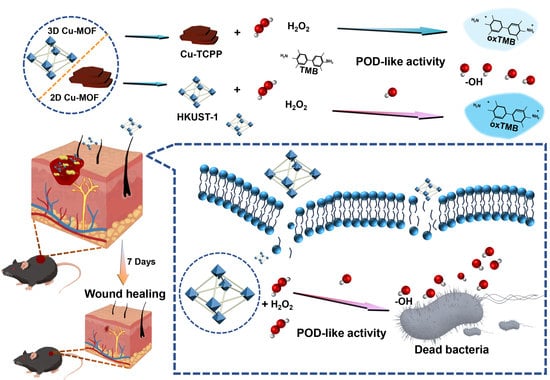
Different Dimensional Copper-Based Metal–Organic Frameworks with Enzyme-Mimetic Activity for Antibacterial Therapy
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of Cu-MOFs
2.2. POD-like Catalytic Activity of Cu-MOFs
2.3. Detection of •OH
2.4. Antibacterial Assay In Vitro
2.5. Evaluation of Antibacterial Activity In Vivo
2.6. Biosafety Assay In Vivo
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Cu-MOFs
3.3. POD-like Catalytic Activity of Cu-MOFs
3.4. Detection of •OH
3.5. Bacterial Culture and Antibacterial Assay In Vitro
3.6. Evaluation of Antibacterial Activity In Vivo
3.7. Biosafety Assay In Vivo
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The chemistry and applications of metal-organic frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef]
- Xie, Y.; Shi, X.; Chen, L.; Lu, J.; Lu, X.; Sun, D.; Zhang, L. Direct electrodeposition of bimetallic nanostructures on Co-based MOFs for electrochemical sensing of hydrogen peroxide. Front. Chem. 2022, 10, 856003. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yang, Y.W. Metal-organic frameworks for biomedical applications. Small 2020, 16, e1906846. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.P.; Yang, D.C.; Wei, P.; Liu, B.; Chen, Z.G.; Zhang, L.Y.; Lu, J. One-step electrodeposition of silver nanostructures on 2D/3D metal-organic framework ZIF-67: Comparison and application in electrochemical detection of hydrogen peroxide. ACS Appl. Mater. Interfaces 2020, 12, 41960–41968. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, F.; Ding, S.; Shen, J.; Zhu, K. Sublethal levels of antibiotics promote bacterial persistence in epithelial cells. Adv. Sci. 2020, 7, 1900840. [Google Scholar] [CrossRef]
- Zhang, S.; Rong, F.L.; Guo, C.P.; Duan, F.H.; He, L.H.; Wang, M.H.; Zhang, Z.H.; Kang, M.M.; Du, M. Metal-organic frameworks (MOFs) based electrochemical biosensors for early cancer diagnosis in vitro. Coord. Chem. Rev. 2021, 439, 213948. [Google Scholar] [CrossRef]
- Dong, L.Z.; Ran, F.S.; Ying, G.C.; Cong, L.W.; Xiang, C.J.; Hong, L.B.; Qiang, L.J. Metal-organic framework (MOF)-based nanomaterials for biomedical applications. Curr. Med. Chem. 2019, 26, 3341–3369. [Google Scholar]
- Xie, X.; Ke, R.; Cheng, C.; Wang, Y.-H.; Song, Z.; Zhang, C.D.; Wang, H.S. Multiple adsorption properties of aptamers on metal-organic frameworks for nucleic acid assay. Biosens. Bioelectron. 2021, 176, 112896. [Google Scholar] [CrossRef]
- Fan, X.; Shi, Q.F.; Nan, Z.D. Facile synthesis of Cu-CuFeO nanozymes for sensitive assay of H2O2 and GSH. Dalton Trans. 2020, 49, 12780–12792. [Google Scholar]
- Lin, C.; Guo, X.; Chen, L.; You, T.; Lu, J.; Sun, D. Ultrathin trimetallic metal–organic framework nanosheets for accelerating bacteria-infected wound healing. J. Colloid Interface Sci. 2022, 628, 731–744. [Google Scholar] [CrossRef]
- Long, Y.; Li, L.; Xu, T.; Wu, X.; Gao, Y.; Huang, J.; He, C.; Ma, T.; Ma, L.; Cheng, C.; et al. Hedgehog artificial macrophage with atomic-catalytic centers to combat Drug-resistant bacteria. Nat. Commun. 2021, 12, 6143. [Google Scholar] [CrossRef] [PubMed]
- Shams, S.; Ahmad, W.; Memon, A.H.; Shams, S.; Wei, Y.; Yuan, Q.; Liang, H. Cu/H3BTC MOF as a potential antibacterial therapeutic agent against Staphylococcus aureus and Escherichia coli. New J. Chem. 2020, 44, 17671–17678. [Google Scholar] [CrossRef]
