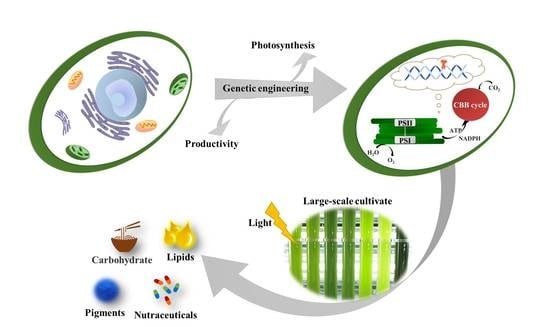
Advances in Genetic Engineering in Improving Photosynthesis and Microalgal Productivity
Abstract
:1. Introduction
2. Strategies for Increasing Photosynthetic Efficiency
2.1. The Light Phase of Photosynthesis
2.2. The Carbon Reactions of Photosynthesis
2.3. Non-Coding RNAs and Transcription Factors Affecting Photosynthesis
| Name | Type | Length | Species | Function | Reference |
|---|---|---|---|---|---|
| ApcZ | sRNA | 137 | Synechocystis 6803 | Inhibiting ocp translation under stress-free conditions | [60] |
| IsaR1 | sRNA | 68 | Conserved in cyanobacteria | Limiting photosynthesis-related gene expression (petJ, petABDC1, hemA, sufBCDS, and several others) under low iron conditions | [61] |
| PsrR1 | sRNA | 131 | Conserved in cyanobacteria | Limiting photosynthesis-related gene expression (psaL, psaJ, chlN, cpcA, and several others) upon shift to HL | [62] |
| RblR | asRNA | 113 | Synechocystis 6803 | Activating rbcL expression | [64] |
| PsbA2R PsbA3R | asRNA | 130, 220 160, 180 | Synechocystis 6803 | Protecting psbA2 and psbA3 mRNA from premature degradation | [65] |
| As1-Flv4 | asRNA | 280, 500 | Synechocystis 6803 | Preventing premature expression of the flv4-2 operon after shift to LC | [67] |
| IsrR | asRNA | 177 | Synechocystis 6803 | Inhibiting isiA expression under iron stress | [69] |
3. Transgenic Microalgae for Improved Biomass Production
3.1. Lipids
3.2. Pigments
3.3. Polysaccharides
4. The Limitations and Future Strategies of Genetic Engineering in Microalgal Productivity
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Benedetti, M.; Vecchi, V.; Barera, S.; Dall’Osto, L. Biomass from microalgae: The potential of domestication towards sustainable biofactories. Microb. Cell Factories 2018, 17, 173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vecchi, V.; Barera, S.; Bassi, R.; Dall’Osto, L. Potential and Challenges of Improving Photosynthesis in Algae. Plants 2020, 9, 67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wobbe, L.; Remacle, C. Improving the sunlight-to-biomass conversion efficiency in microalgal biofactories. J. Biotechnol. 2015, 201, 28–42. [Google Scholar] [CrossRef] [PubMed]
- Barber, J. Photosynthetic energy conversion: Natural and artificial. Chem. Soc. Rev. 2009, 38, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Stirbet, A.; Lazár, D.; Guo, Y.; Govindjee, G. Photosynthesis: Basics, history and modelling. Ann. Bot. 2019, 126, 511–537. [Google Scholar] [CrossRef] [Green Version]
- Perin, G.; Gambaro, F.; Morosinotto, T. Knowledge of Regulation of Photosynthesis in Outdoor Microalgae Cultures Is Essential for the Optimization of Biomass Productivity. Front. Plant Sci. 2022, 13, 846496. [Google Scholar] [CrossRef]
- Vijay, D.; Akhtar, M.K.; Hess, W.R. Genetic and metabolic advances in the engineering of cyanobacteria. Curr. Opin. Biotechnol. 2019, 59, 150–156. [Google Scholar] [CrossRef]
- Trovão, M.; Schüler, L.M.; Machado, A.; Bombo, G.; Navalho, S.; Barros, A.; Pereira, H.; Silva, J.; Freitas, F.; Varela, J. Random Mutagenesis as a Promising Tool for Microalgal Strain Improvement towards Industrial Production. Mar. Drugs 2022, 20, 440. [Google Scholar] [CrossRef]
- Cardona, T.; Shao, S.; Nixon, P.J. Enhancing photosynthesis in plants: The light reactions. Essays Biochem. 2018, 62, 85–94. [Google Scholar] [CrossRef] [Green Version]
- Perrine, Z.; Negi, S.; Sayre, R.T. Optimization of photosynthetic light energy utilization by microalgae. Algal Res. 2012, 1, 134–142. [Google Scholar] [CrossRef]
- Melis, A. Solar energy conversion efficiencies in photosynthesis: Minimizing the chlorophyll antennae to maximize efficiency. Plant Sci. 2009, 177, 272–280. [Google Scholar] [CrossRef]
- Kirst, H.; Gabilly, S.T.; Niyogi, K.K.; Lemaux, P.G.; Melis, A. Photosynthetic antenna engineering to improve crop yields. Planta 2017, 245, 1009–1020. [Google Scholar] [CrossRef] [PubMed]
- Kirst, H.; Melis, A. The chloroplast signal recognition particle (CpSRP) pathway as a tool to minimize chlorophyll antenna size and maximize photosynthetic productivity. Biotechnol. Adv. 2014, 32, 66–72. [Google Scholar] [CrossRef]
