacial Cohesiveness in a Poly(Lactic Acid)/Miscanthus Fibers Biocomposite. Polymers 2021, 13, 2285. [Google Scholar] [CrossRef]
- Dias, P.P.; Jayasinghe, L.B.; Waldmann, D. Investigation of Mycelium-Miscanthus Composites as Building Insulation Material. Results Mater. 2021, 10, 100189. [Google Scholar] [CrossRef]
- Ntimugura, F.; Vinai, R.; Dalzell, M.; Harper, A.; Walker, P. Mechanical Properties and Microstructure of Slag and Fly Ash Alkali-Activated Lightweight Concrete Containing Miscanthus Particles. Mater. Lett. 2022, 312, 131696. [Google Scholar] [CrossRef]
- Wu, F.; Yu, Q.; Brouwers, H.J.H. Long-Term Performance of Bio-Based Miscanthus Mortar. Constr. Build. Mater. 2022, 324, 126703. [Google Scholar] [CrossRef]
- Pons Ribera, S.; Hamzaoui, R.; Colin, J.; Bessette, L.; Audouin, M. Valorization of Vegetal Fibers (Hemp, Flax, Miscanthus and Bamboo) in a Fiber Reinforced Screed (FRS) Formulation. Materials 2023, 16, 2203. [Google Scholar] [CrossRef]
- García-Velásquez, C.; van der Meer, Y. Can We Improve the Environmental Benefits of Biobased PET Production through Local Biomass Value Chains? —A Life Cycle Assessment Perspective. J. Clean. Prod. 2022, 380, 135039. [Google Scholar] [CrossRef]
- Hamzah, M.H.; Bowra, S.; Cox, P. Organosolv Lignin Aggregation Behaviour of Soluble Lignin Extract from Miscanthus x Giganteus at Different Ethanol Concentrations and Its Influence on the Lignin Esterification. Chem. Biol. Technol. Agric. 2021, 8, 65. [Google Scholar] [CrossRef]
- Götz, M.; Rudi, A.; Heck, R.; Schultmann, F.; Kruse, A. Processing Miscanthus to High-value Chemicals: A Techno-economic Analysis Based on Process Simulation. GCB Bioenergy 2022, 14, 447–462. [Google Scholar] [CrossRef]
- Świątek, K.; Gaag, S.; Klier, A.; Kruse, A.; Sauer, J.; Steinbach, D. Acid Hydrolysis of Lignocellulosic Biomass: Sugars and Furfurals Formation. Catalysts 2020, 10, 437. [Google Scholar] [CrossRef]
- Pang, J.; Zhang, B.; Jiang, Y.; Zhao, Y.; Li, C.; Zheng, M.; Zhang, T. Complete Conversion of Lignocellulosic Biomass to Mixed Organic Acids and Ethylene Glycol via Cascade Steps. Green. Chem. 2021, 23, 2427–2436. [Google Scholar] [CrossRef]
- Marín, F.; Sánchez, J.L.; Arauzo, J.; Fuertes, R.; Gonzalo, A. Semichemical Pulping of Miscanthus Giganteus. Effect of Pulping Conditions on Some Pulp and Paper Properties. Bioresour. Technol. 2009, 100, 3933–3940. [Google Scholar] [CrossRef]
- Tsalagkas, D.; Börcsök, Z.; Pásztory, Z.; Gogate, P.; Csóka, L. Assessment of the Papermaking Potential of Processed Miscanthus × Giganteus Stalks Using Alkaline Pre-Treatment and Hydrodynamic Cavitation for Delignification. Ultrason. Sonochemistry 2021, 72, 105462. [Google Scholar] [CrossRef] [PubMed]
- Lexa, A.; Sängerlaub, S.; Zollner-Croll, H. Extraktion von Zellstoff Aus Nicht-Holzpflanzen Und Vergleich Mit Holzpflanzen. Chem. Ing. Tech. 2023, 95. [Google Scholar] [CrossRef]
- Singh, S.S.; Lim, L.-T.; Manickavasagan, A. Enhanced Microfibrillation of Miscanthus × Giganteus Biomass by Binary-Enzymes Pre-Treatment. Ind. Crops Prod. 2022, 177, 114537. [Google Scholar] [CrossRef]
- Cudjoe, E.; Hunsen, M.; Xue, Z.; Way, A.E.; Barrios, E.; Olson, R.A.; Hore, M.J.A.; Rowan, S.J. Miscanthus Giganteus: A Commercially Viable Sustainable Source of Cellulose Nanocrystals. Carbohydr. Polym. 2017, 155, 230–241. [Google Scholar] [CrossRef]
- Chen, M.-H.; Bowman, M.J.; Dien, B.S.; Rausch, K.D.; Tumbleson, M.E.; Singh, V. Autohydrolysis of Miscanthus × Giganteus for the Production of Xylooligosaccharides (XOS): Kinetics, Characterization and Recovery. Bioresour. Technol. 2014, 155, 359–365. [Google Scholar] [CrossRef]
- Lan, K.; Xu, Y.; Kim, H.; Ham, C.; Kelley, S.S.; Park, S. Techno-Economic Analysis of Producing Xylo-Oligosaccharides and Cellulose Microfibers from Lignocellulosic Biomass. Bioresour. Technol. 2021, 340, 125726. [Google Scholar] [CrossRef]
