Staphylococcus aureus Modulates Carotenoid and Phospholipid Content in Response to Oxygen-Restricted Growth Conditions, Triggering Changes in Membrane Biophysical Properties
Abstract
:1. Introduction
2. Results
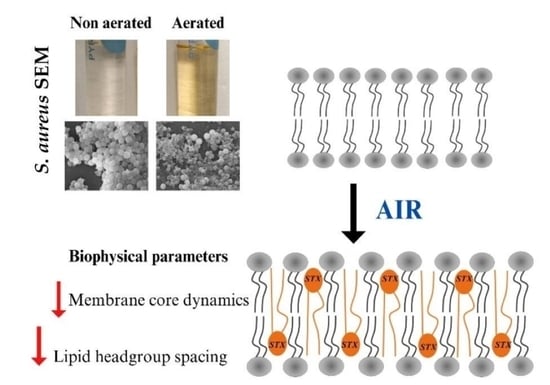
2.1. Cell Morphologies in Biofilm and Planktonic Cells and Production of Exopolysaccharides in Biofilms
2.2. Biofilms Pigmentation Levels Vary When Exposed to Oxygen Stress
2.3. Carotenoid and Phospholipid Content and Composition
2.4. Biophysical Properties of Membranes
3. Discussion
3.1. Regulation of Lipid Composition in S. aureus Biofilms and Planktonic Cells
3.2. Variation in the Biophysical Properties of S. aureus Membranes
4. Materials and Methods
4.1. Materials
4.2. Bacterial Cultures
4.3. Gravity Fed System for Biofilm Growth under Aerobic and Anaerobic Conditions
4.4. Separation of Total Lipids
4.5. Scanning Electron Microscopy (SEM) of Biofilms and Planktonic Cells
4.6. Fluorescence Spectroscopy
4.7. Fourier Transform Infrared (FTIR) Spectroscopy
4.8. Targeted Lipidomics Analysis by LC-MS/MS
4.9. Carotenoids Analysis by LC-DAD-APCI-MS/MS
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tong, S.Y.C.; Davis, J.S.; Eichenberger, E.; Holland, T.L.; Vance, F. Staphylococcus aureus Infections: Epidemiology, Pathophysiology, Clinical Manifestations, and Management. Clin. Microbiol. Rev. 2015, 28, 603–661. [Google Scholar] [CrossRef] [PubMed]
- Lowy, F. Staphylococcus aureus infections. N. Engl. J. Med. 1998, 339, 520–532. [Google Scholar] [CrossRef] [PubMed]
- Kintarak, S.; Whawell, S.A.; Speight, M.; Packer, S.; Nair, S.P. Internalization of Staphylococcus aureus by Human Keratinocytes. Infect. Immun. 2004, 72, 5668–5675. [Google Scholar] [CrossRef]
- Foster, T.J. Antibiotic resistance in Staphylococcus aureus. Current status and future prospects. FEMS Microbiol. Rev. 2017, 41, 430–449. [Google Scholar] [CrossRef] [PubMed]
- Braungardt, H.; Singh, V.K. Impact of Deficiencies in Branched-Chain Fatty Acids and Staphyloxanthin in Staphylococcus aureus. BioMed Res. Int. 2019, 2019, 2603435. [Google Scholar] [CrossRef]
- Perez-Lopez, M.I.; Mendez-Reina, R.; Trier, S.; Herrfurth, C.; Feussner, I.; Bernal, A.; Forero-Shelton, M.; Leidy, C. Variations in carotenoid content and acyl chain composition in exponential, stationary and biofilm states of Staphylococcus aureus, and their influence on membrane biophysical properties. Biochim. Biophys. Acta Biomembr. 2019, 1861, 978–987. [Google Scholar] [CrossRef]
- Gruszecki, W.I.; Strzałka, K. Carotenoids as modulators of lipid membrane physical properties. Biochim. Biophys. Acta Mol. Basis Dis. 2005, 1740, 108–115. [Google Scholar] [CrossRef]
- López, G.-D.; Suesca, E.; Álvarez-Rivera, G.; Rosato, A.E.; Ibáñez, E.; Cifuentes, A.; Leidy, C.; Carazzone, C. Carotenogenesis of Staphylococcus aureus: New insights and impact on membrane biophysical properties. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2021, 1866, 158941. [Google Scholar] [CrossRef]
- Staubitz, P.; Neumann, H.; Schneider, T.; Wiedemann, I.; Peschel, A. MprF-mediated biosynthesis of lysylphosphatidylglycerol, an important determinant in staphylococcal defensin resistance. FEMS Microbiol. Lett. 2006, 231, 67–71. [Google Scholar] [CrossRef]