- Polle, J.E.W.; Kanakagiri, S.-D.; Melis, A. tla1, a DNA insertional transformant of the green alga Chlamydomonas reinhardtii with a truncated light-harvesting chlorophyll antenna size. Planta 2003, 217, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Polle, J.E.W.; Benemann, J.R.; Tanaka, A.; Melis, A. Photosynthetic apparatus organization and function in the wild type and a chlorophyll b-less mutant of Chlamydomonas reinhardtii. Dependence on carbon source. Planta 2000, 211, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Kirst, H.; Garcia-Cerdan, J.G.; Zurbriggen, A.; Ruehle, T.; Melis, A. Truncated Photosystem Chlorophyll Antenna Size in the Green Microalga Chlamydomonas reinhardtii upon Deletion of the TLA3-CpSRP43 Gene. Plant Physiol. 2012, 160, 2251–2260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeong, J.; Baek, K.; Kirst, H.; Melis, A.; Jin, E. Loss of CpSRP54 function leads to a truncated light-harvesting antenna size in Chlamydomonas reinhardtii. Biochim. Et Biophys. Acta (BBA) Bioenerg. 2017, 1858, 45–55. [Google Scholar] [CrossRef]
- Bujaldon, S.; Kodama, N.; Rathod, M.K.; Tourasse, N.; Ozawa, S.-I.; Sellés, J.; Vallon, O.; Takahashi, Y.; Wollman, F.-A. The BF4 and p71 antenna mutants from Chlamydomonas reinhardtii. Biochim. Et Biophys. Acta (BBA) Bioenerg. 2020, 1861, 148085. [Google Scholar] [CrossRef]
- Rathod, M.K.; Nellaepalli, S.; Ozawa, S.-I.; Kuroda, H.; Kodama, N.; Bujaldon, S.; Wollman, F.-A.; Takahashi, Y. Assembly Apparatus of Light-Harvesting Complexes: Identification of Alb3.1–cpSRP–LHCP Complexes in the Green Alga Chlamydomonas reinhardtii. Plant Cell Physiol. 2021, 63, 70–81. [Google Scholar] [CrossRef]
- Jeong, J.; Baek, K.; Yu, J.; Kirst, H.; Betterle, N.; Shin, W.; Bae, S.; Melis, A.; Jin, E. Deletion of the chloroplast LTD protein impedes LHCI import and PSI–LHCI assembly in Chlamydomonas reinhardtii. J. Exp. Bot. 2017, 69, 1147–1158. [Google Scholar] [CrossRef]
- Baek, K.; Kim, D.H.; Jeong, J.; Sim, S.J.; Melis, A.; Kim, J.-S.; Jin, E.; Bae, S. DNA-free two-gene knockout in Chlamydomonas reinhardtii via CRISPR-Cas9 ribonucleoproteins. Sci. Rep. 2016, 6, 30620. [Google Scholar] [CrossRef] [PubMed]
- Bujaldon, S.; Kodama, N.; Rappaport, F.; Subramanyam, R.; de Vitry, C.; Takahashi, Y.; Wollman, F.-A. Functional Accumulation of Antenna Proteins in Chlorophyll b-Less Mutants of Chlamydomonas reinhardtii. Mol. Plant 2017, 10, 115–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Negi, S.; Perrine, Z.; Friedland, N.; Kumar, A.; Tokutsu, R.; Minagawa, J.; Berg, H.; Barry, A.N.; Govindjee, G.; Sayre, R. Light regulation of light-harvesting antenna size substantially enhances photosynthetic efficiency and biomass yield in green algae†. Plant J. 2020, 103, 584–603. [Google Scholar] [CrossRef]
- Kirst, H.; Formighieri, C.; Melis, A. Maximizing photosynthetic efficiency and culture productivity in cyanobacteria upon minimizing the phycobilisome light-harvesting antenna size. Biochim. Et Biophys. Acta (BBA) Bioenerg. 2014, 1837, 1653–1664. [Google Scholar] [CrossRef] [Green Version]
- Cazzaniga, S.; Dall’Osto, L.; Szaub, J.; Scibilia, L.; Ballottari, M.; Purton, S.; Bassi, R. Domestication of the green alga Chlorella sorokiniana: Reduction of antenna size improves light-use efficiency in a photobioreactor. Biotechnol. Biofuels 2014, 7, 157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dall’Osto, L.; Cazzaniga, S.; Guardini, Z.; Barera, S.; Benedetti, M.; Mannino, G.; Maffei, M.E.; Bassi, R. Combined resistance to oxidative stress and reduced antenna size enhance light-to-biomass conversion efficiency in Chlorella vulgaris cultures. Biotechnol. Biofuels 2019, 12, 221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nymark, M.; Volpe, C.; Hafskjold, M.C.G.; Kirst, H.; Serif, M.; Vadstein, O.; Bones, A.M.; Melis, A.; Winge, P. Loss of ALBINO3b Insertase Results in Truncated Light-Harvesting Antenna in Diatoms. Plant Physiol. 2019, 181, 1257–1276. [Google Scholar] [CrossRef]
- Chen, M.; Blankenship, R.E. Expanding the solar spectrum used by photosynthesis. Trends Plant Sci. 2011, 16, 427–431. [Google Scholar] [CrossRef]
- Yang, D.; Qing, Y.; Min, C. Incorporation of the chlorophyll d-binding light-harvesting protein from Acaryochloris marina and its localization within the photosynthetic apparatus of Synechocystis sp. PCC6803. Biochim. Et Biophys. Acta (BBA) Bioenerg. 2010, 1797, 204–211. [Google Scholar] [CrossRef] [Green Version]
- Badshah, S.L.; Mabkhot, Y.; Al-Showiman, S.S. Photosynthesis at the far-red region of the spectrum in Acaryochloris marina. Biol. Res. 2017, 50, 16. [Google Scholar] [CrossRef]
- Ho, M.-Y.; Gan, F.; Shen, G.; Zhao, C.; Bryant, D.A. Far-red light photoacclimation (FaRLiP) in Synechococcus sp. PCC 7335: I. Regulation of FaRLiP gene expression. Photosynth. Res. 2017, 131, 173–186. [Google Scholar] [CrossRef] [PubMed]