- Bhatia, R.; Lad, J.B.; Bosch, M.; Bryant, D.N.; Leak, D.; Hallett, J.P.; Franco, T.T.; Gallagher, J.A. Production of Oligosaccharides and Biofuels from Miscanthus Using Combinatorial Steam Explosion and Ionic Liquid Pretreatment. Bioresour. Technol. 2021, 323, 124625. [Google Scholar] [CrossRef] [PubMed]
- Vilcocq, L.; Crepet, A.; Jame, P.; Carvalheiro, F.; Duarte, L.C. Combination of Autohydrolysis and Catalytic Hydrolysis of Biomass for the Production of Hemicellulose Oligosaccharides and Sugars. Reactions 2021, 3, 30–46. [Google Scholar] [CrossRef]
- Pidlisnyuk, V.; Erickson, L.E.; Wang, D.; Zhao, J.; Stefanovska, T.; Schlup, J.R. Miscanthus as Raw Materials for Bio-Based Products. In Phytotechnology with Biomass Production; CRC Press: Boca Raton, FL, USA, 2021; pp. 201–215. [Google Scholar]
- Nazli, R.I.; Gulnaz, O.; Kafkas, E.; Tansi, V. Comparison of Different Chemical Pretreatments for Their Effects on Fermentable Sugar Production from Miscanthus Biomass. Biomass Convers. Biorefinery 2021, 13, 6471–6479. [Google Scholar] [CrossRef]
- Govil, T.; Wang, J.; Samanta, D.; David, A.; Tripathi, A.; Rauniyar, S.; Salem, D.R.; Sani, R.K. Lignocellulosic Feedstock: A Review of a Sustainable Platform for Cleaner Production of Nature’s Plastics. J. Clean. Prod. 2020, 270, 122521. [Google Scholar] [CrossRef]
- Haldar, D.; Purkait, M.K. A Review on the Environment-Friendly Emerging Techniques for Pretreatment of Lignocellulosic Biomass: Mechanistic Insight and Advancements. Chemosphere 2021, 264, 128523. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Zhao, Y.; Chen, X.; Shao, Q.; Qin, W. Pretreatment of Miscanthus with Biomass-Degrading Bacteria for Increasing Delignification and Enzymatic Hydrolysability. Microb. Biotechnol. 2019, 12, 787–798. [Google Scholar] [CrossRef]
- Rivas, S.; Santos, V.; Parajó, J.C. Effects of Hydrothermal Processing on Miscanthus × Giganteus Polysaccharides: A Kinetic Assessment. Polymers 2022, 14, 4732. [Google Scholar] [CrossRef]
- Mankar, A.R.; Pandey, A.; Modak, A.; Pant, K.K. Pretreatment of Lignocellulosic Biomass: A Review on Recent Advances. Bioresour. Technol. 2021, 334, 125235. [Google Scholar] [CrossRef]
- Tiwari, A.; Chen, C.-W.; Haldar, D.; Patel, A.K.; Dong, C.-D.; Singhania, R.R. Laccase in Biorefinery of Lignocellulosic Biomass. Appl. Sci. 2023, 13, 4673. [Google Scholar] [CrossRef]
- Dai, Y.; Hu, B.; Yang, Q.; Nie, L.; Sun, D. Comparison of the Effects of Different Pretreatments on the Structure and Enzymatic Hydrolysis of Miscanthus. Biotechnol. Appl. Biochem. 2022, 69, 548–557. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.; Larroche, C.; Dussap, C.-G. Comprehensive Assessment of 2G Bioethanol Production. Bioresour. Technol. 2020, 313, 123630. [Google Scholar] [CrossRef]
- Kang, K.E.; Jeong, J.-S.; Kim, Y.; Min, J.; Moon, S.-K. Development and Economic Analysis of Bioethanol Production Facilities Using Lignocellulosic Biomass. J. Biosci. Bioeng. 2019, 128, 475–479. [Google Scholar] [CrossRef] [PubMed]
- Skiba, E.A.; Ovchinnikova, E.V.; Budaeva, V.V.; Banzaraktsaeva, S.P.; Kovgan, M.A.; Chumachenko, V.A.; Mironova, G.F.; Kortusov, A.N.; Parmon, V.N.; Sakovich, G.V. Miscanthus Bioprocessing Using HNO3-Pretreatment to Improve Productivity and Quality of Bioethanol and Downstream Ethylene. Ind. Crops Prod. 2022, 177, 114448. [Google Scholar] [CrossRef]
- Dubis, B.; Bułkowska, K.; Lewandowska, M.; Szempliński, W.; Jankowski, K.J.; Idźkowski, J.; Kordala, N.; Szymańska, K. Effect of Different Nitrogen Fertilizer Treatments on the Conversion of Miscanthus×giganteus to Ethanol. Bioresour. Technol. 2017, 243, 731–737. [Google Scholar] [CrossRef]
- Zhang, Y.; Oates, L.G.; Serate, J.; Xie, D.; Pohlmann, E.; Bukhman, Y.V.; Karlen, S.D.; Young, M.K.; Higbee, A.; Eilert, D.; et al. Diverse Lignocellulosic Feedstocks Can Achieve High Field-scale Ethanol Yields While Providing Flexibility for the Biorefinery and Landscape-level Environmental Benefits. GCB Bioenergy 2018, 10, 825–840. [Google Scholar] [CrossRef]