- Manrique-Moreno, M.; Jemioła-Rzemińska, M.; Múnera-Jaramillo, J.; López, G.-D.; Suesca, E.; Leidy, C.; Strzałka, K. Staphylococcus aureus Carotenoids Modulate the Thermotropic Phase Behavior of Model Systems That Mimic Its Membrane Composition. Membranes 2022, 12, 945. [Google Scholar] [CrossRef]
- Ocampo, J.; Afanador, N.; Vives, M.J.; Moreno, J.C.; Leidy, C. The antibacterial activity of phospholipase A2 type IIA is regulated by the cooperative lipid chain melting behavior in Staphylococcus aureus. Biochim. Biophys. Acta Biomembr. 2010, 1798, 1021–1028. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Gálvez, A.; Viera, I.; Roca, M. Carotenoids and Chlorophylls as Antioxidants. Antioxidants 2020, 9, 505. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Lee, C. Functional expression and extension of staphylococcal staphyloxanthin biosynthetic pathway in Escherichia coli. J. Biol. Chem. 2012, 287, 21575–21583. [Google Scholar] [CrossRef]
- Hall, J.W.; Yang, J.; Guo, H.; Ji, Y. The Staphylococcus aureus AirSR Two-Component System Mediates Reactive Oxygen Species Resistance via Transcriptional Regulation of Staphyloxanthin Production. Infect. Immun. 2017, 85, e00838-16. [Google Scholar] [CrossRef]
- Mishra, N.N.; Liu, G.Y.; Yeaman, M.R.; Nast, C.C.; Proctor, R.A.; McKinnell, J.; Bayer, A.S. Carotenoid-Related Alteration of Cell Membrane Fluidity Impacts Staphylococcus aureus Susceptibility to Host Defense Peptides. Antimicrob. Agents Chemother. 2011, 55, 526–531. [Google Scholar] [CrossRef]
- Chaibenjawong, P.; Foster, S.J. Desiccation tolerance in Staphylococcus aureus. Arch. Microbiol. 2010, 193, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Crass, R.L.; Powell, K.L.; Huang, A.M. Daptomycin for the treatment of Staphylococcus aureus infections complicated by septic pulmonary emboli. Diagn. Microbiol. Infect. Dis. 2019, 93, 131–135. [Google Scholar] [CrossRef]
- Liu, G.Y.; Essex, A.; Buchanan, J.T.; Datta, V.; Hoffman, H.M.; Bastian, J.F.; Fierer, J.; Nizet, V. Staphylococcus aureus golden pigment impairs neutrophil killing and promotes virulence through its antioxidant activity. J. Exp. Med. 2005, 202, 209–215. [Google Scholar] [CrossRef]
- Wölk, C.; Youssef, H.; Guttenberg, T.; Marbach, H.; Vizcay-Barrena, G.; Shen, C.; Brezesinski, G.; Harvey, D.R. Phase Diagram for a Lysyl-Phosphatidylglycerol Analogue in Biomimetic Mixed Monolayers with Phosphatidylglycerol: Insights into the Tunable Properties of Bacterial Membranes. ChemPhysChem 2020, 21, 702–706. [Google Scholar] [CrossRef]
- Zhang, Y.-M.; Rock, C.O. Membrane lipid homeostasis in bacteria. Nat. Rev. Microbiol. 2008, 6, 222–233. [Google Scholar] [CrossRef]
- Hernández-Villa, L.; Manrique-Moreno, M.; Leidy, C.; Jemioła-Rzemińska, M.; Ortíz, C.; Strzałka, K. Biophysical evaluation of cardiolipin content as a regulator of the membrane lytic effect of antimicrobial peptides. Biophys. Chem. 2018, 238, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Renner, L.D.; Weibel, D.B. Cardiolipin microdomains localize to negatively curved regions of Escherichia coli membranes. Proc. Natl. Acad. Sci. USA 2011, 108, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Rocha-Roa, C.; Orjuela, J.D.; Leidy, C.; Cossío, P.; Santamaría, C.A. Cardiolipin prevents pore formation in phosphatidylglycerol bacterial membrane models. FEBS Lett. 2021, 595, 2701–2714. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, S.; Pané-Farré, J.; Kohler, C.; Hecker, M.; Engelmann, S. Anaerobic gene expression in Staphylococcus aureus. J. Bacteriol. 2007, 189, 4275–4289. [Google Scholar] [CrossRef]