- Suarez, J.V.; Mudd, E.A.; Day, A. A Chloroplast-Localised Fluorescent Protein Enhances the Photosynthetic Action Spectrum in Green Algae. Microorganisms 2022, 10, 1770. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Cao, T.; Dautermann, O.; Buschbeck, P.; Cantrell, M.B.; Chen, Y.; Lein, C.D.; Shi, X.; Ware, M.A.; Yang, F.; et al. Green diatom mutants reveal an intricate biosynthetic pathway of fucoxanthin. Proc. Natl. Acad. Sci. USA 2022, 119, e2203708119. [Google Scholar] [CrossRef] [PubMed]
- Murchie, E.H.; Ruban, A.V. Dynamic non-photochemical quenching in plants: From molecular mechanism to productivity. Plant J. 2020, 101, 885–896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghosh, D.; Mohapatra, S.; Dogra, V. Improving photosynthetic efficiency by modulating non-photochemical quenching. Trends Plant Sci. 2022. [Google Scholar] [CrossRef]
- Perozeni, F.; Beghini, G.; Cazzaniga, S.; Ballottari, M. Chlamydomonas reinhardtii LHCSR1 and LHCSR3 proteins involved in photoprotective non-photochemical quenching have different quenching efficiency and different carotenoid affinity. Sci. Rep. 2020, 10, 21957. [Google Scholar] [CrossRef]
- Perozeni, F.; Cazzaniga, S.; Ballottari, M. In vitro and in vivo investigation of chlorophyll binding sites involved in non-photochemical quenching in Chlamydomonas reinhardtii. Plant Cell Environ. 2019, 42, 2522–2535. [Google Scholar] [CrossRef] [Green Version]
- Cantrell, M.; Peers, G. A mutant of Chlamydomonas without LHCSR maintains high rates of photosynthesis, but has reduced cell division rates in sinusoidal light conditions. PLoS ONE 2017, 12, e0179395. [Google Scholar] [CrossRef] [Green Version]
- Barera, S.; Dall’Osto, L.; Bassi, R. Effect of lhcsr gene dosage on oxidative stress and light use efficiency by Chlamydomonas reinhardtii cultures. J. Biotechnol. 2021, 328, 12–22. [Google Scholar] [CrossRef]
- Kromdijk, J.; Głowacka, K.; Leonelli, L.; Gabilly, S.T.; Iwai, M.; Niyogi, K.K.; Long, S.P. Improving photosynthesis and crop productivity by accelerating recovery from photoprotection. Science 2016, 354, 857–861. [Google Scholar] [CrossRef]
- De Souza, A.P.; Burgess, S.J.; Doran, L.; Hansen, J.; Manukyan, L.; Maryn, N.; Gotarkar, D.; Leonelli, L.; Niyogi, K.K.; Long, S.P. Soybean photosynthesis and crop yield are improved by accelerating recovery from photoprotection. Science 2022, 377, 851–854. [Google Scholar] [CrossRef] [PubMed]
- Erb, T.J.; Zarzycki, J. Biochemical and synthetic biology approaches to improve photosynthetic CO2-fixation. Curr. Opin. Chem. Biol. 2016, 34, 72–79. [Google Scholar] [CrossRef] [Green Version]
- Wei, L.; Wang, Q.; Xin, Y.; Lu, Y.; Xu, J. Enhancing photosynthetic biomass productivity of industrial oleaginous microalgae by overexpression of RuBisCO activase. Algal Res. 2017, 27, 366–375. [Google Scholar] [CrossRef]
- Pinto, T.S.; Malcata, F.X.; Arrabaça, J.D.; Silva, J.M.; Spreitzer, R.J.; Esquível, M.G. Rubisco mutants of Chlamydomonas reinhardtii enhance photosynthetic hydrogen production. Appl Microbiol. Biotechnol. 2013, 97, 5635–5643. [Google Scholar] [CrossRef]
- Liang, F.; Lindblad, P. Synechocystis PCC 6803 overexpressing RuBisCO grow faster with increased photosynthesis. Metab. Eng. Commun. 2017, 4, 29–36. [Google Scholar] [CrossRef]
- Ruffing, A.M. Improved Free Fatty Acid Production in Cyanobacteria with Synechococcus sp. PCC 7002 as Host. Front. Bioeng. Biotechnol. 2014, 2, 17. [Google Scholar] [CrossRef] [Green Version]
- Eungrasamee, K.; Incharoensakdi, A.; Lindblad, P.; Jantaro, S. Overexpression of lipA or glpD_RuBisCO in the Synechocystis sp. PCC 6803 Mutant Lacking the Aas Gene Enhances Free Fatty-Acid Secretion and Intracellular Lipid Accumulation. Int. J. Mol. Sci. 2021, 22, 11468. [Google Scholar] [CrossRef] [PubMed]
- Eungrasamee, K.; Incharoensakdi, A.; Lindblad, P.; Jantaro, S. Synechocystis sp. PCC 6803 overexpressing genes involved in CBB cycle and free fatty acid cycling enhances the significant levels of intracellular lipids and secreted free fatty acids. Sci. Rep. 2020, 10, 4515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esquível, M.G.; Matos, A.R.; Marques Silva, J. Rubisco mutants of Chlamydomonas reinhardtii display divergent photosynthetic parameters and lipid allocation. Appl. Microbiol. Biotechnol. 2017, 101, 5569–5580. [Google Scholar] [CrossRef] [PubMed]
- Karkehabadi, S.; Peddi, S.R.; Anwaruzzaman, M.; Taylor, T.C.; Cederlund, A.; Genkov, T.; Andersson, I.; Spreitzer, R.J. Chimeric Small Subunits Influence Catalysis without Causing Global Conformational Changes in the Crystal Structure of Ribulose-1,5-bisphosphate Carboxylase/Oxygenase. Biochemistry 2005, 44, 9851–9861. [Google Scholar] [CrossRef]