- Dwivedi, D.; Rathour, R.K.; Sharma, V.; Rana, N.; Bhatt, A.K.; Bhatia, R.K. Co-Fermentation of Forest Pine Needle Waste Biomass Hydrolysate into Bioethanol. Biomass Convers. Biorefin 2022. [Google Scholar] [CrossRef]
- Ju, Y.; Kim, I.J.; Kim, S.; Olawuyi, I.F.; Kim, K.; Kim, S.R. Deacetylation Kinetics of Promising Energy Crops, Hemp and Kenaf, for Cellulosic Ethanol Production. GCB Bioenergy 2022, 14, 1150–1161. [Google Scholar] [CrossRef]
- El Hage, M.; Louka, N.; Rezzoug, S.-A.; Maugard, T.; Sablé, S.; Koubaa, M.; Debs, E.; Maache-Rezzoug, Z. Bioethanol Production from Woody Biomass: Recent Advances on the Effect of Pretreatments on the Bioconversion Process and Energy Yield Aspects. Energies 2023, 16, 5052. [Google Scholar] [CrossRef]
- Yuan, Z.; Li, G.; Wei, W.; Wang, J.; Fang, Z. A Comparison of Different Pre-Extraction Methods Followed by Steam Pretreatment of Bamboo to Improve the Enzymatic Digestibility and Ethanol Production. Energy 2020, 196, 117156. [Google Scholar] [CrossRef]
- Liang, Z.; Neményi, A.; Kovács, G.P.; Gyuricza, C. Potential Use of Bamboo Resources in Energy Value-added Conversion Technology and Energy Systems. GCB Bioenergy 2023, 15, 936–953. [Google Scholar] [CrossRef]
- Zabed, H.; Sahu, J.N.; Boyce, A.N.; Faruq, G. Fuel Ethanol Production from Lignocellulosic Biomass: An Overview on Feedstocks and Technological Approaches. Renew. Sustain. Energy Rev. 2016, 66, 751–774. [Google Scholar] [CrossRef]
- Lamichhane, G.; Acharya, A.; Poudel, D.K.; Aryal, B.; Gyawali, N.; Niraula, P.; Phuyal, S.R.; Budhathoki, P.; Bk, G.; Parajuli, N. Recent Advances in Bioethanol Production from Lignocellulosic Biomass. Int. J. Green. Energy 2021, 18, 731–744. [Google Scholar] [CrossRef]
- Ferrari, G.; Pezzuolo, A.; Nizami, A.-S.; Marinello, F. Bibliometric Analysis of Trends in Biomass for Bioenergy Research. Energies 2020, 13, 3714. [Google Scholar] [CrossRef]
- Thomas, H.L.; Arnoult, S.; Brancourt-Hulmel, M.; Carrère, H. Methane Production Variability According to Miscanthus Genotype and Alkaline Pretreatments at High Solid Content. BioEnergy Res. 2019, 12, 325–337. [Google Scholar] [CrossRef]
- Jury, C.; Thomas, H.L.; Carrère, H. Life Cycle Assessment of Two Alkaline Pretreatments of Sorghum and Miscanthus and of Their Batch Co-Digestion with Cow Manure. BioEnergy Res. 2022, 15, 810–833. [Google Scholar] [CrossRef]
- Fu, S.-F.; Chen, K.-Q.; Zhu, R.; Sun, W.-X.; Zou, H.; Guo, R.-B. Improved Anaerobic Digestion Performance of Miscanthus Floridulus by Different Pretreatment Methods and Preliminary Economic Analysis. Energy Convers. Manag. 2018, 159, 121–128. [Google Scholar] [CrossRef]
- Baute, K.; Van Eerd, L.; Robinson, D.; Sikkema, P.; Mushtaq, M.; Gilroyed, B. Comparing the Biomass Yield and Biogas Potential of Phragmites Australis with Miscanthus x Giganteus and Panicum Virgatum Grown in Canada. Energies 2018, 11, 2198. [Google Scholar] [CrossRef]
- Peng, X.; Li, C.; Liu, J.; Yi, Z.; Han, Y. Changes in Composition, Cellulose Degradability and Biochemical Methane Potential of Miscanthus Species during the Growing Season. Bioresour. Technol. 2017, 235, 389–395. [Google Scholar] [CrossRef]
- Klimiuk, E.; Pokój, T.; Budzyński, W.; Dubis, B. Theoretical and Observed Biogas Production from Plant Biomass of Different Fibre Contents. Bioresour. Technol. 2010, 101, 9527–9535. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Pei, L.; Chen, G.; Mu, L.; Yan, B.; Li, H.; Zhou, T. Recent Advancements in Strategies to Improve Anaerobic Digestion of Perennial Energy Grasses for Enhanced Methane Production. Sci. Total Environ. 2023, 861, 160552. [Google Scholar] [CrossRef] [PubMed]
- Olatunji, K.O.; Ahmed, N.A.; Ogunkunle, O. Optimization of Biogas Yield from Lignocellulosic Materials with Different Pretreatment Methods: A Review. Biotechnol. Biofuels 2021, 14, 159. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Xin, Y.; Shi, H.; Ai, P.; Yu, L.; Li, X.; Chen, S. Closing Ammonia Loop in Efficient Biogas Production: Recycling Ammonia Pretreatment of Wheat Straw. Biosyst. Eng. 2019, 180, 182–190. [Google Scholar] [CrossRef]