- Coleman, G.; Garbutt, I.; Demnitz, U. Ability of a Staphylococcus aureus isolate from a chronic osteomyelitic lesion to survive in the absence of air. Eur. J. Clin. Microbiol. 1983, 2, 595–597. [Google Scholar] [CrossRef]
- Park, M.; Myers, R.; Marzella, L. Oxygen tensions and infections: Modulation of microbial growth, activity of antimicrobial agents, and immunologic responses. Clin. Infect. Dis. 1992, 14, 720–740. [Google Scholar] [CrossRef]
- Ortiz-Prado, E.; Dunn, J.F.; Vasconez, J.; Castillo, D.; Viscor, G. Partial pressure of oxygen in the human body: A general review. Am. J. Blood Res. 2019, 9, 1–14. [Google Scholar]
- Periasamy, S.; Joo, H.S.; Duong, A.C.; Bach, T.H.L.; Tan, V.Y.; Chatterjee, S.S.; Cheung, G.Y.; Otto, M. How Staphylococcus aureus biofilms develop their characteristic structure. Proc. Natl. Acad. Sci. USA 2012, 109, 1281–1286. [Google Scholar] [CrossRef]
- Archer, N.K.; Mazaitis, M.J.; Costerton, J.G.; Leid, J.G.; Powers, M.E.; Shirtliff, M.E. Staphylococcus aureus biofilms. Virulence 2011, 2, 445–459. [Google Scholar] [CrossRef]
- Heimburg, T. Thermal Biophysics of Membranes; Wiley: Hoboken, NJ, USA, 2007. [Google Scholar]
- Chamberlain, N.R.; Mehrtens, B.G.; Xiong, Z.; Kapral, F.A.; Boardman, J.L.; Rearick, J.I. Correlation of carotenoid production, decreased membrane fluidity, and resistance to oleic acid killing in Staphylococcus aureus 18Z. Infect. Immun. 1991, 59, 4332–4337. [Google Scholar] [CrossRef]
- Seel, W.; Baust, D.; Sons, D.; Albers, M.; Etzbach, L.; Fuss, J.; Lipski, A. Carotenoids are used as regulators for membrane fluidity by Staphylococcus xylosus. Sci. Rep. 2020, 10, 330. [Google Scholar] [CrossRef]
- Lin-Vien, D.; Colthup, N.B.; Fateley, W.G.; Grasselli, J.G. The Handbook of Infrared and Raman Characteristic Frequencies of Organic Molecules; Elsevier Science & Technology: Amsterdam, The Netherlands, 1991. [Google Scholar]
- Lewis, R.N.A.H.; McElhaney, R.N. Membrane lipid phase transitions and phase organization studied by Fourier transform infrared spectroscopy. Biochim. Biophys. Acta Biomembr. 2013, 1828, 2347–2358. [Google Scholar] [CrossRef] [PubMed]
- Costerton, J.W.; Stewart, S.; Greenberg, E.P. Bacterial Biofilms: A Common Cause of Persistent Infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef] [PubMed]
- Oldfield, E.; Feng, X. Resistance-resistant antibiotics. Trends Pharmacol. Sci. 2014, 35, 664–674. [Google Scholar] [CrossRef]
- Clauditz, A.; Resch, A.; Wieland, K.P.; Peschel, A.; Götz, F. Staphyloxanthin Plays a Role in the Fitness of Staphylococcus aureus and Its Ability to Cope with Oxidative Stress. ASM J. 2006, 74, 4950–4953. [Google Scholar] [CrossRef] [PubMed]
- Romantsov, T.; Guan, Z.; Wood, J.M. Cardiolipin and the osmotic stress responses of bacteria. Biochim. Biophys. Acta 2009, 1788, 2092–2100. [Google Scholar] [CrossRef] [PubMed]