- Genkov, T.; Meyer, M.; Griffiths, H.; Spreitzer, R.J. Functional Hybrid Rubisco Enzymes with Plant Small Subunits and Algal Large Subunits: Engineered rbcS cDNA for Expression in Chlamydomonas. J. Biol. Chem. 2010, 285, 19833–19841. [Google Scholar] [CrossRef] [Green Version]
- Mackinder, L.C.M.; Chen, C.; Leib, R.D.; Patena, W.; Blum, S.R.; Rodman, M.; Ramundo, S.; Adams, C.M.; Jonikas, M.C. A Spatial Interactome Reveals the Protein Organization of the Algal CO2-Concentrating Mechanism. Cell 2017, 171, 133–147. [Google Scholar] [CrossRef] [Green Version]
- Meyer, M.T.; Genkov, T.; Skepper, J.N.; Jouhet, J.; Mitchell, M.C.; Spreitzer, R.J.; Griffiths, H. Rubisco small-subunit α-helices control pyrenoid formation in Chlamydomonas. Proc. Natl. Acad. Sci. USA 2012, 109, 19474–19479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, B.; Liu, J.; Ma, X.; Guo, B.; Liu, B.; Wu, T.; Jiang, Y.; Chen, F. Genetic engineering of the Calvin cycle toward enhanced photosynthetic CO2 fixation in microalgae. Biotechnol. Biofuels 2017, 10, 229. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, T.; Tamoi, M.; Kimura, A.; Mine, A.; Sakuyama, H.; Yoshida, E.; Maruta, T.; Suzuki, K.; Ishikawa, T.; Shigeoka, S. Enhancement of photosynthetic capacity in Euglena gracilis by expression of cyanobacterial fructose-1,6-/sedoheptulose-1,7-bisphosphatase leads to increases in biomass and wax ester production. Biotechnol. Biofuels 2015, 8, 80. [Google Scholar] [CrossRef] [Green Version]
- Fang, L.; Lin, H.X.; Low, C.S.; Wu, M.H.; Chow, Y.; Lee, Y.K. Expression of the Chlamydomonas reinhardtii Sedoheptulose-1,7-bisphosphatase in Dunaliella bardawil leads to enhanced photosynthesis and increased glycerol production. Plant Biotechnol. J. 2012, 10, 1129–1135. [Google Scholar] [CrossRef]
- Liang, F.; Lindblad, P. Effects of overexpressing photosynthetic carbon flux control enzymes in the cyanobacterium Synechocystis PCC 6803. Metab. Eng. 2016, 38, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Roussou, S.; Albergati, A.; Liang, F.; Lindblad, P. Engineered cyanobacteria with additional overexpression of selected Calvin-Benson-Bassham enzymes show further increased ethanol production. Metab. Eng. Commun. 2021, 12, e00161. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Wang, Q. The Roles of sRNAs in Regulating Stress Responses in Cyanobacteria. In Microbial Photosynthesis; Wang, Q., Ed.; Springer: Singapore, 2020; pp. 245–259. [Google Scholar] [CrossRef]
- Zhan, J.; Steglich, C.; Scholz, I.; Hess, W.R.; Kirilovsky, D. Inverse regulation of light harvesting and photoprotection is mediated by a 3′-end-derived sRNA in cyanobacteria. Plant Cell 2020, 33, 358–380. [Google Scholar] [CrossRef]
- Georg, J.; Kostova, G.; Vuorijoki, L.; Schon, V.; Kadowaki, T.; Huokko, T.; Baumgartner, D.; Muller, M.; Klahn, S.; Allahverdiyeva, Y.; et al. Acclimation of Oxygenic Photosynthesis to Iron Starvation Is Controlled by the sRNA IsaR1. Curr. Biol. 2017, 27, 1425–1436.e7. [Google Scholar] [CrossRef]
- Georg, J.; Dienst, D.; Schurgers, N.; Wallner, T.; Kopp, D.; Stazic, D.; Kuchmina, E.; Klahn, S.; Lokstein, H.; Hess, W.R.; et al. The small regulatory RNA SyR1/PsrR1 controls photosynthetic functions in cyanobacteria. Plant Cell 2014, 26, 3661–3679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kadowaki, T.; Nagayama, R.; Georg, J.; Nishiyama, Y.; Wilde, A.; Hess, W.R.; Hihara, Y. A Feed-Forward Loop Consisting of the Response Regulator RpaB and the Small RNA PsrR1 Controls Light Acclimation of Photosystem I Gene Expression in the Cyanobacterium Synechocystis sp. PCC 6803. Plant Cell Physiol. 2016, 57, 813–823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, J.; Li, T.; Xu, W.; Zhan, J.; Chen, H.; He, C.; Wang, Q. Small antisense RNA RblR positively regulates RuBisCo in Synechocystis sp. PCC 6803. Front. Microbiol. 2017, 8, 231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakurai, I.; Stazic, D.; Eisenhut, M.; Vuorio, E.; Steglich, C.; Hess, W.R.; Aro, E.M. Positive regulation of psbA gene expression by cis-encoded antisense RNAs in Synechocystis sp. PCC 6803. Plant Physiol. 2012, 160, 1000–1010. [Google Scholar] [CrossRef] [Green Version]
- Bolay, P.; Schlüter, S.; Grimm, S.; Riediger, M.; Hess, W.R.; Klähn, S. The transcriptional regulator RbcR controls ribulose-1,5-bisphosphate carboxylase/oxygenase (RuBisCO) genes in the cyanobacterium Synechocystis sp. PCC 6803. New Phytol. 2022, 235, 432–445. [Google Scholar] [CrossRef]