- Li, L.; Cao, L.J.; Xiang, X.; Wu, X.Z.; Ma, L.; Chen, F.; Cao, S.J.; Cheng, C.; Deng, D.W.; Qiu, L. ROS-Catalytic transition-metal-based enzymatic nanoagents for tumor and bacterial eradication. Adv. Funct. Mater. 2021, 32, 2107530. [Google Scholar] [CrossRef]
- Chakrabarti, S.; Chattopadhyay, P.; Islam, J.; Ray, S.; Raju, P.S.; Mazumder, B. Aspects of nanomaterials in wound healing. Curr. Drug Deliv. 2019, 16, 26–41. [Google Scholar] [CrossRef]
- Mao, Z.H.; Chen, J.; Wang, Y.D.; Xia, J.J.; Zhang, Y.J.; Zhang, W.W.; Zhu, H.; Hu, X.J.; Chen, H.X. Copper metal organic framework as natural oxidase mimic for effective killing of Gram-negative and Gram-positive bacteria. Nanoscale 2022, 14, 9474–9484. [Google Scholar] [CrossRef]
- Guo, L.; Sun, J.; Zhang, W.; Hou, L.; Liang, L.; Liu, Y.; Yuan, C. Bottom-up fabrication of 1D Cu-based conductive metal–organic framework nanowires as a high-rate anode towards efficient lithium storage. ChemSusChem 2019, 12, 5051–5058. [Google Scholar] [CrossRef]
- Guo, X.J.; Lin, C.Y.; Zhang, M.J.; Duan, X.W.; Dong, X.R.; Sun, D.P.; Pan, J.B.; You, T.H. 2D/3D Copper-based metal-organic frameworks for electrochemical detection of hydrogen peroxide. Front. Chem. 2021, 9, 743637. [Google Scholar] [CrossRef]
- Zhao, M.T.; Huang, Y.; Peng, Y.W.; Huang, Z.Q.; Ma, Q.L.; Zhang, H. Two-dimensional metal-organic framework nanosheets: Synthesis and applications. Chem. Soc. Rev. 2018, 47, 6267–6295. [Google Scholar] [CrossRef]
- Cai, X.; Xie, Z.; Li, D.; Kassymova, M.; Zang, S.-Q.; Jiang, H.-L. Nano-sized metal-organic frameworks: Synthesis and applications. Coord. Chem. Rev. 2020, 417, 213366. [Google Scholar] [CrossRef]
- Diring, S.; Furukawa, S.; Takashima, Y.; Tsuruoka, T.; Kitagawa, S. Controlled multiscale synthesis of porous coordination polymer innano/micro regimes. Chem. Mater. 2010, 22, 4531–4538. [Google Scholar] [CrossRef]
- Tsuruoka, T.; Furukawa, S.; Takashima, Y.; Yoshida, K.; Isoda, S.; Kitagawa, S. Nanoporous nanorods fabricated by coordination modulation and oriented attachment growth. Angew. Chem. Int. Ed. 2009, 48, 4739–4743. [Google Scholar] [CrossRef] [PubMed]
- Cun, J.E.; Fan, X.; Pan, Q.Q.; Gao, W.X.; Luo, K.; He, B.; Pu, Y.J. Copper-based metal-organic frameworks for biomedical applications. Adv. Colloid Interface Sci. 2022, 305, 102686. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Y.; Yan, F.; Ren, P.; Li, Y.; Wu, Q.; Fang, X.D.; Chen, F.F.; Wang, C. Incorporation of metal-organic frameworks into electrospun chitosan/poly (vinyl alcohol) nanofibrous membrane with enhanced antibacterial activity for wound dressing application. Int. J. Biol. Macromol. 2020, 158, 9–17. [Google Scholar] [CrossRef]
- Shanmugapriya, K.; Kang, H.W. Engineering pharmaceutical nanocarriers for photodynamic therapy on wound healing: Review. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 105, 110110. [Google Scholar] [CrossRef] [PubMed]