- Ohniwa, R.L.; Kitabayashi, K.; Morikawa, K. Alternative cardiolipin synthase Cls1 compensates for stalled Cls2 function in Staphylococcus aureus under conditions of acute acid stress. FEMS Microbiol. Lett. 2013, 338, 141–146. [Google Scholar] [CrossRef]
- Zhang, T.H.; Muraih, J.K.; Nasim, T.; Hershowitz, J.; Victor, R.L.; Silverman, J.; Uwumarenogie, S.; Taylor, S.D.; Palmer, M. Cardiolipin prevents membrane translocation and permeabilization by Daptomycin. J. Biol. Chem. 2014, 289, 11584–11591. [Google Scholar] [CrossRef]
- Ernst, C.M.; Peschel, A. Broad-spectrum antimicrobial peptide resistance by MprF-mediated aminoacylation and flipping of phospholipids. Mol. Microbiol. 2011, 80, 290–299. [Google Scholar] [CrossRef]
- Kossakowska-Zwierucho, M.; Kaźmierkiewicz, R.; Bielawski, K.P.; Nakonieczna, J. Factors Determining Staphylococcus aureus Susceptibility to Photoantimicrobial Chemotherapy: RsbU Activity, Staphyloxanthin Level, and Membrane Fluidity. Front. Microbiol. 2016, 7, 1141. [Google Scholar] [CrossRef]
- Grudzinski, W.; Nierzwicki, L.; Welc, R.; Reszczynska, E.; Luchowski, R.; Czub, J.; Gruszecki, W.I. Localization and Orientation of Xanthophylls in a Lipid Bilayer. Sci. Rep. 2017, 7, 9619. [Google Scholar] [CrossRef]
- Lewis, R.N.A.H.; McElhaney, N. The physicochemical properties of cardiolipin bilayers and cardiolipin-containing lipid membranes. Biomembranes 2009, 1788, 2069–2079. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.; Tang, X.; Dong, W.; Sun, N.; Yuan, W. A Review of Biofilm Formation of Staphylococcus aureus and Its Regulation Mechanism. Antibiotics 2022, 12, 12. [Google Scholar] [CrossRef] [PubMed]
- Fleming, K.G. Fluorescence Theory, de Encyclopedia of Spectroscopy and Spectrometry; Elsevier: Amsterdam, The Netherlands, 2017; pp. 647–653. [Google Scholar]
- Rodríguez–Beltrán, É.; López, G.-D.; Anzola, J.M.; Rodríguez–Castillo, J.G.; Carazzone, C.; Murcia, M.I. Heterogeneous fitness landscape cues, pknG high expression, and phthiocerol dimycocerosate low production of Mycobacterium tuberculosis ATCC25618 rpoB S450L in enriched broth. Tuberculosis 2022, 132, 102156. [Google Scholar] [CrossRef] [PubMed]
- Castro-Puyana, M.; Pérez-Sánchez, A.; Valdésb, A.; Ibrahim, O.H.M.; Suárez-Álvarez, O.S. Pressurized liquid extraction of Neochloris oleoabundans for the recovery of bioactive carotenoids with anti-proliferative activity against human colon cancer cells. Food Res. Int. 2017, 99, 1048–1055. [Google Scholar] [CrossRef] [PubMed]
- Dhooghe, L.; Mesia, K.; Kohtala, E.; Tona, L.; Pieters, L.; Vlietinck, A.; Apers, S. Development and validation of an HPLC-method for the determination of alkaloids in the stem bark extract of Nauclea pobeguinii. Talanta 2007, 76, 462–468. [Google Scholar] [CrossRef]







| Growth Condition | |
|---|---|
| Aerated biofilms | 0.41 ± 0.12 |
| Non-aerated biofilms | 0.29 ± 0.05 |
| Aerated planktonic cells | 0.37 ± 0.04 |
| Non-aerated planktonic cells | 0.28 ± 0.04 |
| No. | Compound | Biof-Aero | Biof-NoAero | Plant-Aer | Plant-NoAer |
|---|---|---|---|---|---|
| (μg/g Dry Cell Weight ± SD) | |||||