- Eisenhut, M.; Georg, J.; Klahn, S.; Sakurai, I.; Mustila, H.; Zhang, P.; Hess, W.R.; Aro, E.M. The antisense RNA As1_flv4 in the cyanobacterium Synechocystis sp. PCC 6803 prevents premature expression of the flv4-2 operon upon shift in inorganic carbon supply. J. Biol. Chem. 2012, 287, 33153–33162. [Google Scholar] [CrossRef] [Green Version]
- Zhang, P.; Eisenhut, M.; Brandt, A.-M.; Carmel, D.; Silén, H.M.; Vass, I.; Allahverdiyeva, Y.; Salminen, T.A.; Aro, E.-M. Operon flv4-flv2 provides cyanobacterial photosystem II with flexibility of electron transfer. Plant Cell 2012, 24, 1952–1971. [Google Scholar] [CrossRef] [Green Version]
- Dühring, U.; Axmann, I.M.; Hess, W.R.; Wilde, A. An internal antisense RNA regulates expression of the photosynthesis gene isiA. Proc. Natl. Acad. Sci. USA 2006, 103, 7054–7058. [Google Scholar] [CrossRef] [Green Version]
- Fayyaz, M.; Chew, K.W.; Show, P.L.; Ling, T.C.; Ng, I.S.; Chang, J.S. Genetic engineering of microalgae for enhanced biorefinery capabilities. Biotechnol. Adv. 2020, 43, 107554. [Google Scholar] [CrossRef]
- Bajhaiya, A.K.; Ziehe Moreira, J.; Pittman, J.K. Transcriptional Engineering of Microalgae: Prospects for High-Value Chemicals. Trends Biotechnol. 2017, 35, 95–99. [Google Scholar] [CrossRef]
- Muñoz, C.F.; Südfeld, C.; Naduthodi, M.I.S.; Weusthuis, R.A.; Barbosa, M.J.; Wijffels, R.H.; D’Adamo, S. Genetic engineering of microalgae for enhanced lipid production. Biotechnol. Adv. 2021, 52, 107836. [Google Scholar] [CrossRef] [PubMed]
- Li-Beisson, Y.; Thelen, J.J.; Fedosejevs, E.; Harwood, J.L. The lipid biochemistry of eukaryotic algae. Prog. Lipid Res. 2019, 74, 31–68. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.H.; Wang, X.; Niu, Y.F.; Yang, Z.K.; Zhang, M.H.; Wang, Z.M.; Yang, W.D.; Liu, J.S.; Li, H.Y. Antisense knockdown of pyruvate dehydrogenase kinase promotes the neutral lipid accumulation in the diatom Phaeodactylum tricornutum. Microb. Cell Factories 2014, 13, 100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, X.; Yao, L.; Yang, B.; Lee, Y.K.; Chen, F.; Liu, J. RNAi-mediated silencing of a pyruvate dehydrogenase kinase enhances triacylglycerol biosynthesis in the oleaginous marine alga Nannochloropsis salina. Sci. Rep. 2017, 7, 11485. [Google Scholar] [CrossRef] [Green Version]
- Ma, X.-N.; Liu, B.; Yang, B.; Guo, B.-B.; Liu, J.; Chen, F. Physiochemical and gene expression analyses reveal differential responses of the marine oleaginous alga Nannochloropsis salina under different lipid-induction conditions. J. Appl. Phycol. 2018, 30, 909–919. [Google Scholar] [CrossRef]
- Liang, M.H.; Wang, L.; Wang, Q.; Zhu, J.; Jiang, J.G. High-value bioproducts from microalgae: Strategies and progress. Crit. Rev. Food Sci. Nutr. 2019, 59, 2423–2441. [Google Scholar] [CrossRef]
- Niu, Y.F.; Wang, X.; Hu, D.X.; Balamurugan, S.; Li, D.W.; Yang, W.D.; Liu, J.S.; Li, H.Y. Molecular characterization of a glycerol-3-phosphate acyltransferase reveals key features essential for triacylglycerol production in Phaeodactylum tricornutum. Biotechnol. Biofuels 2016, 9, 60. [Google Scholar] [CrossRef] [Green Version]
- Balamurugan, S.; Wang, X.; Wang, H.L.; An, C.J.; Li, H.; Li, D.W.; Yang, W.D.; Liu, J.S.; Li, H.Y. Occurrence of plastidial triacylglycerol synthesis and the potential regulatory role of AGPAT in the model diatom Phaeodactylum tricornutum. Biotechnol. Biofuels 2017, 10, 97. [Google Scholar] [CrossRef] [Green Version]
- Chungjatupornchai, W.; Areerat, K.; Fa-Aroonsawat, S. Increased triacylglycerol production in oleaginous microalga Neochloris oleoabundans by overexpression of plastidial lysophosphatidic acid acyltransferase. Microb. Cell Factories 2019, 18, 53. [Google Scholar] [CrossRef]
- Zou, L.G.; Chen, J.W.; Zheng, D.L.; Balamurugan, S.; Li, D.W.; Yang, W.D.; Liu, J.S.; Li, H.Y. High-efficiency promoter-driven coordinated regulation of multiple metabolic nodes elevates lipid accumulation in the model microalga Phaeodactylum tricornutum. Microb. Cell Factories 2018, 17, 54. [Google Scholar] [CrossRef] [Green Version]
- Lin, J.-Y.; Sri Wahyu Effendi, S.; Ng, I.S. Enhanced carbon capture and utilization (CCU) using heterologous carbonic anhydrase in Chlamydomonas reinhardtii for lutein and lipid production. Bioresour. Technol. 2022, 351, 127009. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.-R.; Lai, Y.-C.; Sung, P.-K.; Tan, S.-I.; Chang, C.-H.; Chen, C.-Y.; Chang, J.-S.; Ng, I.S. Enhancing carbon capture and lipid accumulation by genetic carbonic anhydrase in microalgae. J. Taiwan Inst. Chem. Eng. 2018, 93, 131–141. [Google Scholar] [CrossRef]