- Akman, H.E.; Perendeci, N.A.; Ertekin, C.; Yaldiz, O. Energy Crops and Methane: Process Optimization of Ca(OH)2 Assisted Thermal Pretreatment and Modeling of Methane Production. Molecules 2022, 27, 6891. [Google Scholar] [CrossRef]
- Yao, Y.; Bergeron, A.D.; Davaritouchaee, M. Methane Recovery from Anaerobic Digestion of Urea-Pretreated Wheat Straw. Renew. Energy 2018, 115, 139–148. [Google Scholar] [CrossRef]
- Zhong, C. Industrial-Scale Production and Applications of Bacterial Cellulose. Front. Bioeng. Biotechnol. 2020, 8, 605374. [Google Scholar] [CrossRef]
- Kim, H.; Son, J.; Lee, J.; Yoo, H.Y.; Lee, T.; Jang, M.; Oh, J.; Park, C. Improved Production of Bacterial Cellulose through Investigation of Effects of Inhibitory Compounds from Lignocellulosic Hydrolysates. GCB Bioenergy 2021, 13, 436–444. [Google Scholar] [CrossRef]
- Son, J.; Lee, K.H.; Lee, T.; Kim, H.S.; Shin, W.H.; Oh, J.-M.; Koo, S.-M.; Yu, B.J.; Yoo, H.Y.; Park, C. Enhanced Production of Bacterial Cellulose from Miscanthus as Sustainable Feedstock through Statistical Optimization of Culture Conditions. Int. J. Environ. Res. Public. Health 2022, 19, 866. [Google Scholar] [CrossRef]
- Skiba, E.A.; Gladysheva, E.K.; Golubev, D.S.; Budaeva, V.V.; Aleshina, L.A.; Sakovich, G.V. Self-Standardization of Quality of Bacterial Cellulose Produced by Medusomyces Gisevii in Nutrient Media Derived from Miscanthus Biomass. Carbohydr. Polym. 2021, 252, 117178. [Google Scholar] [CrossRef]
- Urbina, L.; Corcuera, M.Á.; Gabilondo, N.; Eceiza, A.; Retegi, A. A Review of Bacterial Cellulose: Sustainable Production from Agricultural Waste and Applications in Various Fields. Cellulose 2021, 28, 8229–8253. [Google Scholar] [CrossRef]
- Ogrizek, L.; Lamovšek, J.; Čuš, F.; Leskovšek, M.; Gorjanc, M. Properties of Bacterial Cellulose Produced Using White and Red Grape Bagasse as a Nutrient Source. Processes 2021, 9, 1088. [Google Scholar] [CrossRef]
- Cazón, P.; Puertas, G.; Vázquez, M. Production and Characterization of Active Bacterial Cellulose Films Obtained from the Fermentation of Wine Bagasse and Discarded Potatoes by Komagateibacter Xylinus. Polymers 2022, 14, 5194. [Google Scholar] [CrossRef]
- Abdelraof, M.; Hasanin, M.S.; El -Saied, H. Ecofriendly Green Conversion of Potato Peel Wastes to High Productivity Bacterial Cellulose. Carbohydr. Polym. 2019, 211, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, C.; Bai, F.; Zhao, X. Overproduction of Cellulase by Trichoderma Reesei RUT C30 through Batch-Feeding of Synthesized Low-Cost Sugar Mixture. Bioresour. Technol. 2016, 216, 503–510. [Google Scholar] [CrossRef]
- Xiang, J.; Wang, X.; Sang, T. Cellulase Production from Trichoderma Reesei RUT C30 Induced by Continuous Feeding of Steam-Exploded Miscanthus Lutarioriparius. Ind. Crops Prod. 2021, 160, 113129. [Google Scholar] [CrossRef]
- Ma, L.; Li, C.; Yang, Z.; Jia, W.; Zhang, D.; Chen, S. Kinetic Studies on Batch Cultivation of Trichoderma Reesei and Application to Enhance Cellulase Production by Fed-Batch Fermentation. J. Biotechnol. 2013, 166, 192–197. [Google Scholar] [CrossRef]
- Dey, P.; Singh, J.; Scaria, J.; Anand, A.P. Improved Production of Cellulase by Trichoderma Reesei (MTCC 164) from Coconut Mesocarp-Based Lignocellulosic Wastes under Response Surface-Optimized Condition. 3 Biotech. 2018, 8, 402. [Google Scholar] [CrossRef]
- Lee, H.; Lee, Y.M.; Heo, Y.M.; Lee, J.; Kim, J.S.; Kang, K.Y.; Kim, J.-J. Utilization of Agricultural Residues for Enhancement of Cellulolytic Enzyme Production and Enzymatic Saccharification by Trichoderma Harzianum KUC1716. Ind. Crops Prod. 2017, 109, 185–191. [Google Scholar] [CrossRef]
- Trulea, A.; Vintila, T.; Pop, G.; Monica, D. Effects of Bioprocess Parameters on Production of Cellulase Using Miscanthus as Substrate. Anim. Sci. Biotechnol. 2013, 46, 149–154. [Google Scholar]
- Modenbach, A.A.; Nokes, S.E. Enzymatic Hydrolysis of Biomass at High-Solids Loadings—A Review. Biomass Bioenergy 2013, 56, 526–544. [Google Scholar] [CrossRef]