| 1 | 4,4-DPE-Iso1 | 556.72 ± 22.29 | 1129.74 ± 56.27 | 67.36 ± 2.51 | 4069.07 ± 125.34 |
| 2 | 4,4-DPE-Iso2 | 42.78 ± 3.45 | 252.12 ± 7.31 | n.d. | 346.36 ± 3.85 |
| 3 | 4,4-DNPA | 1742.18 ± 88.96 | 437.63 ± 19.86 | 802.07 ± 20.87 | 111.24 ± 11.81 |
| 4 | STX-Iso-1 | n.d. | n.d. | n.d. | 146.78 ± 6.91 |
| 5 | STX-Iso-2 | 132.17 ± 11.52 | 22.06 ± 2.29 | 591.55 ± 32.91 | n.d. |
| 6 | STX-Iso-3 | 64.23 ± 4.48 | 17.76 ± 2.16 | 49.72 ± 4.87 | n.d. |
| 7 | STX-Iso-4 | 42.20 ± 2.83 | n.d. | 121.79 ± 4.80 | n.d. |
| 8 | STX-Iso-5 | n.d. | n.d. | 243.92 ± 6.30 | n.d. |
| 9 | STX-Iso-6 | 520.01 ± 36.17 | 119.57 ± 9.81 a | 2232.06 ± 112.15 | 41.83 ± 3.73 a |
| 10 | STX-Iso-7 | 491.00 ± 15.36 | 109.58 ± 4.50 | 1316.68 ± 34.64 | n.d. |
| 11 | STX-Iso-8 | 93.78 ± 4.66 | n.d. | 307.91 ± 5.40 | n.d. |
| 12 | STX-C17 | 80.20 ± 5.40 b | n.d. | 92.29 ± 4.72 b | n.d. |
| Total carotenoids precursor | 599.50 ± 36.34 | 1376.86 ± 61.70 | 67.36 ± 2.51 | 4415.43 ± 263.23 | |
| Total carotenoids | 3168.78 ± 57.73 | 706.59 ± 15.69 | 5758.00 ± 72.50 | 299.85 ± 53.38 | |
| Biophysical | Planktonic | Biofilm | ||
|---|---|---|---|---|
| Membrane Parameters | Aerated | Non-Aerated | Aerated | Non-Aerated |
| 10 °C | 0.539 ± 0.001 | 0.498 ± 0.002 | 0.535 ± 0.001 | 0.545 ± 0.001 |
| Laurdan GP | ||||
| 37 °C | 0.327 ± 0.006 | 0.168 ± 0.001 | 0.274 ± 0.004 | 0.233 ± 0.002 |
| 10 °C | 0.231 ± 0.006 | 0.189 ± 0.008 | 0.259 ± 0.004 | 0.234 ± 0.014 |
| DPH Anisotropy | ||||
| 37 °C | 0.178 ± 0.008 | 0.096 ± 0.006 | 0.181 ± 0.003 | 0.143 ± 0.007 |
| 10 °C | 2851.36 | 2850.74 | 2852.08 | 2851.20 |
| Assy. Strech | ||||
| 37 °C | 2853.11 | 2853.21 | 2852.97 | 2853.04 |
| [°C] | 12 | 18 | 15 | 24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zamudio-Chávez, L.; Suesca, E.; López, G.-D.; Carazzone, C.; Manrique-Moreno, M.; Leidy, C. Staphylococcus aureus Modulates Carotenoid and Phospholipid Content in Response to Oxygen-Restricted Growth Conditions, Triggering Changes in Membrane Biophysical Properties. Int. J. Mol. Sci. 2023, 24, 14906. https://doi.org/10.3390/ijms241914906
Zamudio-Chávez L, Suesca E, López G-D, Carazzone C, Manrique-Moreno M, Leidy C. Staphylococcus aureus Modulates Carotenoid and Phospholipid Content in Response to Oxygen-Restricted Growth Conditions, Triggering Changes in Membrane Biophysical Properties. International Journal of Molecular Sciences. 2023; 24(19):14906. https://doi.org/10.3390/ijms241914906
Chicago/Turabian StyleZamudio-Chávez, Laura, Elizabeth Suesca, Gerson-Dirceu López, Chiara Carazzone, Marcela Manrique-Moreno, and Chad Leidy. 2023. "Staphylococcus aureus Modulates Carotenoid and Phospholipid Content in Response to Oxygen-Restricted Growth Conditions, Triggering Changes in Membrane Biophysical Properties" International Journal of Molecular Sciences 24, no. 19: 14906. https://doi.org/10.3390/ijms241914906
APA StyleZamudio-Chávez, L., Suesca, E., López, G.-D., Carazzone, C., Manrique-Moreno, M., & Leidy, C. (2023). Staphylococcus aureus Modulates Carotenoid and Phospholipid Content in Response to Oxygen-Restricted Growth Conditions, Triggering Changes in Membrane Biophysical Properties. International Journal of Molecular Sciences, 24(19), 14906. https://doi.org/10.3390/ijms241914906