- Esmaeili, E.; Eslami-Arshaghi, T.; Hosseinzadeh, S.; Elahirad, E.; Jamalpoor, Z.; Hatamie, S.; Soleimani, M. The biomedical potential of cellulose acetate/polyurethane nanofibrous mats containing reduced graphene oxide/silver nanocomposites and curcumin: Antimicrobial performance and cutaneous wound healing. Int. J. Biol. Macromol. 2020, 152, 418–427. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Y.; Xu, J.; Chai, Y.S.; Zhang, J.; Hu, Z.Q.; Zhou, H.Y. Nano-silver modified porcine small intestinal submucosa for the treatment of infected partial-thickness burn wounds. Burns 2019, 45, 950–956. [Google Scholar] [CrossRef] [PubMed]
- Mihai, M.M.; Dima, M.B.; Dima, B.; Holban, A.M. Nanomaterials for wound healing and infection control. Materials 2019, 12, 2176. [Google Scholar] [CrossRef]
- Qian, W.; Yan, C.; He, D.F.; Yu, X.Z.; Yuan, L.; Liu, M.L.; Luo, G.X.; Deng, J. pH-triggered charge-reversible of glycol chitosan conjugated carboxyl graphene for enhancing photothermal ablation of focal infection. Acta Biomater. 2018, 69, 256–264. [Google Scholar] [CrossRef]
- Mirzahosseinipour, M.; Khorsandi, K.; Hosseinzadeh, R.; Ghazaeian, M.; Shahidi, F.K. Antimicrobial photodynamic and wound healing activity of curcumin encapsulated in silica nanoparticles. Photodiagn. Photodyn. Ther. 2020, 29, 101639. [Google Scholar] [CrossRef]
- Wang, S.G.; Chen, Y.C.; Chen, Y.C. Antibacterial gold nanoparticle-based photothermal killing of vancomycin-resistant bacteria. Nanomedicine 2018, 13, 1405–1416. [Google Scholar] [CrossRef]
- Yu, X.Z.; He, D.F.; Zhang, X.M.; Zhang, H.M.; Song, J.L.; Shi, D.Z.; Fan, Y.H.; Luo, G.X.; Deng, J. Surface-adaptive and initiator-loaded graphene as a light-induced generator with free radicals for drug-resistant bacteria eradication. ACS Appl. Mater. Interfaces 2019, 11, 1766–1781. [Google Scholar] [CrossRef] [PubMed]
- Alavi, M.; Nokhodchi, A. An overview on antimicrobial and wound healing properties of ZnO nanobiofilms, hydrogels, and bionanocomposites based on cellulose, chitosan, and alginate polymers. Carbohydr. Polym. 2020, 227, 115349. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.T.; Wang, Y.X.; Ma, Q.L.; Huang, Y.; Zhang, X.; Ping, J.F.; Zhang, Z.C.; Lu, Q.P.; Yu, Y.F.; Xu, H.; et al. Ultrathin 2D metal-organic framework nanosheets. Adv. Mater. 2015, 27, 7372–7378. [Google Scholar] [CrossRef] [PubMed]
- Chui, S.S.Y.; Lo, S.M.F.; Charmant, J.P.H.; Orpen, A.G.; Williams, I.D. A chemically functionalizable nanoporous material Cu-3(TMA)(2)(H2O)(3)(n). Science 1999, 283, 1148–1150. [Google Scholar] [CrossRef]
- Wang, S.J.; Xu, D.P.; Ma, L.; Qiu, J.X.; Wang, X.; Dong, Q.L.; Zhang, Q.; Pan, J.; Liu, Q. Ultrathin ZIF-67 nanosheets as a colorimetric biosensing platform for peroxidase-like catalysis. Anal. Bioanal. Chem. 2018, 410, 7145–7152. [Google Scholar] [CrossRef]
- Wang, C.; Gao, J.; Tan, H. Integrated antibody with catalytic metal–organic framework for colorimetric immunoassay. ACS Appl. Mater. Interfaces 2018, 10, 25113–25120. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Y.; Li, W.Y.; Zheng, Y.Q. Nitro-functionalized metal-organic frameworks with catalase mimic properties for glutathione detection. Analyst 2019, 144, 6041–6047. [Google Scholar] [CrossRef]
- Yu, H.; Wu, H.L.; Tian, X.M.; Zhou, Y.F.; Ren, C.G.; Wang, Z.H. A nano-sized Cu-MOF with high peroxidase-like activity and its potential application in colorimetric detection of H2O2 and glucose. RSC Adv. 2021, 11, 26963–26973. [Google Scholar] [CrossRef]