- Padella, M.; O’Connell, A.; Prussi, M. What Is Still Limiting the Deployment of Cellulosic Ethanol? Analysis of the Current Status of the Sector. Appl. Sci. 2019, 9, 4523. [Google Scholar] [CrossRef]
- Isikgor, F.H.; Becer, C.R. Lignocellulosic Biomass: A Sustainable Platform for the Production of Bio-Based Chemicals and Polymers. Polym. Chem. 2015, 6, 4497–4559. [Google Scholar] [CrossRef]
- Ojo, A.O.; de Smidt, O. Lactic Acid: A Comprehensive Review of Production to Purification. Processes 2023, 11, 688. [Google Scholar] [CrossRef]
- Ajala, E.O.; Olonade, Y.O.; Ajala, M.A.; Akinpelu, G.S. Lactic Acid Production from Lignocellulose—A Review of Major Challenges and Selected Solutions. ChemBioEng Rev. 2020, 7, 38–49. [Google Scholar] [CrossRef]
- López-Gómez, J.P.; Alexandri, M.; Schneider, R.; Venus, J. A Review on the Current Developments in Continuous Lactic Acid Fermentations and Case Studies Utilising Inexpensive Raw Materials. Process Biochem. 2019, 79, 1–10. [Google Scholar] [CrossRef]
- Gunes, K.; Sargin, S.; Celiktas, M.S. Investigation of Lactic Acid Production by Pressurized Liquid Hot Water from Cultivated Miscanthus × Giganteus. Prep. Biochem. Biotechnol. 2023, 53, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Boakye-Boaten, N.A.; Xiu, S.; Shahbazi, A.; Wang, L.; Li, R.; Schimmel, K. Uses of Miscanthus Press Juice within a Green Biorefinery Platform. Bioresour. Technol. 2016, 207, 285–292. [Google Scholar] [CrossRef]
- Biofuels Digest. Available online: https://www.biofuelsdigest.com/bdigest/2014/03/13/direvo-industrial-biotechnology-biofuels-digests-2014-5-minute-guide/ (accessed on 2 June 2023).
- Adhikari, D.K.; Trivedi, J.; Agraval, D. Consolidated Bio Processing of Lignocellulosic Biomass for L-Lactic Acid Production. U.S. Patent Application No. 14/415,652, 16 July 2015. [Google Scholar]
- van der Pol, E.C.; Eggink, G.; Weusthuis, R.A. Production of l(+)-Lactic Acid from Acid Pretreated Sugarcane Bagasse Using Bacillus Coagulans DSM2314 in a Simultaneous Saccharification and Fermentation Strategy. Biotechnol. Biofuels 2016, 9, 248. [Google Scholar] [CrossRef]
- Ma, K.; Hu, G.; Pan, L.; Wang, Z.; Zhou, Y.; Wang, Y.; Ruan, Z.; He, M. Highly Efficient Production of Optically Pure L-Lactic Acid from Corn Stover Hydrolysate by Thermophilic Bacillus Coagulans. Bioresour. Technol. 2016, 219, 114–122. [Google Scholar] [CrossRef]
- Luo, H.; Klein, I.M.; Jiang, Y.; Zhu, H.; Liu, B.; Kenttämaa, H.I.; Abu-Omar, M.M. Total Utilization of Miscanthus Biomass, Lignin and Carbohydrates, Using Earth Abundant Nickel Catalyst. ACS Sustain. Chem. Eng. 2016, 4, 2316–2322. [Google Scholar] [CrossRef]
- Dias, C.; Nobre, B.P.; Santos, J.A.L.; Lopes da Silva, T.; Reis, A. Direct Lipid and Carotenoid Extraction from Rhodosporidium Toruloides Broth Culture after High Pressure Homogenization Cell Disruption: Strategies, Methodologies, and Yields. Biochem. Eng. J. 2022, 189, 108712. [Google Scholar] [CrossRef]
- Martins, J.A.; Lopes da Silva, T.; Marques, S.; Carvalheiro, F.; Roseiro, L.B.; Duarte, L.C.; Gírio, F. The Use of Flow Cytometry to Assess Rhodosporidium Toruloides NCYC 921 Performance for Lipid Production Using Miscanthus Sp. Hydrolysates. Biotechnol. Rep. 2021, 30, e00639. [Google Scholar] [CrossRef]
- Mast, B.; Zöhrens, N.; Schmidl, F.; Hernandez, R.; French, W.T.; Merkt, N.; Claupein, W.; Graeff-Hönninger, S. Lipid Production for Microbial Biodiesel by the Oleagenious Yeast Rhodotorula Glutinis Using Hydrolysates of Wheat Straw and Miscanthus as Carbon Sources. Waste Biomass Valorization 2014, 5, 955–962. [Google Scholar] [CrossRef]
- Tasselli, G.; Filippucci, S.; Borsella, E.; D’Antonio, S.; Gelosia, M.; Cavalaglio, G.; Turchetti, B.; Sannino, C.; Onofri, A.; Mastrolitti, S.; et al. Yeast Lipids from Cardoon Stalks, Stranded Driftwood and Olive Tree Pruning Residues as Possible Extra Sources of Oils for Producing Biofuels and Biochemicals. Biotechnol. Biofuels 2018, 11, 147. [Google Scholar] [CrossRef] [PubMed]