- Yusuf, M.; Shabbir, M.; Mohammad, F. Natural Colorants: Historical, Processing and Sustainable Prospects. Nat. Prod. Bioprospecting 2017, 7, 123–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pagels, F.; Guedes, A.C.; Amaro, H.M.; Kijjoa, A.; Vasconcelos, V. Phycobiliproteins from cyanobacteria: Chemistry and biotechnological applications. Biotechnol. Adv. 2019, 37, 422–443. [Google Scholar] [CrossRef]
- Wang, L.; Liu, Z.; Jiang, H.; Mao, X. Biotechnology advances in β-carotene production by microorganisms. Trends Food Sci. Technol. 2021, 111, 322–332. [Google Scholar] [CrossRef]
- Capa-Robles, W.; García-Mendoza, E. Enhanced β-carotene and Biomass Production by Induced Mixotrophy in Dunaliella salina across a Combined Strategy of Glycerol, Salinity, and Light. Metabolites 2021, 11, 866. [Google Scholar] [CrossRef]
- Ren, Y.; Deng, J.; Huang, J.; Wu, Z.; Yi, L.; Bi, Y.; Chen, F. Using green alga Haematococcus pluvialis for astaxanthin and lipid co-production: Advances and outlook. Bioresour. Technol. 2021, 340, 125736. [Google Scholar] [CrossRef]
- Pereira, A.G.; Otero, P. Xanthophylls from the Sea: Algae as Source of Bioactive Carotenoids. Mar. Drugs 2021, 19, 188. [Google Scholar] [CrossRef]
- Black, H.S.; Boehm, F.; Edge, R. The Benefits and Risks of Certain Dietary Carotenoids that Exhibit both Anti- and Pro-Oxidative Mechanisms-A Comprehensive Review. Antioxidants 2020, 9, 264. [Google Scholar] [CrossRef]
- Bohn, T. Carotenoids, Chronic Disease Prevention and Dietary Recommendations. Int. J. Vitam. Nutr. Res. 2017, 87, 121–130. [Google Scholar] [CrossRef]
- Bouyahya, A.; El Omari, N.; Hakkur, M.; El Hachlafi, N.; Charfi, S.; Balahbib, A.; Guaouguaou, F.-E.; Rebezov, M.; Maksimiuk, N.; Shariati, M.A.; et al. Sources, health benefits, and biological properties of zeaxanthin. Trends Food Sci. Technol. 2021, 118, 519–538. [Google Scholar] [CrossRef]
- Di Lena, G.; Casini, I.; Lucarini, M.; Lombardi-Boccia, G. Carotenoid profiling of five microalgae species from large-scale production. Food Res. Int. (Ott. Ont.) 2019, 120, 810–818. [Google Scholar] [CrossRef] [PubMed]
- Mohamadnia, S.; Tavakoli, O.; Faramarzi, M.A. Enhancing production of fucoxanthin by the optimization of culture media of the microalga Tisochrysis lutea. Aquaculture 2021, 533, 736074. [Google Scholar] [CrossRef]
- Li, Y.; Sun, H.; Wu, T.; Fu, Y.; He, Y.; Mao, X.; Chen, F. Storage carbon metabolism of Isochrysis zhangjiangensis under different light intensities and its application for co-production of fucoxanthin and stearidonic acid. Bioresour. Technol. 2019, 282, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.; da Silva, G.M.; Silva, A.; Lima, L.R.A.; Bezerra, R.P. Bioactive Compounds of Arthrospira spp. (Spirulina) with Potential Anticancer Activities: A Systematic Review. ACS Chem. Biol. 2021, 16, 2057–2067. [Google Scholar] [CrossRef]
- Braune, S.; Krüger-Genge, A.; Kammerer, S. Phycocyanin from Arthrospira platensis as Potential Anti-Cancer Drug: Review of In Vitro and In Vivo Studies. Life 2021, 11, 91. [Google Scholar] [CrossRef]
- Langi, P.; Kiokias, S.; Varzakas, T.; Proestos, C. Carotenoids: From Plants to Food and Feed Industries. Methods Mol. Biol. (Clifton N. J.) 2018, 1852, 57–71. [Google Scholar] [CrossRef]
- Bhalamurugan, G.L.; Valérie, O.; Mark, L. Valuable bioproducts obtained from microalgal biomass and their commercial applications: A review. Environ. Eng. Res. 2018, 23, 229–241. [Google Scholar] [CrossRef] [Green Version]
- Saini, D.K.; Chakdar, H.; Pabbi, S.; Shukla, P. Enhancing production of microalgal biopigments through metabolic and genetic engineering. Crit. Rev. Food Sci. Nutr. 2020, 60, 391–405. [Google Scholar] [CrossRef]
- Zhu, Q.L.; Zheng, J.L.; Liu, J. Transcription activation of β-carotene biosynthetic genes at the initial stage of stresses as an indicator of the increased β-carotene accumulation in isolated Dunaliella salina strain GY-H13. Aquat. Toxicol. (Amst. Neth.) 2020, 222, 105472. [Google Scholar] [CrossRef]
- Chavarriaga-Aguirre, P.; Prías, M.; López, D.; Ortiz, D.; Toro-Perea, N.; Tohme, J. Molecular analysis of the expression of a crtB transgene and the endogenous psy2-y (1) and psy2-y (2) genes of cassava and their effect on root carotenoid content. Transgenic Res. 2017, 26, 639–651. [Google Scholar] [CrossRef] [PubMed]