- Yang, H.J.; Liu, J.; Feng, X.; Nie, F.; Yang, G.P. A novel copper-based metal-organic framework as a peroxidase-mimicking enzyme and its glucose chemiluminescence sensing application. Anal. Bioanal. Chem. 2021, 413, 4407–4416. [Google Scholar] [CrossRef]
- Liao, X.; Xu, Q.; Sun, H.; Liu, W.; Chen, Y.; Xia, X.H.; Wang, C. Plasmonic nanozymes: Localized surface plasmonic resonance regulates reaction kinetics and antibacterial performance. J. Phys. Chem. Lett. 2022, 13, 312–323. [Google Scholar] [CrossRef]
- Gao, L.; Zhuang, J.; Nie, L.; Zhang, J.; Zhang, Y.; Gu, N.; Wang, T.; Feng, J.; Yang, D.; Perrett, S.; et al. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat. Nanotechnol. 2007, 2, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Cao, S.; Ma, T.; Xiang, X.; Luo, H.; Borovskikh, P.; Rodriguez, R.D.; Guo, Q.; Qiu, L.; Cheng, C. Engineering biofunctional enzyme-mimics for catalytic therapeutics and diagnostics. Adv. Funct. Mater. 2020, 31, 2007475. [Google Scholar] [CrossRef]
- Wu, R.B.; Qian, X.K.; Yu, F.; Liu, H.; Zhou, K.; Wei, J.; Huang, Y.Z. MOF-templated formation of porous CuO hollow octahedra for lithium-ion battery anode materials. J. Mater. Chem. A 2013, 1, 11126–11129. [Google Scholar] [CrossRef]






| Catalyst | Substance | Vmax (mol L−1 s−1) | References | |
|---|---|---|---|---|
| ZIF-67 | TMB | 13.69 | 3.5 × 10−7 | [35] |
| H2O2 | 3.52 | 2.8 × 10−7 | ||
| Antibody@Cu-MOFs | TMB | 3.91 | 5.445 × 10−7 | [36] |
| H2O2 | 7.37 | 1.075 × 10−7 | ||
| Cu-MOFs (CuCl2) | TMB | 4.11 | 5.556 × 10−7 | [36] |
| H2O2 | 6.41 | 1.02 × 10−7 | ||
| Cu-MOFs (Cu(NO3)2) | TMB | 2.56 | 2.5 × 10−7 | [37] |
| H2O2 | 4.34 | 1.82 × 10−7 | ||
| Cu-MOFs (Cu(NO3)2) | TMB | 0.456 | 2.478 × 10−8 | [38] |
| H2O2 | 28.58 | 5.45 × 10−8 | ||
| Cu-MOFs (CuI) | TMB | 2.4862 | 7.517 × 10−8 | [39] |
| H2O2 | 0.163 | 6.736 × 10−8 | ||
| CuFe2O4 | TMB | 2.26 | 2.07 × 10−8 | [9] |
| H2O2 | 0.5 | 2.61 × 10−8 | ||
| (Ni2Co1)0.5Cu0.5 MOFs | TMB | 0.34 | 1.81 × 10−8 | [10] |
| H2O2 | 1.08 | 1.29 × 10−8 | ||
| AuNPs/Cu-MOFs | TMB | 0.29 | 2.96 × 10−7 | [40] |
| H2O2 | 0.65 | 2.25 × 10−7 | ||
| HRP | TMB | 0.434 | 10.0 × 10−8 | [41] |
| H2O2 | 3.702 | 8.71 × 10−8 | ||
| HKUST-1 | TMB | 0.545 | 0.833 × 10−8 | This work |
| H2O2 | 2.036 | 1.757 × 10−8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, C.; Guo, X.; Mo, F.; Sun, D. Different Dimensional Copper-Based Metal–Organic Frameworks with Enzyme-Mimetic Activity for Antibacterial Therapy. Int. J. Mol. Sci. 2023, 24, 3173. https://doi.org/10.3390/ijms24043173
Lin C, Guo X, Mo F, Sun D. Different Dimensional Copper-Based Metal–Organic Frameworks with Enzyme-Mimetic Activity for Antibacterial Therapy. International Journal of Molecular Sciences. 2023; 24(4):3173. https://doi.org/10.3390/ijms24043173
Chicago/Turabian StyleLin, Chuyan, Xiangjian Guo, Fayin Mo, and Duanping Sun. 2023. "Different Dimensional Copper-Based Metal–Organic Frameworks with Enzyme-Mimetic Activity for Antibacterial Therapy" International Journal of Molecular Sciences 24, no. 4: 3173. https://doi.org/10.3390/ijms24043173
APA StyleLin, C., Guo, X., Mo, F., & Sun, D. (2023). Different Dimensional Copper-Based Metal–Organic Frameworks with Enzyme-Mimetic Activity for Antibacterial Therapy. International Journal of Molecular Sciences, 24(4), 3173. https://doi.org/10.3390/ijms24043173