- Sitepu, I.R.; Jin, M.; Fernandez, J.E.; da Costa Sousa, L.; Balan, V.; Boundy-Mills, K.L. Identification of Oleaginous Yeast Strains Able to Accumulate High Intracellular Lipids When Cultivated in Alkaline Pretreated Corn Stover. Appl. Microbiol. Biotechnol. 2014, 98, 7645–7657. [Google Scholar] [CrossRef]
- Xu, Q.; Li, S.; Huang, H.; Wen, J. Key Technologies for the Industrial Production of Fumaric Acid by Fermentation. Biotechnol. Adv. 2012, 30, 1685–1696. [Google Scholar] [CrossRef] [PubMed]
- Martin-Dominguez, V.; Garcia-Montalvo, J.; Garcia-Martin, A.; Ladero, M.; Santos, V.E. Fumaric Acid Production by R. Arrhizus NRRL 1526 Using Apple Pomace Enzymatic Hydrolysates: Kinetic Modelling. Processes 2022, 10, 2624. [Google Scholar] [CrossRef]
- Sebastian, J.; Rouissi, T.; Brar, S.K. Miscanthus Sp.—Perennial Lignocellulosic Biomass as Feedstock for Greener Fumaric Acid Bioproduction. Ind. Crops Prod. 2022, 175, 114248. [Google Scholar] [CrossRef]
- Swart, R.M.; Brink, H.; Nicol, W. Rhizopus Oryzae for Fumaric Acid Production: Optimising the Use of a Synthetic Lignocellulosic Hydrolysate. Fermentation 2022, 8, 278. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Gurav, R.; Choi, T.-R.; Jung, H.-R.; Yang, S.-Y.; Moon, Y.-M.; Song, H.-S.; Jeon, J.-M.; Choi, K.-Y.; Yang, Y.-H. Bioconversion of Plant Biomass Hydrolysate into Bioplastic (Polyhydroxyalkanoates) Using Ralstonia Eutropha 5119. Bioresour. Technol. 2019, 271, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Sohn, Y.J.; Son, J.; Lim, H.J.; Lim, S.H.; Park, S.J. Valorization of Lignocellulosic Biomass for Polyhydroxyalkanoate Production: Status and Perspectives. Bioresour. Technol. 2022, 360, 127575. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Oh, Y.H.; Jang, Y.-A.; Kang, K.H.; David, Y.; Yu, J.H.; Song, B.K.; Choi, J.; Chang, Y.K.; Joo, J.C.; et al. Recombinant Ralstonia Eutropha Engineered to Utilize Xylose and Its Use for the Production of Poly(3-Hydroxybutyrate) from Sunflower Stalk Hydrolysate Solution. Microb. Cell Factories 2016, 15, 95. [Google Scholar] [CrossRef]
- Annamalai, N.; Sivakumar, N. Production of Polyhydroxybutyrate from Wheat Bran Hydrolysate Using Ralstonia Eutropha through Microbial Fermentation. J. Biotechnol. 2016, 237, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Vance, C.; Sweeney, J.; Murphy, F. Space, Time, and Sustainability: The Status and Future of Life Cycle Assessment Frameworks for Novel Biorefinery Systems. Renew. Sustain. Energy Rev. 2022, 159, 112259. [Google Scholar] [CrossRef]


| Miscanthus Species and Country of Habitat | Cellulose | Hemicelluloses | Lignin | Ash | Others | Ref. |
|---|---|---|---|---|---|---|
| M. × gigantus (France) | 41.08 | 24.52 | 27.00 | - | 7.4 | [26] |
| M. × giganteus (Canada) | 55.01 | 17.42 | 16.90 | - | 10.67 | [27] |
| M. × giganteus (UK) | 45.5 | 29.2 | 23.8 | - | - | [28] |
| M. × giganteus (different climate regions of Russia) | 43.2–55.5 | 17.9–22.9 | 17.1–25.1 | 0.90–2.95 | 0.3–1.2% | [29] |
| M. × giganteus (Poland) | 45.12 | 29.30 | 22.21 | - | - | [22] |
| M. sinensis (Poland) | 44.12 | 29.79 | 19.52 | - | - | |
| M. sacchariflorus (Poland) | 44.57 | 29.11 | 20.34 | - | - | |
| M. lutarioriparius (China) | 41.89 | 18.21 | 16.77 | - | - | [30] |
| M. sinensis (Russia) | 49.1 | 20.7 | 23.3 | 3.00 | 2.6 | [31] |
| M. sacchariflorus (Russia) | 53.3 | 21.3 | 28.1 | 5.66 | 2.4 | |
| M. sinensis (China) | 37.66 (av.), 48.52 (max.) | 22.94 (av.) | 17.35 (av.) | 2.47 (av.), 4.5 (max.), 1.43 (min) | 15.83 (av.) | [24] |
| M. floridulus (China) | 36.28 (av.) | 21.95 (av.) | 16.94 (av.) | 2.74 (av.) | 18.41 (av.) | |
| M. nudipes (China) | 36.07 (av.) | 22.39 (av.) | 17.21 (av.) | 2.51 (av.) | 21.10 (av.) | |
| M. sacchariflorus (China) | 39.25 (av.), | 26.35 (av.), 34.23 (max.) | 18.11 (av.), 23.75 (max.) | 2.51 (av.), | 11.62 (av), 5.38 (min.) | |
| M. lutarioriparius (China) | 39.96 (av.) | 22.85 (av.) | 18.69 (av.) | 2.43 (av.) | 12.43 (av.) | |