- Chakdar, H.; Pabbi, S. Chapter 9—Algal Pigments for Human Health and Cosmeceuticals. In Algal Green Chemistry; Rastogi, R.P., Madamwar, D., Pandey, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 171–188. [Google Scholar] [CrossRef]
- Chi, S.; He, Y.; Ren, J.; Su, Q.; Liu, X.; Chen, Z.; Wang, M.; Li, Y.; Li, J. Overexpression of a bifunctional enzyme, CrtS, enhances astaxanthin synthesis through two pathways in Phaffia rhodozyma. Microb. Cell Factories 2015, 14, 90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perozeni, F.; Cazzaniga, S.; Baier, T.; Zanoni, F.; Zoccatelli, G.; Lauersen, K.J.; Wobbe, L.; Ballottari, M. Turning a green alga red: Engineering astaxanthin biosynthesis by intragenic pseudogene revival in Chlamydomonas reinhardtii. Plant Biotechnol. J. 2020, 18, 2053–2067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cazzaniga, S.; Perozeni, F.; Baier, T.; Ballottari, M. Engineering astaxanthin accumulation reduces photoinhibition and increases biomass productivity under high light in Chlamydomonas reinhardtii. Biotechnol. Biofuels Bioprod. 2022, 15, 77. [Google Scholar] [CrossRef]
- Anila, N.; Simon, D.P.; Chandrashekar, A.; Ravishankar, G.A.; Sarada, R. Metabolic engineering of Dunaliella salina for production of ketocarotenoids. Photosynth. Res. 2016, 127, 321–333. [Google Scholar] [CrossRef]
- Patel, V.K.; Soni, N.; Prasad, V.; Sapre, A.; Dasgupta, S.; Bhadra, B. CRISPR-Cas9 System for Genome Engineering of Photosynthetic Microalgae. Mol. Biotechnol. 2019, 61, 541–561. [Google Scholar] [CrossRef]
- Baek, K.; Yu, J.; Jeong, J.; Sim, S.J.; Bae, S.; Jin, E. Photoautotrophic production of macular pigment in a Chlamydomonas reinhardtii strain generated by using DNA-free CRISPR-Cas9 RNP-mediated mutagenesis. Biotechnol. Bioeng. 2018, 115, 719–728. [Google Scholar] [CrossRef]
- Kao, P.H.; Ng, I.S. CRISPRi mediated phosphoenolpyruvate carboxylase regulation to enhance the production of lipid in Chlamydomonas reinhardtii. Bioresour. Technol. 2017, 245, 1527–1537. [Google Scholar] [CrossRef]
- Lin, P.-C.; Pakrasi, H.B. Engineering cyanobacteria for production of terpenoids. Planta 2019, 249, 145–154. [Google Scholar] [CrossRef]
- Chaves, J.E.; Melis, A. Biotechnology of cyanobacterial isoprene production. Appl. Microbiol Biotechnol 2018, 102, 6451–6458. [Google Scholar] [CrossRef]
- Formighieri, C.; Melis, A. A phycocyanin·phellandrene synthase fusion enhances recombinant protein expression and β-phellandrene (monoterpene) hydrocarbons production in Synechocystis (cyanobacteria). Metab. Eng. 2015, 32, 116–124. [Google Scholar] [CrossRef]
- Tounsi, L.; Hentati, F.; Ben Hlima, H.; Barkallah, M.; Smaoui, S.; Fendri, I.; Michaud, P.; Abdelkafi, S. Microalgae as feedstock for bioactive polysaccharides. Int. J. Biol. Macromol. 2022, 221, 1238–1250. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Gu, X.; Yang, D.; Xu, S. Bioactive substances and potentiality of marine microalgae. Food Sci. Nutr. 2021, 9, 5279–5292. [Google Scholar] [CrossRef] [PubMed]
- Pierre, G.; Delattre, C. What Is in Store for EPS Microalgae in the Next Decade? Molecules 2019, 24, 4296. [Google Scholar] [CrossRef] [Green Version]
- Lakatos, G.E.; Ranglová, K.; Manoel, J.C.; Grivalský, T.; Kopecký, J.; Masojídek, J. Bioethanol production from microalgae polysaccharides. Folia Microbiol. 2019, 64, 627–644. [Google Scholar] [CrossRef] [PubMed]
- Yi, Z.; Su, Y.; Brynjolfsson, S.; Olafsdóttir, K.; Fu, W. Chapter 3—Bioactive polysaccharides and their derivatives from microalgae: Biosynthesis, applications, and challenges. In Studies in Natural Products Chemistry; Atta ur, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2021; Volume 71, pp. 67–85. [Google Scholar]
- Qi, J.; Kim, S.M. Characterization and immunomodulatory activities of polysaccharides extracted from green alga Chlorella ellipsoidea. Int. J. Biol. Macromol. 2017, 95, 106–114. [Google Scholar] [CrossRef]
- Uppin, V.; Dharmesh, S.M. Polysaccharide from Spirulina platensis Evokes Antitumor Activity in Gastric Cancer Cells via Modulation of Galectin-3 and Exhibited Cyto/DNA Protection: Structure-Function Study. J. Agric. Food Chem. 2022, 70, 7058–7069. [Google Scholar] [CrossRef]
- Netanel Liberman, G.; Ochbaum, G.; Mejubovsky-Mikhelis, M.; Bitton, R.; Malis Arad, S. Physico-chemical characteristics of the sulfated polysaccharides of the red microalgae Dixoniella grisea and Porphyridium aerugineum. Int. J. Biol. Macromol. 2020, 145, 1171–1179. [Google Scholar] [CrossRef]
- Goyal, M.; Baranwal, M. Hetero-Polysaccharides Secreted from Dunaliella salina Exhibit Immunomodulatory Activity Against Peripheral Blood Mononuclear Cells and RAW 264.7 Macrophages. Indian J. Microbiol. 2019, 59, 428–435. [Google Scholar] [CrossRef]
- Zhu, J.; Wakisaka, M. Growth promotion of Euglena gracilis by ferulic acid from rice bran. AMB Express 2018, 8, 16. [Google Scholar] [CrossRef]
- Gaignard, C.; Gargouch, N.; Dubessay, P.; Delattre, C.; Pierre, G.; Laroche, C.; Fendri, I.; Abdelkafi, S.; Michaud, P. New horizons in culture and valorization of red microalgae. Biotechnol. Adv. 2019, 37, 193–222. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Xu, X. The functional divergence of two glgP homologues in Synechocystis sp. PCC 6803. FEMS Microbiol. Lett. 2006, 260, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Kumaraswamy, G.K.; Guerra, T.; Qian, X.; Zhang, S.; Bryant, D.A.; Dismukes, G.C. Reprogramming the glycolytic pathway for increased hydrogen production in cyanobacteria: Metabolic engineering of NAD+-dependent GAPDH. Energy Environ. Sci. 2013, 6, 3722–3731. [Google Scholar] [CrossRef]
- Pancha, I.; Shima, H.; Higashitani, N.; Igarashi, K.; Higashitani, A.; Tanaka, K.; Imamura, S. Target of rapamycin-signaling modulates starch accumulation via glycogenin phosphorylation status in the unicellular red alga Cyanidioschyzon merolae. Plant J. 2019, 97, 485–499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pancha, I.; Tanaka, K.; Imamura, S. Overexpression of a glycogenin, CmGLG2, enhances floridean starch accumulation in the red alga Cyanidioschyzon merolae. Plant Signal. Behav. 2019, 14, 1596718. [Google Scholar] [CrossRef]
- Mularczyk, M.; Michalak, I. Astaxanthin and other Nutrients from Haematococcus pluvialis-Multifunctional Applications. Mar. Drugs 2020, 18, 459. [Google Scholar] [CrossRef]
- Zhang, Y.-T.; Jiang, J.-Y.; Shi, T.-Q.; Sun, X.-M.; Zhao, Q.-Y.; Huang, H.; Ren, L.-J. Application of the CRISPR/Cas system for genome editing in microalgae. Appl. Microbiol. Biotechnol. 2019, 103, 3239–3248. [Google Scholar] [CrossRef]
- Yang, R.; Wei, D.; Xie, J. Diatoms as cell factories for high-value products: Chrysolaminarin, eicosapentaenoic acid, and fucoxanthin. Crit. Rev. Biotechnol. 2020, 40, 993–1009. [Google Scholar] [CrossRef]

| Pigment | Microalgae Strains | Application | Reference |
|---|---|---|---|
| β-carotene | Dunaliella salina, Chlorella zofingiensis, Spirulina spp. | The precursor of vitamin A, its antioxidant property, and its use to prevent macular degeneration, asthma, pharmaceutical, and cosmetics | [86,87] |
| Astaxanthin | Haematococus pluvialis, Nannochloropsis oculate, Chlorococcus spp. | UV protection, food colorant, anti-aging, immune enhancement, pharmaceutical, anti-hypertensive, and anti-cancer properties; anti-inflammatory | [88,89] |
| Lutein | Chlorella vulgaris, Chlorococcum citroforme | Feed additive and food colorant aid in the regulation of cancers, cardiovascular diseases, cognitive function, and age-related macular degeneration in humans | [90,91] |
| Zeaxanthin | Nannochloropsis oculate, Porphyridium cruentum | Food additives, amelioration of age-related macular degeneration, antioxidants, anti-inflammatory agents, and prevention of neurological disease | [92,93] |
| Fucoxanthin | Phaeodactylum tricornutum | Anti-cancer, anti-inflammatory, and anti-obesity effects | [94,95] |
| Phycocyanin | Spirulina spp., Arthrospira platensis | Used as fluorescent reagents for hepatoprotective activity, antioxidant activity, anti-inflammatory activity, and neuroprotective activity | [96,97] |
| Lycopene | Chlorella marina | Antioxidants are used as treatments for cardiovascular diseases and prostate cancer | [98,99] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, J.; Wang, D.; Chen, H.; Wang, Q. Advances in Genetic Engineering in Improving Photosynthesis and Microalgal Productivity. Int. J. Mol. Sci. 2023, 24, 1898. https://doi.org/10.3390/ijms24031898
Hu J, Wang D, Chen H, Wang Q. Advances in Genetic Engineering in Improving Photosynthesis and Microalgal Productivity. International Journal of Molecular Sciences. 2023; 24(3):1898. https://doi.org/10.3390/ijms24031898
Chicago/Turabian StyleHu, Jinlu, Dan Wang, Hui Chen, and Qiang Wang. 2023. "Advances in Genetic Engineering in Improving Photosynthesis and Microalgal Productivity" International Journal of Molecular Sciences 24, no. 3: 1898. https://doi.org/10.3390/ijms24031898
APA StyleHu, J., Wang, D., Chen, H., & Wang, Q. (2023). Advances in Genetic Engineering in Improving Photosynthesis and Microalgal Productivity. International Journal of Molecular Sciences, 24(3), 1898. https://doi.org/10.3390/ijms24031898