| Hybrid (China) | 37.14 (av.) | 21.21 (av.), 15.71 (min.) | 16.37 (av.), 13.01 (min.) | 2.56 (av.) | 20.67 (av.), 34.88 (max.) |
| Feedstock and Country of Habitat | Pretreatment | Enzymes for Hydrolysis | Microbial Producer | Ethanol Concentration and Yield | Year, Ref. |
|---|---|---|---|---|---|
| M. sacchariflorus (Korea) | 0.4 M NaOH at 95 °C | Cellic® CTec2 and HTec2 | Saccharomyces cerevisiae | 45.5 g/L, 165 L/t miscanthus | 2019, [76] |
| M. × giganteus (UK) | 1% H2SO4 and autoclaved at 121 °C | Celluclast® | S. cerevisiae | 13.58 g/L, 0.148 g/g miscanthus | 2021, [28] |
| M. sacchariflorus (Russia) | 4% HNO3 at 94−96 °C | CelloLux®-A and BrewZyme BGX | S. cerevisiae | 40 g/L, 260 L/t miscanthus | 2022, [77] |
| M. × giganteus (France) | 20% DMSO and 80% DES (Choline chloride/glycerol) at 373 K | Celluclast® 1.5L | S. cerevisiae | 18.03 g/L, 138.4 g/kg miscanthus | 2022, [26] |
| M. × giganteus (USA) | AFEX-pretreatment at 100 °C | CTec2 and HTec2 | S. cerevisiae | 33.7 g/L, 252 L/t miscanthus | 2018, [79] |
| Zymomonas mobilis | 38.0 g/L, 284 L/t miscanthus | ||||
| Corn stover (USA) | S. cerevisiae | 32.1 g/L, 233 L/t corn stover | |||
| Zymomonas mobilis | 45.1 g/L, 327 L/t corn stover | ||||
| Pine needle waste biomass (India) | 1.0% NaOH + Microwave (900 W for 12 min) | Xylanase from Bacillus pumilus and cellulase from Bacillus subtilis | Schizosaccharomyces sp. EF-3 and Kluyveromyces marxianus (co-fermentation) | 17.65 g/L | 2022, [80] |
| Hemp (South Korea) | 0.2–1.6% NaOH at 65 °C, 1% H2SO4 at 121 °C | Cellic® CTec2 | S. cerevisiae | 18.9 g/L | 2022, [81,82] |
| Kenaf (South Korea) | 16.2 g/L | ||||
| Bamboo (Phyllostachys edulis) (China) | NaOH, acid catalyzed steam pretreatment 190 °C | Cellic® CTec3 and β-glucosidase | S. cerevisiae | 50.10 g/L | 2020, [83,84] |
| Feedstock and Country of Habitat | Pretreatment | Inoculum | Methane Concentration, mL CH4/g Volatile Solids | Year, Ref. |
|---|---|---|---|---|
| M. sinensis (France) | no | Anaerobic sludge (from UASB treating sugar industry wastewater) | 202 | 2019, [88] |
| M. sacchariflorus (France) | no | 195 | ||
| M. × giganteus Floridulus (France) | no | 184 | ||
| 10% NaOH at 23−26 °C, 6 days | 291 | |||
| 10% CaO at 23−26 °C, 6 days | 245 | |||
| M. floridulus (China) | no | Biogas slurry, collected from biogas plant used corn straw as feedstock (China) | 229.5 | 2018, [90] |
| 6% NaOH at 35 °C, 3 h | 284.9 | |||
| 2% H2O2 at 35 °C, 24 h | 327.4 | |||
| Hot water at 95 °C, 10 h | 260.0 | |||
| Microaerobic pretreatment | 271.6 | |||
| HCl at 99 °C, 0.5 h | 260.3 | |||
| Switchgrass (Turkey) | no | Anaerobic sludge | 217.1 | 2022, [97] |
| 3% solid loading, 100 °C, 6 h | 248.7 | |||
| Wheat straw (USA) | no | Inoculum from a mesophilic anaerobic digester in Pullman Wastewater Treatment Plant (USA) | 407.8 | 2019, [96] |
| 0.7% NH3 and thermal at 105 °C | 538.1 | |||
| Wheat straw (USA) | no | Effluent from a wastewater treatment plant (USA) | 210.4 | 2018, [98] |
| 1% urea at 20 °C, 6 d | 305.5 |
| Feedstock and Country of Habitat (If Known) | Pretreatment | Enzymes for Hydrolysis | Microbial Producer | BC Concentration | Year, Ref. |
|---|---|---|---|---|---|
| Miscanthus (Korea) | hydrothermal pretreatment in the presence of H2SO4 + detoxified by adsorption on activated carbon | Celic® CTec2 | Gluconacetobacter xylinus | 16.70 g/L | 2021, [100] |
| M. sacchariflorus Maxim. (Russia) | two stages using 4% NaOH and 4% HNO3 at 90−96 °C | CelloLux®-A and BrewZyme BGX | Medusomyces gisevii | 1.24 g/L | 2021, [102] |
| Barley straw | hydrothermal pretreatment in the presence of H2SO4 + detoxified by adsorption on activated carbon | Celic® CTec2 | G. xylinus | 13.09 g/L | 2021, [100] |
| Pine tree | 12.54 g/L | ||||
| Grape pomace + potatoes (Spain) | 2% H2SO4 at 125 °C + neutralization with CaCO3 | no | Komagateibacter xylinus | 4.0 g/L | 2022, [105] |
| Potato peel waste | 2.0 M of each of nitric, sulfuric, hydrochloric and phosphoric acid at 100 °C for 2, 3, 4 and 6 h | no | G. xylinum | 4.7 g/L | 2019, [106] |
| Feedstock and Country of Habitat (If Known) | Pretreatment | Microbial Producer | Biotech Product | Enzyme Activity | Year, Ref. |
|---|---|---|---|---|---|
| M. lutarioriparius (China) | steam explosion at 195 °C, 10 min | T. reesei | Cellulase | Cellulase activity 19.85 FPU/mL | 2021, [108] |
| M. sacchariflorus | no | Pseudomonas | Laccase | Laccase activity 8091 U/L | 2019, [70] |
| Coconut mesocarp (India) | liquid hot water treatment at 210 °C for 20 min + 0.5% NaOH at 180 °C for 40 min | T. reesei | Cellulase | Cellulase activity 54 FPU/mL | 2018, [110] |
| Wheat bran | no | Bacillus sp. | Laccase | Laccase activity 246.7 U/L | 2019, [70] |
| Feedstock and Country of Habitat | Pretreatment | Enzymes for Hydrolysis | Microbial Producer | Lactic Acid Concentration | Year, Ref. |
|---|---|---|---|---|---|
| M.× giganteus (Turkey) | LHW at 140 °C, 100 bar and 45 min | C2730 | Rhizopus oryzae | 6.8 g/L | 2023, [119] |
| Sugarcane bagasse (Australia) | 0.72% H2SO4 170 °C for 15 min + steam explosion | Genencor GC220 (Denmark) | Bacillus coagulans | 70.4 g/L | 2016, [123] |
| Corn stover (China) | simultaneous bio-delignification and saccharification with lignocellulolytic enzyme system obtained from co-fungi culture | B. coagulans | 92 g/L | 2016, [124] | |
| Feedstock and Country of Habitat (If Known) | Pretreatment | Enzymes for Hydrolysis | Microbial Producer | Lipids Concentration and Lipid Content | Year, Ref. |
|---|---|---|---|---|---|
| Miscanthus (Netherlands) | hydrothermal pretreatment at 190 °C for 15 min | Cellic® CTec2 | Rhodosporidium toruloides | 1.64 g/L, 30.67% of cell dry weight | 2021, [127] |
| Cardoon stalks (Italy) | 0.6% H2SO4 solution for 10 min + steam explosion at 195 °C, 7.5 min | CTEC2 | Solicoccozyma terricola | 13.20 g/L, 55.60% of cell dry weight | 2018, [129] |
| Residues from olive tree pruning (Italy) | Steam explosion at 210 °C for 25 min | NS-22192 (Novozyme, Denmark) | Naganishia adeliensis | 4.90 g/L, 44.38% of cell dry weight | |
| Corn stover | AFEX pretreatment at 140 °C for 30 min | Cellic® Ctec3 and Cellic® Htec3 | Cryptococcus humicola | 15.5 g/L, 40% of cell dry weight | 2014, [130] |
| Feedstock | Pretreatment | Enzymes for Hydrolysis | Microbial Producer | PHAs Concentration | Year, Ref. |
|---|---|---|---|---|---|
| Miscanthus | Hydrolyzate prepared by dilute H2SO4 pretreatment and enzymatic digestion | Ralstonia eutropha | 2.0 g/L | 2019, [135] | |
| Barley | 1.8 g/L | ||||
| Pine | 1.7 g/L | ||||
| Sunflower stalk | Hydrothermal treatment at 190 °C for 5 min | Cellic® CTec3 | R. eutropha | 7.86 g/L | 2016, [137] |
| Wheat bran | 1% NaOH | Commercial cellulase of T. reesei and β—glucosidase of Aspergillus niger | R. eutropha | 0.319 g/L | 2016, [138] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mironova, G.F.; Budaeva, V.V.; Skiba, E.A.; Gismatulina, Y.A.; Kashcheyeva, E.I.; Sakovich, G.V. Recent Advances in Miscanthus Macromolecule Conversion: A Brief Overview. Int. J. Mol. Sci. 2023, 24, 13001. https://doi.org/10.3390/ijms241613001
Mironova GF, Budaeva VV, Skiba EA, Gismatulina YA, Kashcheyeva EI, Sakovich GV. Recent Advances in Miscanthus Macromolecule Conversion: A Brief Overview. International Journal of Molecular Sciences. 2023; 24(16):13001. https://doi.org/10.3390/ijms241613001
Chicago/Turabian StyleMironova, Galina F., Vera V. Budaeva, Ekaterina A. Skiba, Yulia A. Gismatulina, Ekaterina I. Kashcheyeva, and Gennady V. Sakovich. 2023. "Recent Advances in Miscanthus Macromolecule Conversion: A Brief Overview" International Journal of Molecular Sciences 24, no. 16: 13001. https://doi.org/10.3390/ijms241613001
APA StyleMironova, G. F., Budaeva, V. V., Skiba, E. A., Gismatulina, Y. A., Kashcheyeva, E. I., & Sakovich, G. V. (2023). Recent Advances in Miscanthus Macromolecule Conversion: A Brief Overview. International Journal of Molecular Sciences, 24(16), 13001. https://doi.org/10.3390/ijms241613001